SU5416 and SU6668 are potent antiangiogenic small-molecule inhibitors of receptor tyrosine kinases, including those of the vascular endothelial growth factor and platelet-derived growth factor receptor families. The stem cell factor (SCF) receptor, c-kit, is structurally related to these receptors and, although not expressed on mature peripheral blood cells, is expressed in leukemic blasts derived from 60% to 80% of acute myeloid leukemia (AML) patients. The c-kit kinase inhibitory activity of SU5416 and SU6668 was evaluated in MO7E cells, a human myeloid leukemia cell line. Tyrosine autophosphorylation of the receptor, induced by SCF, was inhibited in these cells by SU5416 and SU6668 in a dose-dependent manner (inhibitory concentration of 50% [IC50] 0.1-1 μM). Inhibition of extracellular signal–regulated kinase 1/2 (ERK1/2) phosphorylation, a signaling event downstream of c-kit activation, was also inhibited in a dose-dependent manner. Both compounds also inhibited SCF-induced proliferation of MO7E cells (IC50 0.1 μM for SU5416; 0.29 μM for SU6668). Furthermore, both SU5416 and SU6668 induced apoptosis in a dose- and time-dependent manner as measured by the increase in activated caspase-3 and the enhanced cleavage of its substrate poly(ADP-ribose) polymerase. These findings with MO7E cells were extended to leukemic blasts from c-kit+ patients. In patient blasts, both SU5416 and SU6668 inhibited SCF-induced phosphorylation of c-kit and ERK1/2 and induced apoptosis. These studies indicate that SU5416 and SU6668 inhibit biologic functions of c-kit in addition to exhibiting antiangiogenic properties and suggest that the combination of these activities may provide a novel therapeutic approach for the treatment of AML.
Introduction
The receptor tyrosine kinase (RTK) c-kit is essential for the development of normal hematopoietic cells and has been proposed to play a functional role in acute myeloid leukemia (AML).1,2 c-Kit is a member of the class III family of RTKs, characterized by an extracellular ligand binding region containing 5 immunoglobulin repeats, a hydrophobic transmembrane domain, and an intracellular kinase domain split by an insert.3 Binding of the c-kit ligand, stem cell factor (SCF), initiates a signal transduction cascade that includes receptor autophosphorylation and subsequent phosphorylation on numerous intracellular substrates. c-Kit and its ligand play a pivotal role in normal hematopoiesis, as evidenced by naturally occurring murine mutations at the Sl locus, which encodes SCF, as well as in the c-kit receptor itself. These mutations result in varying degrees of macrocytic anemia and a loss of mast cells in addition to deficiencies in gametogenesis and melanogenesis.4-6
In addition to its role in normal hematopoiesis, c-kit is expressed in leukemic blasts in approximately 60% to 80% of AML patients, as assessed by surface immunostaining using antibodies specific to c-kit or by expression of c-kit messenger RNA.1,7,8 Supporting a functional role for c-kit in AML, increased tyrosine phosphorylation of the receptor, as well as a proliferative response upon SCF stimulation, has been demonstrated in leukemic blasts in most AML cases that were c-kit+.1 2 The proliferative response to SCF has been shown to be synergistic with granulocyte-macrophage colony-stimulating factor (GM-CSF) or interleukin (IL)-3, both of which are known to promote the growth of leukemic cells in vitro.
SU5416 and SU6668 are small-molecule inhibitors of RTKs such as Flk-1/KDR that have structural and sequence similarity to c-kit. SU5416 is a more selective and potent inhibitor of the Flk-1/KDR receptor; in contrast, SU6668 exhibits a broader inhibitory target profile, with effects on platelet-derived growth factor (PDGF) receptor and fibroblast growth factor (FGF) receptor in addition to Flk-1/KDR.9,10 Both compounds have been shown to exhibit selectivity with respect to other tyrosine kinases, for example, with inhibitory concentration of 50% (IC50) above 10 μM for epidermal growth factor (EGF) receptor, Src, Met, and ZAP-70.9,10 In cell-based and preclinical animal models, both compounds have also been shown to exhibit antiangiogenic properties. SU5416 inhibits vascular endothelial growth factor (VEGF)-induced and SU6668 VEGF- and FGF-induced proliferation of human umbilical vein endothelial cells in culture; however, neither compound potently inhibits the growth of tumor cells grown in culture.9,10 In addition, both compounds inhibit the growth of a variety of tumor cells grown as subcutaneous xenografts in athymic mice; furthermore, SU6668 has been shown to regress established xenograft tumors in mice.9,10 Intravital fluoresence videomicroscopy in mouse tumor xenograft models has demonstrated that SU5416 and SU6668 also inhibit tumor angiogenesis in vivo.10 11
Currently, SU5416 is in late-stage clinical trials, while SU6668 is in early-stage clinical development. Importantly, these compounds have been very well tolerated to date. Furthermore, no hematologic side effects have been observed with SU5416 or SU6668 in either preclinical models or in patients enrolled in clinical trials.
In addition to the central role of angiogenesis in the growth of solid tumors, there is increasing evidence that angiogenic factors and increased vessel density also play a role in the growth of hematopoietic malignancies, including AML.12,13 High VEGF levels have been shown to be a poor prognostic indicator in AML.14 In addition, it has recently been reported that functional VEGF receptors were present on approximately 50% of leukemic samples analyzed.15 Thus, compounds that have both antiangiogenic and anti–c-kit activities may provide a novel therapeutic approach for the treatment of AML.
To provide support for exploring a clinical role for SU5416 and SU6668 in AML, the effect of these compounds on c-kit was determined. In vitro studies were performed using MO7E cells, a human myeloid leukemia cell line that expresses c-kit, as well as blasts derived from c-kit+ leukemia patients. Results presented here demonstrate that SU5416 and SU6668 inhibited biologic functions of c-kit in MO7E cells, including ligand-induced tyrosine phosphorylation and proliferation. Additionally, both compounds induced apoptosis in these cells. Finally, SU5416 and SU6668 inhibited SCF-induced phosphorylation of c-kit in blasts from several AML patients, one T-cell acute lymphoblastic leukemia (T-ALL) and one chronic myeloid leukemia (CML) patient, and also induced apoptosis in these cells. These results suggest that the combination of angiogenesis inhibition through the VEGF receptor and c-kit inhibition may provide a useful means to treat AML. To address this, SU5416 is currently in phase II clinical development in AML and a variety of other hematologic malignancies.
Materials and methods
Cell culture, patient samples, and compounds
MO7E cells, a human myeloid leukemia cell line, were obtained from James Griffin (Dana Farber Cancer Institute, Boston, MA) and were maintained in RPMI 1640 medium containing 10% fetal bovine serum (FBS) supplemented with 10 ng/mL each of IL-3 and GM-CSF (R&D Systems, Minneapolis, MN). AML, T-ALL, and CML blasts were obtained fresh from peripheral blood of patients at M. D. Anderson Cancer Center (Table1). These patients were selected randomly rather than from a particular therapeutic regimen. The specimens contained 23% to 97% blast cells at the time of collection. Cells were isolated by Ficoll-Hypaque (Pharmacia) density gradient centrifugation. Unless otherwise indicated, cell culture reagents were obtained from Life Technologies, Inc, Gaithersburg, MD. SU5416 and SU6668 were synthesized as described previously.9 10 SU-A, a structurally related indolinone, was synthesized using similar conditions (unpublished data, 2000).
Summary of SU5416 and SU6668 activity in patient blast samples
| Patient no. . | Diagnosis . | Cytogenetics . | % Blasts . | % c-kit . | c-kit positivity inhibition . | Apoptosis . | ||
|---|---|---|---|---|---|---|---|---|
| SU5416 . | SU6668 . | SU5416 . | SU6668 . | |||||
| 1 | AML relapsed | Trisomy 4, trisomy 21, trisomy 22 | 90 | 80 | +* | + | +1-153 | NT |
| 2 | AML refractory | t(1;2), -5, del(6), del(8), del(13), del(17), del(18), del(21) | 60 | 50 | + | NT | +1-153 | NT |
| 3 | Myelodysplastic syndrome, T-ALL | t(5;13), t(22;21) | 87 | 19 | + | NT | + | NT |
| 4 | CML Blast crisis | t(9;22) | 31 | 13 | NT | NT | +‡,1-155 | +‡,1-155 |
| 5 | AML relapsed | −16, t(1;21) Trisomy 21 | 94 | 12 | NT | NT | NT | +‡ |
| 6 | AML newly diagnosed | t(3;9), t(8;21) | 23 | 43 | NT | NT | +1-155 | NT |
| 7 | AML refractory | −5, −6 | 36 | 47 | NT | NT | + | + |
| 8 | AML relapsed | Trisomy 8, trisomy 13 | 97 | 40 | +† | + | +‡,1-153 | +‡,1-153 |
| 9 | AML refractory | Not determined | 75 | 81 | NT | NT | +1-155 | NT |
| Patient no. . | Diagnosis . | Cytogenetics . | % Blasts . | % c-kit . | c-kit positivity inhibition . | Apoptosis . | ||
|---|---|---|---|---|---|---|---|---|
| SU5416 . | SU6668 . | SU5416 . | SU6668 . | |||||
| 1 | AML relapsed | Trisomy 4, trisomy 21, trisomy 22 | 90 | 80 | +* | + | +1-153 | NT |
| 2 | AML refractory | t(1;2), -5, del(6), del(8), del(13), del(17), del(18), del(21) | 60 | 50 | + | NT | +1-153 | NT |
| 3 | Myelodysplastic syndrome, T-ALL | t(5;13), t(22;21) | 87 | 19 | + | NT | + | NT |
| 4 | CML Blast crisis | t(9;22) | 31 | 13 | NT | NT | +‡,1-155 | +‡,1-155 |
| 5 | AML relapsed | −16, t(1;21) Trisomy 21 | 94 | 12 | NT | NT | NT | +‡ |
| 6 | AML newly diagnosed | t(3;9), t(8;21) | 23 | 43 | NT | NT | +1-155 | NT |
| 7 | AML refractory | −5, −6 | 36 | 47 | NT | NT | + | + |
| 8 | AML relapsed | Trisomy 8, trisomy 13 | 97 | 40 | +† | + | +‡,1-153 | +‡,1-153 |
| 9 | AML refractory | Not determined | 75 | 81 | NT | NT | +1-155 | NT |
Blasts were isolated from peripheral blood samples from patients with the indicated diagnoses; % blasts and % c-kit positivity are indicated. For phosphorylation assays, SU5416 was used at 5 μM, except where indicated, and SU6668 was tested at 0.5 and 5 μM. Apoptosis was assessed by flow cytometric analysis of activated caspase-3 except where indicated. Apoptosis assays were done with 25 μM SU5416 or SU6668 except where indicated.
+ indicates that inhibition of phosphorylation or induction of apoptosis was observed.
NT indicates the sample was not tested, due to limiting cell number.
SU5416 was tested at 0.05, 0.5, and 5 μM.
SU5416 was tested at 0.5 and 5 μM.
Compounds were tested at 5 and 25 μM.
Apoptosis was assessed using assays measuring caspase-3 activation and PARP cleavage.
Apoptosis was assessed with PARP cleavage assay only.
Stimulation with SCF and detection of c-kit and ERK1/2 phosphorylation
Prior to ligand stimulation, MO7E cells (approximately 107 cells per sample) were quiesced by serum starvation overnight in medium containing 0.1% FBS. Subsequently, cells were stimulated for 15 minutes with 250 ng/mL recombinant human SCF (R&D Systems). The SCF neutralizing antibody (R&D Systems) was preincubated with SCF (100 ng/mL) for 2 hours prior to addition to cells. To determine the inhibitory effect of SU5416 or the E-cadherin control antibody (Santa Cruz Biotechnology Inc, Santa Cruz, CA), quiesced cells were pretreated for 2 hours prior to addition of SCF. To determine the inhibitory effect of SU6668 and SU-A, MO7E cells were pretreated with compound for 48 hours in medium containing 0.1% FBS. Leukemic blasts were pretreated with SU5416 for 1 hour or SU6668 for 24 hours in medium containing 0.1% FBS prior to SCF stimulation. Incubation times for the compounds were chosen based on uptake experiments that demonstrated SU5416 is maximally taken up by cells by 2 hours, while SU6668 uptake is not as rapid16 (unpublished data, 2000). Following stimulation with SCF, MO7E cells and blasts were lysed with radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris, pH 7.2;150 mM NaCl; 1% deoxycholic acid; 0.1% sodium dodecyl sulfate [SDS]; 1% Triton X-100) containing protease and phosphatase inhibitors (0.60 μM aprotinin, 20 μM bestatin, 2.8 μM E-64, 2 μM leupeptin, 2 μM pepstatin A, 0.5 mM sodium fluoride, 0.1 mM phenylmethylsulfonyl fluoride, 0.1 mM sodium orthovanadate). For analysis of c-kit phosphorylation, equivalent amounts of protein from each sample were immunoprecipitated overnight at 4°C with an anti–c-kit antibody (Santa Cruz Biotechnology), followed by a 2-hour incubation with protein G agarose (Upstate Biotechnology Inc, Lake Placid, NY). The immune complexes were then centrifuged, and the pellets were washed with RIPA lysis buffer. Bound proteins were eluted from the protein G agarose by boiling the sample in 25 μL 1 × SDS sample buffer with 2-mercaptoethanol. The proteins were separated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with an antiphosphotyrosine monocloncal antibody (UB40). The membranes were stripped using ImmunoPure IgG Elution buffer (Pierce, Rockford, IL) and reprobed with the anti–c-kit antibody. For analysis of extracellular signal–regulated kinase 1/2 (ERK1/2) phosphorylation, equivalent amounts of total protein lysate were boiled with 5 × SDS sample buffer with 2-mercaptoethanol. The proteins were separated by SDS-PAGE and immunoblotted with an antiphospho ERK1/2 antibody (New England BioLabs, Beverly, MA). The membranes were stripped using ImmunoPure IgG Elution buffer (Pierce) and reprobed with an anti-ERK1/2 antibody (New England BioLabs).
Cell proliferation assays
MO7E cells were quiesced by serum starvation overnight in medium containing 0.1% FBS. Cells were plated in triplicate in 96-well plates at a density of 4 × 105 cells per well and pretreated for 30 minutes with 0 to 10 μM SU5416 or overnight with 0 to 10 μM SU6668 or SU-A. Following serum starvation, SCF was added to a final concentration of 10 ng/mL, and the cells were incubated for 48 hours. After 48 hours, 10 μL of a 5 mg/mL stock of MTT ([3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide]; Sigma Chemical) was added and incubated for 4 hours. Acid isopropanol (0.04 N HCl in isopropanol) was added, and the plates were read at a wavelength of 550 nm. Percent growth relative to the untreated control was calculated based on the MTT readout.
Apoptosis assays
Apoptosis was measured using 2 assays: flow cytometry to detect activated caspase-3, and Western blotting to detect cleavage of poly(ADP-ribose) polymerase (PARP). MO7E cells were quiesced by serum starvation overnight in medium containing 0.1% FBS. Cells were pretreated for 30 minutes with 0 to 25 μM SU5416, 5 μM camptothecin (Sigma Chemical) or the E-cadherin control antibody, or 24 hours with 0 to 25 μM SU6668, followed by the addition of 100 ng/mL SCF. The SCF neutralizing antibody was preincubated with SCF for 2 hours prior to addition to cells. Samples were assayed after 24 hours in culture. Leukemic blasts were pretreated with SU5416 for 30 minutes in 0.1% or 10% FBS or with SU6668 overnight in 0.1% FBS. Then, 100 ng/mL SCF was added, and samples were assayed after 24 hours in culture.
Activated caspase-3.
To measure activated caspase-3, MO7E and blast samples were washed once with PBS and permeabilized by addition of ice-cold 70% ethanol. The cells were then stained with phycoerythrin (PE)-conjugated polyclonal rabbit antiactive caspase-3 (Pharmingen, San Diego, CA), and the fluorescence was quantitated using a FACScan flow cytometer (Becton Dickinson, San Jose, CA).
PARP.
To measure cleavage of PARP, cells were lysed with RIPA buffer, and equivalent amounts of protein were separated by SDS-PAGE. The proteins were transferred to nitrocellulose and immunoblotted with an anti-PARP antibody (R&D Systems).
Results
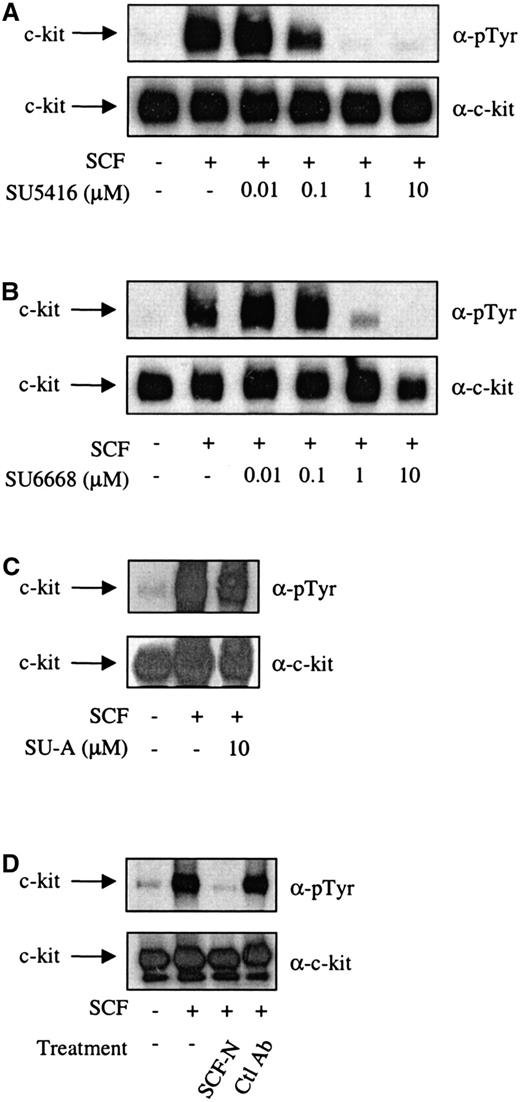
It has been demonstrated previously that the c-kit ligand, SCF, was able to induce tyrosine autophosphorylation of c-kit and proliferation of MO7E cells.1 17 To investigate the ability of SU5416 and SU6668 to inhibit the initial events in c-kit signal transduction, SCF-induced tyrosine phosphorylation of c-kit in the presence of varying concentrations of either SU5416 or SU6668 was examined by Western blotting analysis. As shown in Figure1A, SU5416 inhibited the SCF-induced tyrosine phosphorylation of c-kit in MO7E cells in a dose-dependent manner, with an IC50 value between 0.1 and 1 μM. In a similar experiment conducted with SU6668, SCF-induced tyrosine phosphorylation of c-kit was also inhibited, with a similar estimated IC50 value between 0.1 and 1 μM (Figure 1B). In contrast to the effects of SU5416 and SU6668, treatment with a structurally related compound, SU-A, which possesses strong inhibitory activity against the EGF receptor (IC50 4 nM) but not flk-1 or PDGF receptor (IC50 values > 20 μM; unpublished data, 2000), did not inhibit SCF-induced phosphorylation of c-kit at drug concentrations and under conditions in which EGF receptor responses are significantly inhibited (Figure 1C). To validate the c-kit inhibitory results observed with SU5416 and SU6668, similar experiments were performed with a neutralizing SCF antibody. Using this reagent, SCF-induced tyrosine phosphorylation of c-kit was inhibited, similar to the effect observed with SU5416 and SU6668 treatment (Figure 1D). No inhibition was observed using an isotype control antibody that recognized E-cadherin.
SU5416 and SU6668 inhibit c-kit tyrosine phosphorylation in MO7E cells.
Serum-starved MO7E cells were treated with the indicated concentrations of SU5416 (A) or isotype control E-cadherin antibody (D) for 2 hours, or SU6668 (B) or SU-A (C) for 48 hours, prior to stimulation with 250 ng/mL SCF for 15 minutes. SCF (100 ng/mL) was incubated with the SCF neutralizing antibody for 2 hours prior to addition to cells (D). Cells were harvested, lysed, and immunoprecipitated with an anti–c-kit antibody. After SDS-PAGE and transfer to nitrocellulose, the blots were probed with an anti-phosphotyrosine antibody (top) and subsequently stripped and reprobed with an anti–c-kit antibody (bottom).
SU5416 and SU6668 inhibit c-kit tyrosine phosphorylation in MO7E cells.
Serum-starved MO7E cells were treated with the indicated concentrations of SU5416 (A) or isotype control E-cadherin antibody (D) for 2 hours, or SU6668 (B) or SU-A (C) for 48 hours, prior to stimulation with 250 ng/mL SCF for 15 minutes. SCF (100 ng/mL) was incubated with the SCF neutralizing antibody for 2 hours prior to addition to cells (D). Cells were harvested, lysed, and immunoprecipitated with an anti–c-kit antibody. After SDS-PAGE and transfer to nitrocellulose, the blots were probed with an anti-phosphotyrosine antibody (top) and subsequently stripped and reprobed with an anti–c-kit antibody (bottom).
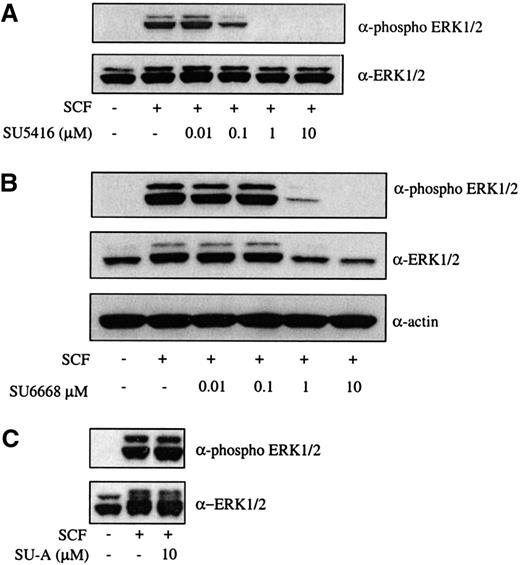
To examine an SCF-mediated signaling event downstream of c-kit activation, ERK1/2 phosphorylation in the presence of SU5416 and SU6668 was analyzed. As shown in Figure 2A,B, SU5416 and SU6668 inhibited ERK1/2 phosphorylation following SCF stimulation in a dose-dependent manner, with IC50 values consistent with those observed for inhibition of c-kit phosphorylation. Expression of ERK1/2 protein was also slightly inhibited by higher concentrations of SU6668 in this cell line (as assessed by the actin immunoblot), but not SU5416, as shown in Figure 2B. No inhibition of ERK1/2 phosphorylation was observed using SU-A, consistent with the inactivity observed for c-kit tyrosine phosphorylation (Figure 2C).
SU5416 and SU6668 inhibit SCF-induced ERK1/2 phosphorylation in MO7E cells.
Serum-starved MO7E cells were treated with the indicated concentrations of SU5416 (A) or SU-A (C) for 2 hours, or SU6668 for 48 hours (B), prior to stimulation with 250 ng/mL SCF for 15 minutes. Cells were harvested and lysed. After SDS-PAGE and transfer to nitrocellulose, the blots were probed with an anti-phosphoERK1/2 antibody (top) and subsequently stripped and reprobed with an anti-ERK1/2 (bottom) antibody. The blot in panel B was subsequently reprobed with an antiactin antibody.
SU5416 and SU6668 inhibit SCF-induced ERK1/2 phosphorylation in MO7E cells.
Serum-starved MO7E cells were treated with the indicated concentrations of SU5416 (A) or SU-A (C) for 2 hours, or SU6668 for 48 hours (B), prior to stimulation with 250 ng/mL SCF for 15 minutes. Cells were harvested and lysed. After SDS-PAGE and transfer to nitrocellulose, the blots were probed with an anti-phosphoERK1/2 antibody (top) and subsequently stripped and reprobed with an anti-ERK1/2 (bottom) antibody. The blot in panel B was subsequently reprobed with an antiactin antibody.
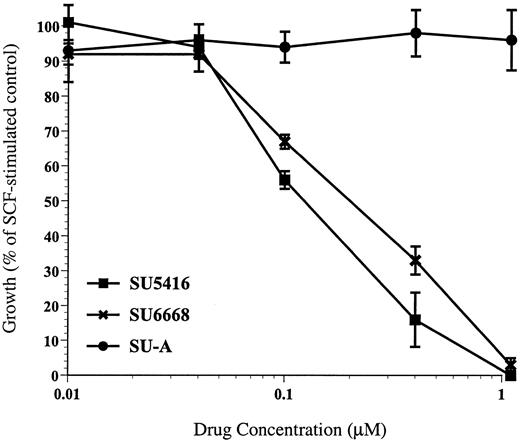
In addition to inducing tyrosine phosphorylation of c-kit, SCF also has been shown to induce proliferation in MO7E cells.17Consistent with the demonstration that SU5416 and SU6668 inhibited SCF-induced tyrosine phosphorylation of c-kit, both compounds were also shown to inhibit SCF-induced proliferation in MO7E cells. SCF induced a proliferative response of approximately 3.5-fold in MO7E cells. As shown in a representative experiment in Figure3, SU5416 inhibition of MO7E cell proliferation was dose dependent, with an IC50 value of 0.1 μM. SU6668 inhibition of MO7E cell proliferation was also dose dependent, with an IC50 value of 0.29 μM. Importantly in this assay, concentrations of SU5416 or SU6668 below 1 μM did not result in absorbance readings below the level of the untreated control cells, indicating that at these concentrations of drug the MTT assay is measuring inhibition of proliferation and not a cytotoxic response. SU-A, consistent with its lack of activity in receptor and ERK1/2 phosphorylation assays, did not inhibit SCF-induced proliferation of MO7E cells (Figure 3). IL-3 and GM-CSF, alone and in combination, also induced proliferation in MO7E cells approximately 3- to 4-fold. SU5416 did not inhibit proliferation mediated by either IL-3 or GM-CSF (IC50 > 10 μM), while SU6668 did show some inhibition of IL-3 and GM-CSF–mediated proliferation (IC50approximately 1 μM) (data not shown). The activity of SU6668 in response to IL-3 and GM-CSF stimulation may be explained by the broader target profile of that compound compared to SU5416.
SU5416 and SU6668 inhibit SCF-induced proliferation of MO7E cells.
MO7E cells were quiesced by serum starvation overnight in medium containing 0.1% FBS. Cells were plated in triplicate in 96-well plates and pretreated for 30 minutes with the indicated concentrations of SU5416 or overnight with SU6668 or SU-A. Cells were incubated with 10 ng/mL SCF in the presence of compound for 48 hours, followed by MTT analysis. Percent growth relative to the untreated control was calculated based on the MTT readout.
SU5416 and SU6668 inhibit SCF-induced proliferation of MO7E cells.
MO7E cells were quiesced by serum starvation overnight in medium containing 0.1% FBS. Cells were plated in triplicate in 96-well plates and pretreated for 30 minutes with the indicated concentrations of SU5416 or overnight with SU6668 or SU-A. Cells were incubated with 10 ng/mL SCF in the presence of compound for 48 hours, followed by MTT analysis. Percent growth relative to the untreated control was calculated based on the MTT readout.
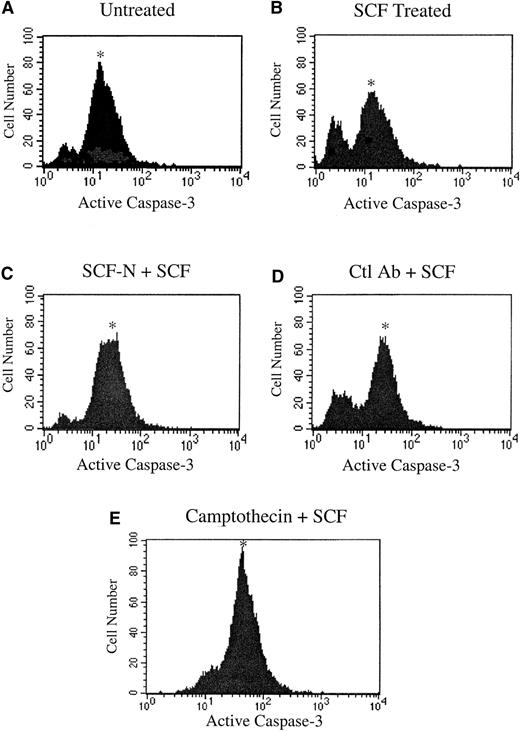
SCF has been reported to act as a survival factor in myeloid cells, preventing cells from undergoing apoptosis.18 To determine the effect of inhibition of c-kit signaling on induction of apoptosis in MO7E cells, the SCF neutralizing antibody reagent was used. Using a flow cytometric analysis, apoptosis was assessed by measuring the level of active caspase-3, a key protease activated during the early stages of apoptosis.19 As shown in Figure4A, a significant number of MO7E cells cultured in the absence of SCF expressed activated caspase-3. This number was greatly diminished by the addition of SCF, demonstrating the survival benefit of SCF treatment (Figure 4B). Inhibition of c-kit activity with the SCF neutralizing antibody reversed the effects of SCF, resulting in an increase in active caspase-3 levels (Figure 4C), while treatment with the isotype control antibody was similar to the SCF-treated sample (Figure 4D). The apoptosis-inducing cytotoxic agent camptothecin was included as a positive control in the assay and resulted in increased levels of activated caspase-3 (Figure 4E).
Inhibition of c-kit signaling induces apoptosis in MO7E cells.
MO7E cells were quiesced by serum starvation overnight in medium containing 0.1% FBS and were untreated (A) or treated with 100 ng/mL SCF (B), SCF plus an SCF-neutralizing antibody (SCF-N) (C) or an isotype control E-cadherin antibody (Ctl Ab) (D), or camptothecin (E). At 24 hours, samples were stained with PE-conjugated antiactivated caspase-3 antibody and quantitated by fluorescence-activated cell sorter analysis. The asterisk indicates the apoptotic population.
Inhibition of c-kit signaling induces apoptosis in MO7E cells.
MO7E cells were quiesced by serum starvation overnight in medium containing 0.1% FBS and were untreated (A) or treated with 100 ng/mL SCF (B), SCF plus an SCF-neutralizing antibody (SCF-N) (C) or an isotype control E-cadherin antibody (Ctl Ab) (D), or camptothecin (E). At 24 hours, samples were stained with PE-conjugated antiactivated caspase-3 antibody and quantitated by fluorescence-activated cell sorter analysis. The asterisk indicates the apoptotic population.
Having established that inhibition of c-kit signaling induced apoptosis in MO7E cells using the SCF neutralizing antibody, the activity of SU5416 and SU6668 in the caspase-3 flow cytometric assay was determined. As shown in Figure 5, similar to treatment with the neutralizing antibody, incubation with either SU5416 or SU6668 resulted in an increased number of cells expressing activated caspase-3 in a dose-dependent manner. Treatment with SU5416 or SU6668 at concentrations 1 μM and above resulted in a marked increase in the number of cells expressing activated caspase-3. Similar results with each compound were also obtained when apoptosis was assayed by detecting the cleavage of PARP in whole-cell lysates by Western blotting and also by TUNEL (data not shown). Consistent with other SCF-mediated responses, SU-A (25 μM) did not induce apoptosis in MO7E cells.
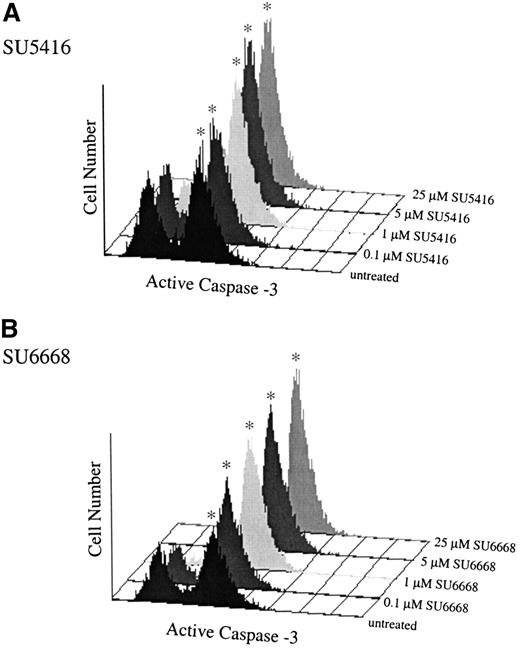
SU5416- and SU6668-treated MO7E cells demonstrate increased expression of activated caspase-3.
MO7E cells were quiesced by serum starvation overnight in medium containing 0.1% FBS and were pretreated for 30 minutes with the indicated concentrations of SU5416 (A), or 24 hours with SU6668 (B), followed by addition of 100 ng/mL SCF; untreated samples were treated with SCF only. At 24 hours, samples were stained with PE-conjugated antiactivated caspase-3 antibody and quantitated by fluorescence-activated cell sorter analysis. The asterisk indicates the apoptotic population.
SU5416- and SU6668-treated MO7E cells demonstrate increased expression of activated caspase-3.
MO7E cells were quiesced by serum starvation overnight in medium containing 0.1% FBS and were pretreated for 30 minutes with the indicated concentrations of SU5416 (A), or 24 hours with SU6668 (B), followed by addition of 100 ng/mL SCF; untreated samples were treated with SCF only. At 24 hours, samples were stained with PE-conjugated antiactivated caspase-3 antibody and quantitated by fluorescence-activated cell sorter analysis. The asterisk indicates the apoptotic population.
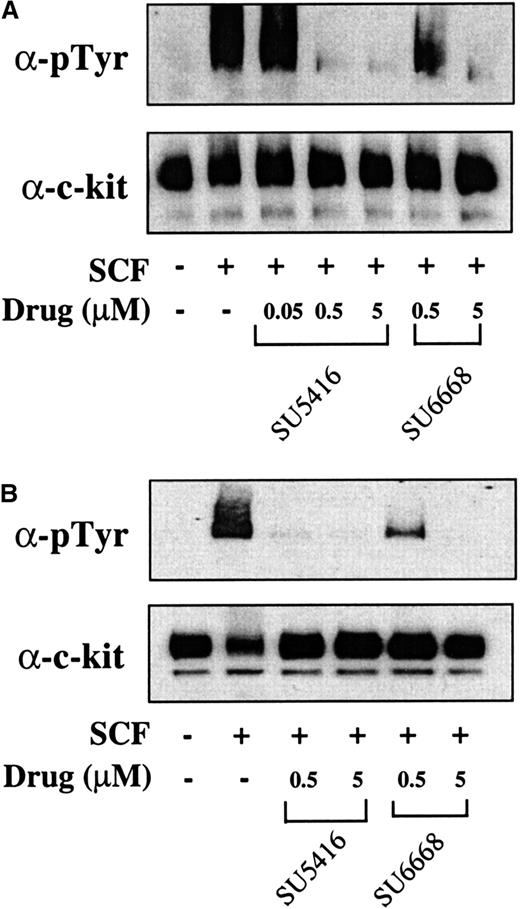
To determine if results obtained in the MO7E cell line could also be observed in leukemic blast cells from patients, the ability of SU5416 and SU6668 to inhibit SCF-induced c-kit and ERK1/2 phosphorylation and to induce apoptosis was investigated. Leukemic blasts were isolated from peripheral blood from a total of 9 patients and stimulated with SCF in the absence or presence of SU5416 or SU6668. Results from 2 patients (No. 1 and 8) are presented; similar results were obtained for all patients tested and are summarized in Table 1. As shown in Figure6, SU5416 and SU6668 inhibited the SCF-induced phosphorylation of c-kit in a dose-dependent manner in both patient samples, with complete inhibition observed at 0.5 and 5 μM SU5416. In the 2 samples represented, SU6668 treatment at 5 μM resulted in complete inhibition of c-kit phosphorylation, while 0.5 μM treatment resulted in partial inhibition.
SU5416 and SU6668 inhibit c-kit tyrosine phosphorylation in AML blast cells.
AML blast cells (panel A: patient No. 1; panel B: patient No. 8) were quiesced by serum starvation and treated with the indicated concentrations of SU5416 for 1 hour or SU6668 for 24 hours prior to stimulation with 250 ng/mL SCF for 15 minutes. Cells were harvested, lysed, and immunoprecipitated with an anti–c-kit antibody. After SDS-PAGE and transfer to nitrocellulose, the blots were probed with an antiphosphotyrosine antibody (top) and subsequently stripped and reprobed with an anti–c-kit antibody (bottom).
SU5416 and SU6668 inhibit c-kit tyrosine phosphorylation in AML blast cells.
AML blast cells (panel A: patient No. 1; panel B: patient No. 8) were quiesced by serum starvation and treated with the indicated concentrations of SU5416 for 1 hour or SU6668 for 24 hours prior to stimulation with 250 ng/mL SCF for 15 minutes. Cells were harvested, lysed, and immunoprecipitated with an anti–c-kit antibody. After SDS-PAGE and transfer to nitrocellulose, the blots were probed with an antiphosphotyrosine antibody (top) and subsequently stripped and reprobed with an anti–c-kit antibody (bottom).
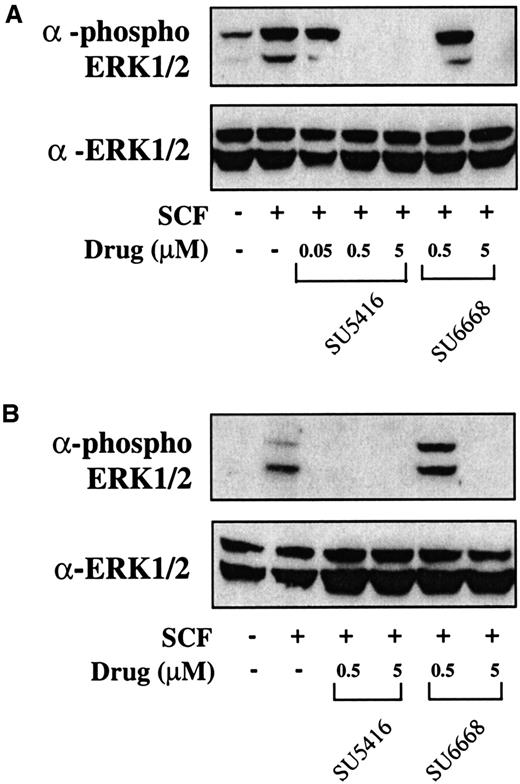
To determine whether downstream signaling pathways of c-kit are also inhibited in AML blasts treated with SU5416 and SU6668, inhibition of ERK1/2 phosphorylation was measured. As shown in Figure7, similar to the c-kit phosphorylation results, ERK1/2 phosphorylation is inhibited by 0.5 and 5 μM SU5416 in both patient samples and partially inhibited by 0.05 μM SU5416 where tested. SU6668 also inhibited ERK1/2 phosphorylation completely at 5 μM and partially at 0.5 μM in both patient samples. To summarize, in blast samples from the 9 leukemia patients analyzed to date (Table 1), SU5416 inhibited c-kit phosphorylation in 3 of 3 AML patients and 1of 1 T-ALL patients tested and ERK1/2 phosphorylation in 2 of 2 AML patients tested; SU6668 inhibited c-kit and ERK1/2 phosphorylation in 2 of 2 AML patients tested.
SU5416 and SU6668 inhibit ERK1/2 phosphorylation in AML blast cells.
AML blast cells (panel A: patient No. 1; panel B: patient No. 8) were quiesced by serum starvation and treated with the indicated concentrations of SU5416 for 1 hour or SU6668 for 24 hours prior to stimulation with 250 ng/mL SCF for 15 minutes. Cells were harvested and lysed. After SDS-PAGE and transfer to nitrocellulose, the blots were probed with an anti-phosphoERK1/2 antibody (top) and subsequently stripped and reprobed with an anti-ERK1/2 antibody (bottom).
SU5416 and SU6668 inhibit ERK1/2 phosphorylation in AML blast cells.
AML blast cells (panel A: patient No. 1; panel B: patient No. 8) were quiesced by serum starvation and treated with the indicated concentrations of SU5416 for 1 hour or SU6668 for 24 hours prior to stimulation with 250 ng/mL SCF for 15 minutes. Cells were harvested and lysed. After SDS-PAGE and transfer to nitrocellulose, the blots were probed with an anti-phosphoERK1/2 antibody (top) and subsequently stripped and reprobed with an anti-ERK1/2 antibody (bottom).
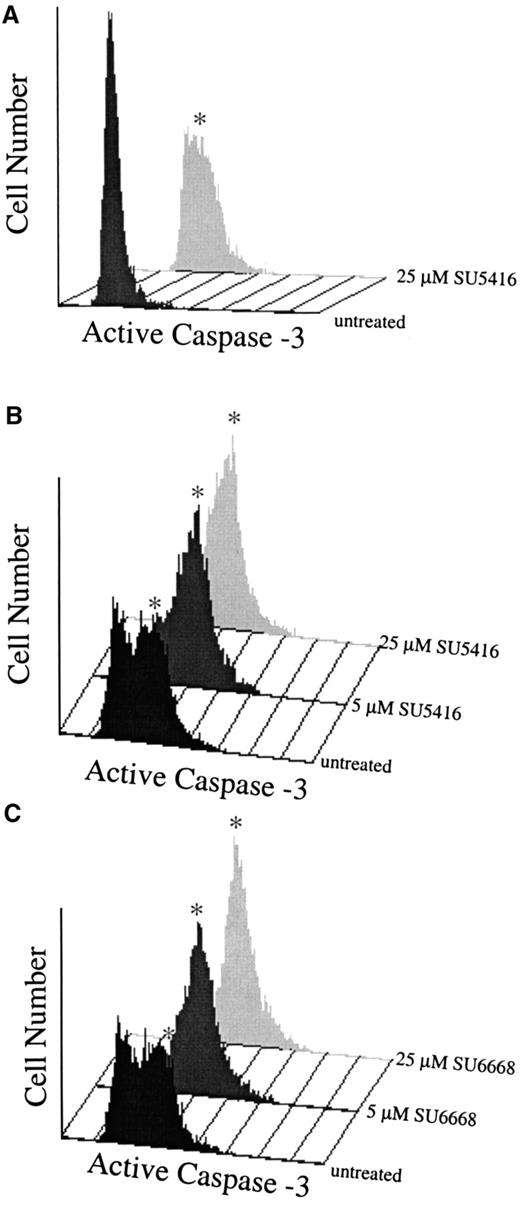
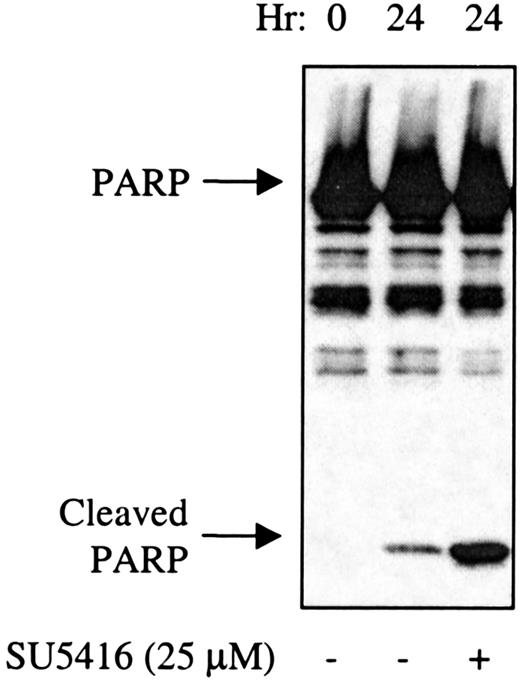
To determine if the compounds also induced apoptosis in leukemic blasts, levels of activated caspase-3 and the appearance of cleaved PARP were assayed, As shown in Figure 8A (patient no. 1) and Figure 8B,C (patient No. 8), SU5416 and SU6668 induced apoptosis in AML blasts in a dose-dependent manner as measured by the increased number of cells expressing activated caspase-3 after 24 hours of treatment with 5 or 25 μM SU5416 or SU6668. SU5416 and SU6668 induced apoptosis in AML blasts also as measured by the appearance of cleaved PARP. As shown in a representative experiment in Figure 9 for patient no. 1, an increase in the cleaved PARP fragment was observed after 24 hours of treatment with 25 μM SU5416. To summarize the apoptosis results from leukemic blast samples that have been analyzed, SU5416 induced apoptosis in 5 of 5 AML patients tested and in 1 of 1 each of c-kit+ T-ALL and CML patients. SU6668 induced apoptosis in 3 of 3 AML patients tested and in 1 of 1 c-kit+ CML patient tested.
SU5416- and SU6668-treated AML blast cells demonstrate increased expression of activated caspase-3.
AML blasts in medium containing 10% FBS (panel A: patient No. 1) or 0.1% FBS (panels B,C: patient No. 8) were pretreated with SU5416 for 1 hour or SU6668 for 24 hours prior to incubation with 100 ng/mL SCF; untreated samples were incubated with SCF only. At 24 hours, samples were stained with PE-conjugated antiactivated caspase-3 antibody and quantitated by fluorescence-activated cell sorter analysis. The asterisk indicates the apoptotic population.
SU5416- and SU6668-treated AML blast cells demonstrate increased expression of activated caspase-3.
AML blasts in medium containing 10% FBS (panel A: patient No. 1) or 0.1% FBS (panels B,C: patient No. 8) were pretreated with SU5416 for 1 hour or SU6668 for 24 hours prior to incubation with 100 ng/mL SCF; untreated samples were incubated with SCF only. At 24 hours, samples were stained with PE-conjugated antiactivated caspase-3 antibody and quantitated by fluorescence-activated cell sorter analysis. The asterisk indicates the apoptotic population.
SU5416-treated AML blast cells demonstrate increased cleavage of PARP.
AML blasts (patient No. 1) in medium containing 10% FBS were pretreated with SU5416 for 1 hour prior to incubation with 100 ng/mL SCF. At 24 hours, cells were lysed and proteins separated by SDS-PAGE. After transfer to nitrocellulose, the blots were probed with an anti-PARP antibody. The full-length 116-kd PARP and the 26-kd cleaved PARP fragment are indicated.
SU5416-treated AML blast cells demonstrate increased cleavage of PARP.
AML blasts (patient No. 1) in medium containing 10% FBS were pretreated with SU5416 for 1 hour prior to incubation with 100 ng/mL SCF. At 24 hours, cells were lysed and proteins separated by SDS-PAGE. After transfer to nitrocellulose, the blots were probed with an anti-PARP antibody. The full-length 116-kd PARP and the 26-kd cleaved PARP fragment are indicated.
Discussion
The results of the studies described here show that both SU5416 and SU6668 inhibit biochemical and biologic functions of c-kit in MO7E cells, a human myeloid leukemia cell line that expresses c-kit. At the biochemical level, both compounds inhibit ligand-induced phosphorylation of the receptor and ERK1/2 in MO7E cells and in freshly isolated c-kit+ blasts from AML, T-ALL, and CML patients. Cell proliferation, previously shown to be a downstream biologic effect of receptor stimulation and phosphorylation in MO7E cells,1 17 is also inhibited by SU5416 and SU6668 treatment.
SU5416 and SU6668 are small-molecule indolinone RTK inhibitors currently being evaluated as antiangiogenic agents in clinical trials in patients with advanced malignancies.10,16 SU5416 is more advanced in clinical development than SU6668 and has demonstrated evidence of biologic activity in a number of tumor types. Of relevance to the studies described here, SU5416 treatment has not been associated with myelosuppression or anemia.16 Both compounds inhibit their respective RTK targets via binding in the conserved adenosine triphosphate binding site within the kinase domain of the receptor. SU5416 is a more selective compound than SU6668 and is a potent inhibitor of the Flk-1/KDR RTK. SU6668 exhibits a broader range of activities, inhibiting PDGF receptor and FGF receptor in addition to Flk-1/KDR. The intracellular domain of these 3 RTKs contains a kinase domain split by a unique insert region, similar to the intracellular domain of c-kit. It has been shown previously that indolinone derivatives related to SU5416 and SU6668 inhibit ligand-induced c-kit phosphorylation in transiently transfected COS cells and kill neoplastic mast cells expressing constitutively activated forms of c-kit.20
SU5416 and SU6668 inhibition of c-kit in a human myeloid leukemia cell line and in AML blasts suggests a therapeutic potential for these compounds in the treatment of hematopoietic malignancies, because leukemic blasts in 60% to 80% of AML patients express functional c-kit.8 Colony growth of c-kit+ blasts can be stimulated by treatment with SCF, either alone or in combination with other hematopoietic growth factors such as GM-CSF or IL-3.1,2 In addition, in one study, baseline tyrosine phosphorylation of c-kit was detected in 7 of 12 c-kit+ AML blast samples, and SCF-inducible tyrosine phosphorylation was observed in 4 of 5 samples tested.1 Thus, c-kit inhibition may provide a novel target for the treatment of most AML patients. Previous studies regarding the prognostic significance of c-kit expression in AML have yielded conflicting results. However, these studies have analyzed clinical outcomes resulting from standard induction therapies for AML, such as the combination of the anthracycline daunorubicin and the antimetabolite cytosine arabinoside and have not focused on therapies that specifically target c-kit.21 22
SU5416 and SU6668 may also prove beneficial in treating AML because of the antiangiogenic properties of these compounds. Angiogenesis, the growth of new blood vessels from preexisting vasculature, is required for the growth of solid tumors beyond a minimal size and is a key component of sustained tumor growth.23 There is also growing evidence that angiogenic factors such as VEGF and basic FGF (bFGF) play a role in the growth of leukemias. VEGF messenger RNA was detected in fresh leukemic blasts in 23 of 33 acute myeloid leukemia (AML) patients, and cellular VEGF protein was found to be higher in AML bone marrow samples than in normal marrow.13,14 In addition, a high VEGF level in AML bone marrow was shown to be a poor prognostic indicator in newly diagnosed AML.14 KDR and Flt-1, VEGF receptors expressed on endothelial cells and monocytes and also inhibited by SU5416 and SU6668 16 (data not shown), were expressed on blasts from approximately 30% to 50% of AML patients studied.13 Levels of bFGF have been shown to be elevated in urine from patients with acute lymphocytic leukemia and lymphoma and were correlated with increased microvessel densities.24,25 Increased vessel density was also observed in bone marrow from AML patients compared with normal controls.12
Furthermore, a potential paracrine interaction of growth factors has been described that may contribute to the growth of AML blasts. VEGF has been shown to stimulate the production of several hematopoietic growth factors from human umbilical vein endothelial cells, including GM-CSF, SCF, M-CSF, G-CSF, and IL-6.13 26 The close proximity of bone marrow endothelial cells and AML blasts may allow for a synergistic relationship between the endothelial cells that secrete growth factors, such as SCF and GM-CSF, and the blast cells that express receptors for these factors.
SCF and FLT3 ligand, whose receptors belong to the same family of RTKs, have been shown to inhibit apoptosis in several myeloid leukemia cell lines established from patients with AML.18 Both ligands were also shown to promote the survival of human fetal liver myeloid progenitor cells.27 Thus, inhibition of c-kit in a myeloid cell line, or in AML blasts, may be predicted to induce apoptosis. Consistent with this prediction, SU5416 and SU6668 induced apoptosis in MO7E cells and in leukemic blasts, at concentrations similar to those required to inhibit receptor phosphorylation and proliferation in these cells. Treatment of AML blasts with 25 μM SU5416 was sufficient to induce apoptosis; importantly, peak plasma levels of approximately 20 μM have been measured at the end of the 1-hour infusion in patients receiving the phase II recommended dose of SU5416 (145 mg/m2), suggesting that an efficacious concentration of drug is achievable in humans at a dose that has been very well tolerated to date.
The data presented here demonstrate inhibition of c-kit functions by SU5416 and SU6668. Combined with the increasing evidence suggesting a role for angiogenesis in AML, this suggests 2 distinct mechanisms by which SU5416 and SU6668 treatment of c-kit+ AML patients may be beneficial. First, inhibition of c-kit may lead to a reduction in blast cell proliferation and overall cell number. Second, inhibition of VEGF receptor–mediated signaling in endothelial cells, in addition to inhibiting growth of endothelial cells, may inhibit the production of growth factors (such as SCF) that are part of a paracrine system that stimulates proliferation of blast cells. SU5416 and SU6668 thus represent a potential novel treatment strategy for AML and are currently being pursued in ongoing phase II clinical trials in hematologic malignancies. In addition to determining standard clinical response criteria, testing these agents in leukemias enables one to apply the experimental strategies used in the preclinical studies described here to clinical samples. By undertaking such studies, mechanism-of-action questions can be addressed that in turn may lead to correlations that aid in predicting clinical response.
The authors thank Sara Courtneidge, Laura Shawver, Jerry McMahon, Alyssa Morimoto, Dirk Mendel, Douglas Laird, Robert Wild, Ken Lipson, and David Kiewlich for helpful scientific interactions, and Sean Paxton and Christina Mirabile for assistance with the figures.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Beverly D. Smolich, Preclinical Therapeutics, Sugen, 230 East Grand Ave, South San Francisco, CA 94080; e-mail: beverly-smolich@sugen.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal