Vaccination with tumor-specific immunoglobulin or idiotype (Id) is a promising new form of immunotherapy for B-cell malignancies. Id protein vaccination has demonstrated clinical activity in B-cell lymphomas, yet it requires the laborious and time-consuming procedures of tumor–myeloma cell hybridization, large-scale in vitro culture, and protein purification. Recombinant adenoviruses are highly efficient and immunogenic gene transfer vehicles from which individualized vaccines can be rapidly assembled using polymerase chain reaction–amplified tumor Id genes. Id-encoding adenoviruses were evaluated as vaccines in 2 murine B-cell lymphoma models. A single injection of recombinant Id adenovirus provided protection from subsequent tumor challenge that was equivalent or superior to that afforded by Id protein vaccination. Protected mice had substantial serum titers of Id-specific antibodies. When used in conjunction with chemotherapy, vaccination also prolonged the survival of mice bearing pre-existing tumor. Mechanistic studies demonstrated that tumor protection was not dependent upon T cells. Importantly, in mice prevaccinated with an irrelevant adenovirus, tumor protection following vaccination with Id adenovirus was not significantly impaired. These findings have implications for the design of future lymphoma immunotherapy trials.
Introduction
Each B lymphocyte expresses a clonal immunoglobulin (Ig) whose heavy and light chain variable regions (VH and VL, respectively) comprise a unique collection of antigenic determinants known as the idiotype (Id). Maintenance of the Id by B cells following malignant transformation offers opportunities for the development of tumor-specific immunotherapies for B-cell malignancies. The Ig Id sequences contain epitopes that can be recognized by antibodies1-3 and CD4+2,4-6 and CD8+ T cells.7-10 Active immunization with tumor-derived Id protein can protect mice against subsequent lymphoma challenge2,11 and, in conjunction with chemotherapy, can cure established tumors.12
In lymphoma patients, Id vaccination has been found to result in humoral and cellular anti-Id responses4 that correlate with improved relapse-free and overall survival.13 Durable tumor regressions have also been observed in several trials.4,5,14,15 These clinical studies have relied on Id protein purified from cultures of tumor–myeloma cell hybridomas, the production of which is rate-limiting for large-scale application in human trials. The ability to rapidly amplify Id-encoding DNA sequences from lymphoma cells using polymerase chain reaction (PCR) has offered the possibility of streamlining the production of custom-made Id vaccines through the application of “naked” DNA vaccination techniques.16 Vaccination with Id-encoding DNA has proven efficacy in eliciting tumor-protective immunity in several murine lymphoma9,16-19 and myeloma19 models. While this approach eliminates the need for production and purification of Id protein, repetitive immunizations are required, and immune responses are weaker than those elicited by protein vaccines, even when augmented by co-administered cytokine DNAs.17 18
Recombinant adenoviruses offer the practical advantages of DNA vaccination in a highly efficient and immunogenic vector system. Replication-defective adenoviruses encoding an antigen of interest can be rapidly produced in high titers.20 Adenoviruses efficiently infect a variety of target cell types in vitro or in vivo, leading to high levels of protein production in situ. Vaccination with recombinant adenoviruses has been shown to induce potent humoral21-28 and cellular23-25,27,28 immune responses to transgene-encoded products. In some cases, vaccination has resulted in breaking of immunologic tolerance to self-antigens.27,29 In a variety of infectious disease models, adenoviral vaccination can stimulate potent, protective immunity to disease challenge.21,22,26,28 Immunization with adenoviruses encoding model or xenogeneic tumor antigens has also been shown to induce humoral30 and cellular29-31 immune responses to the tumor antigen transgene product and to provide protective29,30 and therapeutic30 31 antitumor immunity.
We therefore sought to determine if Id-encoding adenoviruses could induce antitumor immunity against murine lymphomas. In this report we demonstrate that in 2 different B-cell lymphoma models, a single intramuscular (IM) injection of Id-encoding adenovirus can induce high titers of anti-Id antibodies. Vaccination provides protective immunity from lethal tumor challenge that is equal to or superior to that afforded by protein Id vaccination. Pre-immunization with an irrelevant adenovirus did not significantly impair the protective response following Id adenovirus vaccination. In at least one model, tumor protection is not dependent upon effector T cells.
Materials and methods
Mice and cell lines
Six- to 8-week-old female C3H/HeN and BALB/c mice (Harlan-Sprague-Dawley, San Diego, CA), C3H/HeJ β2-microglobulin knockout mice (β2M−/−)32 and control wild type C3H/HeJ mice (The Jackson Laboratory, Bar Harbor, ME) were housed at the Laboratory Animal Facility at Stanford University Medical Center (Stanford, CA). The carcinogen-induced murine B-cell lymphoma 38C13 expressing a clonal IgM/κ on its surface has been previously described.33 The 38C13 cells were maintained in Roswell Park Memorial Institute medium (RPMI 1640) supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 50 μM 2-mercaptoethanol (cRPMI-10).The spontaneously arising murine B-cell lymphoma BCL134 (gift of Dr Samuel Strober, Stanford, CA) was serially passaged in vivo in BALB/c mice. The adenovirus-transformed human embryonic kidney cell line 29335 (gift of Dr Inder Verma, Salk Institute, La Jolla, CA) was maintained in Dulbecco modified Eagle medium (DMEM) high glucose supplemented with 10% heat-inactivated FCS, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. All media and supplements were obtained from Gibco BRL Life Technologies (Frederick, MD).
Production of recombinant adenoviruses
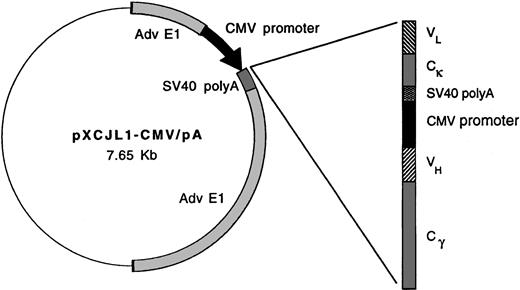
Replication-defective viruses derived from adenovirus type 5 (AdV-5) were prepared according to the methods of Graham and Prevec.20 To construct Id-encoding adenoviruses, murine γ2a heavy chain and κ light chain or human γ1 heavy chain and κ light chain sequences were first introduced into plasmid transfer vector pXCJL1 containing AdV-5 sequences (gift of Dr Inder Verma)24 (Figure 1). Tumor-derived Ig VH and VL region (Id) segments cloned by PCR were then introduced sequentially in the pXCJL1 vector, with each Ig chain being flanked by cytomegalovirus (CMV) promoter and SV40 poly(A) sequences. Transgene insertion into pXCJL1 disrupts theAD5E1 gene, rendering the final recombinant virus replication-defective. Transfer vectors for viruses Ad-IdhuCR (human constant region) and Ad-IdmuCR (murine CR) encode the 38C13 tumor VH and VL regions together with human and murine constant regions, respectively. Ad-CTRLhuCR encodes the VH and VL regions of the Ramos human Burkitt lymphoma cell line (American Type Culture Collection, Rockville, MD) together with human constant regions. Transfer vectors were cotransfected with AdV-5 plasmid pJM1736 into 293 cells by calcium-phosphate precipitation to generate recombinant infectious viral particles.
Schematic representation of idiotype adenoviral constructs.
In adenoviral plasmid transfer vector pXCJL1, heavy and light Ig chains are under the control of individual CMV promoters. The 38C13 VH and VL (Id) sequences are paired with murine γ2a heavy chain and κ light chain or human γ1 heavy chain and κ light chain sequences in viruses Ad-IdmuCR and Ad-IdhuCR, respectively. Ad-CTRLhuCR and Ad-BCL1huCR contain Ramos Id and BCL1 tumor Id sequences, respectively, along with human constant regions.
Schematic representation of idiotype adenoviral constructs.
In adenoviral plasmid transfer vector pXCJL1, heavy and light Ig chains are under the control of individual CMV promoters. The 38C13 VH and VL (Id) sequences are paired with murine γ2a heavy chain and κ light chain or human γ1 heavy chain and κ light chain sequences in viruses Ad-IdmuCR and Ad-IdhuCR, respectively. Ad-CTRLhuCR and Ad-BCL1huCR contain Ramos Id and BCL1 tumor Id sequences, respectively, along with human constant regions.
The Ad-BCL1huCR virus was produced by an alternative method using homologous recombination in BJ5183 bacteria.37 Plasmid pXCJL-BCL1-HCR was first created by inserting the BCL1 tumor VH and VL regions into the pXCJL plasmid containing human constant regions. Plasmids pXCJL-BCL1-HCR and pTG3652 (containing the AdV-5 genome) were then linearized and transfected into BJ5183 cells by heat shock. Plasmids resulting from homologous recombination of the BCL1-HCR–containing fragment into the E1 region of pTG3652 were selected by restriction digest pattern, amplified, and transfected into 293 cells using Lipofectamine Plus (Gibco). Recombinant AdV-5 viruses used as controls included Ad-C6VL-TCR (T-cell receptor) encoding TCR-Id38 and Adβgal encoding β-galactosidase.
Viral plaques were isolated and amplified on 293 cells, with expression of the correct product being confirmed by enzyme-linked immunosorbent assays (ELISAs) for the relevant Ig segments. High-titer virus was obtained by freeze-thaw lysis of infected 293 cells and cesium-chloride banding. Purified virus was dialyzed against phosphate-buffered saline (PBS) (pH 7.4) containing 10% glycerol, and aliquots were stored at −70°C. Viral titers were determined by plaque or limiting dilution assays on 293 cells.
In vitro protein expression by adenovirus-infected cells
Supernatants of infected 293 cells or HeLa cells were assayed by ELISA for the appropriate Ig determinants. Microtiter plates (Nunc, Naperville, IL) were coated with goat antihuman or antimurine IgG antibodies (Caltag, South San Francisco, CA). After blocking, test supernatants and appropriate standards were titered over 8 wells. Bound proteins were detected with horseradish peroxidase (HRPO)-conjugated goat antihuman κ, goat antihuman λ, or goat antimurine κ antibodies (Southern Biotechnology, Birmingham, AL). Monoclonal antibodies (mAbs) against the 38C13 Id,39 BCL1 Id,3 or human variable region VH4-34expressed by Ramos cells (9G4; Zymed Inc, South San Francisco, CA) were used to further confirm the conformational integrity of virus-encoded Ig proteins by ELISA.
Production and modification of Id proteins
An isotype-matched IgM/κ protein,4C5 38C13, and BCL1 IgM Id proteins were derived from tumor–myeloma cell hybridomas as previously described2,3,40 and affinity purified using mannose binding protein columns (Pierce, Rockford, IL) to more than 95% purity as determined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Where indicated, proteins were coupled to keyhole limpet hemocyanin (KLH) (Calbiochem, San Diego, CA) using glutaraldehyde as previously described.2
Vaccinations
Purified virus was diluted in PBS to the desired concentration and injected IM at a dose of 106 to 1010 PFU (plaque-forming unit) as indicated in a volume of 100 μL (50 μL each quadricep). A dose of 108 PFU was used in most experiments, as described in “Results.” Adenovirus vaccines were compared to protein vaccines consisting of 38C13 or BCL1 idiotypic Ig conjugated to KLH (100 μg total; 50 μg each protein) injected subcutaneously (SQ) twice at 2-week intervals along with either SAF141,42 or 10 μg QS2143adjuvants as indicated. QS21 was kindly provided by Aquila Biopharmaceuticals, Framingham, MA.
To determine the effect of previous exposure to adenovirus on the development of anti-Id immunity following Id adenovirus vaccination, groups of 10 mice were first injected IM with either PBS or 108 PFU of an irrelevant virus Adβgal. Three weeks later, mice were bled, and sera were stored at −20°C. Both groups of mice were then vaccinated with 108 PFU Ad-IdhuCR. Nineteen days later, mice were bled for determination of serum anti-38C13 Id titers and then challenged 2 days later with 38C13 tumor cells SQ (as described below).
Humoral immune response assessments
Eighteen to 21 days following the last immunization, blood was collected by tail vein. Serum anti-38C13 Id antibodies were quantitated by ELISA as previously described.2 Briefly, 96-well Maxisorb plates (Nunc, Aperville, IL) were coated with 38C13 IgM and incubated with serially diluted immune sera. Bound antibodies were detected with horseradish peroxidase–conjugated goat antimouse IgG (Caltag) using the 2,2′-azinobis(3-ethyl)-benzthiazoline sulfonic acid (ABTS) substrate and absorbance determination at 405 nm with a Vmax microplate reader (Molecular Devices, Menlo Park, CA). A mixture of affinity-purified anti-38C13 Id mAbs containing IgG1, IgG2a, and IgG2b isotypes in a 2:1:1 ratio was used to generate a standard curve for total anti-Id IgG. Isotype-specific detectors were used for the quantitation of anti-Id antibodies of the IgG1 and IgG2a subclasses (Southern Biotech) using relevant isotype standards. Purified 4C5 (IgM/κ) and BCL1 (IgM/λ) Igs were used to check the specificity of the anti-Id response. Serum anti-BCL1 Id antibodies were measured as previously described.3
Assay for neutralizing anti-adenovirus antibodies
To verify the presence of neutralizing anti-adenovirus antibodies in the serum of mice previously exposed to Adβgal, sera from all 10 mice were pooled for comparison to adenovirus-naive (PBS-treated) mice. Sera were serially-diluted in PBS and 0.1 mL was added to 50 PFU Ad-BCL1huCR in 0.1 mL PBS and incubated for one hour at room temperature. Adenovirus-serum mixtures were then added to 293 cell monolayers in 6-well plates and incubated for 45 minutes at room temperature with frequent swirling. Monolayers were then overlayed with agar and incubated at 37°C. Viral plaques were counted 6 and 8 days later by direct inspection using an inverted microscope.
Tumor challenge
The 38C13 tumor cells were thawed from a common dedicated frozen stock 2 days before tumor challenge and split on the day prior to use. On the day of tumor challenge, cells were washed 3 times in serum-free RPMI and diluted to the appropriate concentration in Hank balanced salt solution (HBSS). Three weeks after adenovirus vaccination and the second protein vaccination, groups of mice received either 1000 cells SQ above the base of the tail or 200 cells intraperitoneally (IP) in a volume of 0.2 mL. For experiments involving vaccination of mice with pre-existing tumor, mice were first injected with 1000 cells SQ above the base of the tail, as detailed above. Two to 12 hours later, mice were immunized with 108 PFU adenovirus IM, and 8 days later, mice received 100 mg/kg cyclophosphamide IP in sterile saline. Animals were then followed for survival as described above. For BCL1 tumor challenge, a cryopreserved lot of tumor cells was prepared by mechanical dissociation of a late-stage tumor-infiltrated spleen; Ficoll (Amersham Pharmacia, Uppsala, Sweden) sedimentation to remove red cells, dead cells, and debris; and liquid nitrogen storage in FCS plus 10% DMSO. On the day of tumor challenge, cells were rapidly thawed, washed in RPMI, resuspended in HBSS, and injected immediately (5 × 104 cells IP per mouse). Thereafter, mice were followed daily for survival. Survival analysis was performed using Prism software (GraphPad, San Diego, CA), and P values were calculated using the log-rank statistical test.
In vivo depletion of T-lymphocyte subsets
Groups of 15 mice were vaccinated with either Ad-IdhuCR or Ad-CTRLId-huCR as described above and challenged with tumor 3 weeks later on day 0. On days −6,−5,−4, 0, and +7 with respect to tumor challenge, mice were injected IP with 200 μg T-cell depleting or control mAbs from ascites as previously described.44Antibodies used were the CD4+ T-cell depleting mAb GK1.5 (rat IgG2b), control rat IgG2a mAb H22-15-5, CD8+T-cell–depleting mAb HB129 (mouse IgG2a), or control mouse IgG2a mAb 17F12. On days −1, +7, and +13 with respect to tumor challenge, peripheral blood was collected from 2 representative mice from each group, and lymphocytes were analyzed by flow cytometry for depletion of each T-cell subset. Staining antibodies CT-CD4 (anti-CD4, Caltag) and 53-5.8 (anti-CD8β.2; PharMingen, San Diego, CA) were not cross-blocked by the depleting antibodies and demonstrated more than 98% depletion of the target T-cell population (data not shown).
Results
Construction of 38C13 Id-encoding recombinant adenoviruses
To evaluate the efficacy of Id-encoding adenoviruses in eliciting anti-Id immunity, recombinant AdV-5 vectors were constructed containing tumor Id or control Ig variable region sequences along with syngeneic murine or xenogeneic (human) Ig constant region sequences (Figure 1). These vectors direct the production of full-length, secreted, tetrameric Igs. Ad-IdmuCR and Ad-IdhuCR encode the 38C13 tumor Id sequences plus murine and human constant regions, respectively. The xenogeneic constant regions are included as previous studies of vaccination with recombinant Id proteins,45 or naked plasmid DNA17 have demonstrated dependence on foreign constant regions for induction of a humoral anti-Id response and tumor protection. The control adenovirus Ad-CTRLhuCR encodes the Id sequences from a human B-cell lymphoma (Ramos) plus human constant regions. Expression of the appropriately encoded Ig products was confirmed by ELISA to detect Id and constant region determinants in supernatants of infected 293 or HeLa cells 3-6 days after infection (data not shown).
Humoral anti-Id response following vaccination with 38C13 Id adenoviruses
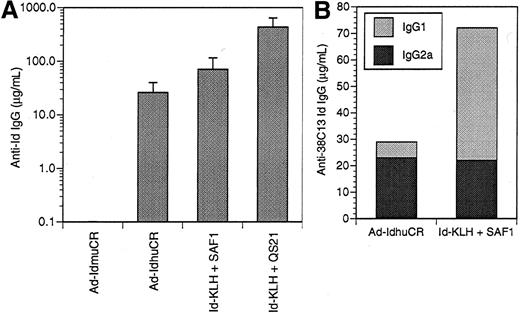
Anti-Id antibodies are the principal effectors of immunity to the 38C13 B-cell lymphoma following vaccination with Id-KLH protein,2,42 Id plasmid DNA,44 and Id-pulsed dendritic cells.46 Therefore, we evaluated the humoral anti-38C13 Id response following vaccination with 38C13 Id adenoviruses. Initial experiments investigated the dose-response relationship of Id adenovirus for the induction of anti-Id antibodies. Groups of mice were given a single IM injection of 106, 107, 108, 109, or 1010PFU Ad-IdhuCR. Three weeks later, levels of anti-38C13 Id antibodies in the sera of vaccinated mice did not differ significantly between the various dose groups (data not shown). Thus, the intermediate dose of 108 PFU was selected for subsequent studies. The humoral anti-Id response to a single dose of Ad-IdhuCR and Ad-IdmuCR was then compared to 2 biweekly vaccinations with Id-KLH along with the SAF141 or QS2143 adjuvants (Figure2A). The Ad-IdmuCR vector containing 38C13 Id sequences and murine constant regions failed to elicit any detectable anti-Id antibodies. Vaccination with adenovirus Ad-CTRLhuCR encoding an unrelated Id (Ramos) also failed to elicit any 38C13-specific antibodies. In contrast, Ad-IdhuCR induced substantial levels of anti-38C13 Id antibodies (26.1 ± 14.0 μg/mL), while Id-KLH plus SAF1 and Id-KLH plus QS21 resulted in still higher titers (70.5 ± 44.9 μg/mL and 430.2 ± 209.5 μg/mL, respectively). The specificity of the immune response was demonstrated by the lack of detectable antibodies against a class-matched control Id (data not shown).
Humoral anti-idiotypic response after vaccination with 38C13 Id adenoviruses versus Id-KLH protein plus adjuvant.
(A) Groups of 10 mice were given a single injection of 38C13 Id-encoding adenovirus containing either syngeneic murine constant regions or xenogeneic human constant regions (Ad-IdhuCR) or 2 biweekly vaccinations of 38C13 Id-KLH protein along with SAF1 or QS21 adjuvant. Three weeks later, sera were collected and analyzed for anti-38C13 Id IgG antibodies by ELISA. Bars represent mean determinations in 10 animals, with error bars showing the mean ± SD. Results are representative of 3 individual experiments. (B) Isotype profiles of anti-Id antibodies elicited by adenovirus versus Id-KLH protein vaccines. Sera collected as above were pooled, and 38C13 Id-specific antibodies were quantitated by ELISA using γ1- or γ2a-specific detectors. Results are representative of 2 experiments.
Humoral anti-idiotypic response after vaccination with 38C13 Id adenoviruses versus Id-KLH protein plus adjuvant.
(A) Groups of 10 mice were given a single injection of 38C13 Id-encoding adenovirus containing either syngeneic murine constant regions or xenogeneic human constant regions (Ad-IdhuCR) or 2 biweekly vaccinations of 38C13 Id-KLH protein along with SAF1 or QS21 adjuvant. Three weeks later, sera were collected and analyzed for anti-38C13 Id IgG antibodies by ELISA. Bars represent mean determinations in 10 animals, with error bars showing the mean ± SD. Results are representative of 3 individual experiments. (B) Isotype profiles of anti-Id antibodies elicited by adenovirus versus Id-KLH protein vaccines. Sera collected as above were pooled, and 38C13 Id-specific antibodies were quantitated by ELISA using γ1- or γ2a-specific detectors. Results are representative of 2 experiments.
To further characterize the anti-Id immune response to vaccination with Ad-IdhuCR, we examined the isotype profiles of IgG anti-Id antibodies following vaccination (Figure 2B). Vaccination of mice with Id-KLH plus SAF1 adjuvant induced anti-Id antibodies of primarily the IgG1 isotype (50 μg/mL), with a lower proportion of the IgG2a isotype (22 μg/mL) (γ1:γ2a ratio = 2.27). In contrast, vaccination with Ad-IdhuCR induced lower total levels of anti-Id antibodies, but the vaccination predominantly those of the IgG2a isotype (23 μg/mL vs 6 μg/mL IgG1) (γ1:γ2a ratio = 0.26), which is consistent with the induction of a Th1-type response.47
Tumor protection following vaccination with 38C13 Id adenoviruses
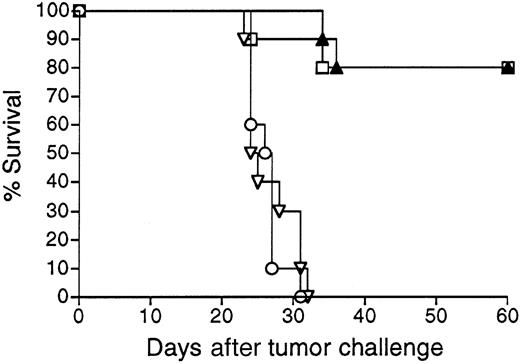
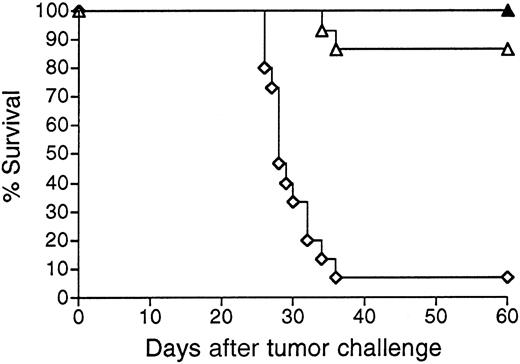
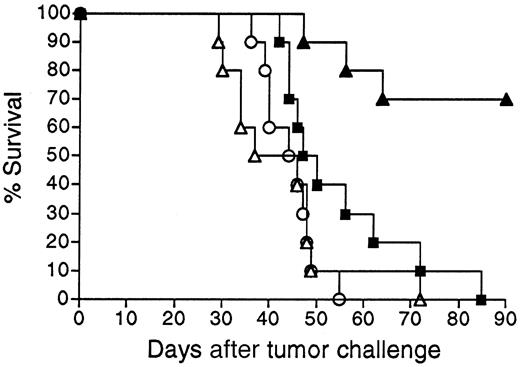
Mice vaccinated with 38C13 Id adenoviruses were next evaluated for resistance to tumor challenge (Figure 3). Mice treated with only PBS and challenged with 1000 38C13 lymphoma cells SQ all died within 32 days. In concordance with their lack of detectable anti-38C13 Id antibodies, mice immunized with Ad-IdmuCR were not protected from tumor challenge and died at the same rate as PBS-treated mice. However, a single IM injection of 108 PFU Ad-IdhuCR protected 80% of mice from the lethal tumor inoculum (P < .0001 for comparison with PBS or Ad-IdmuCR). This level of protection matched that afforded by 2 biweekly vaccinations with Id-KLH protein plus QS21 adjuvant (P = .934). Comparable results were obtained in 3 additional experiments, with protection following 38C13 Id adenovirus vaccination always equivalent to that of Id-KLH plus either QS21 or SAF1 adjuvants. No significant protection from 38C13 tumor challenge was observed following vaccination with Ad-CTRLhuCR (data not shown).
Survival following vaccination with 38C13 Id adenoviruses versus Id-KLH protein.
Groups of 10 mice were vaccinated with a single IM injection of 108 PFU Ad-IdhuCR (▴) or Ad-IdmuCR (▿) or 2 biweekly injections of PBS (○) or 38C13 Id-KLH plus QS21 adjuvant (■). Three weeks following vaccinations, mice were injected SQ with 1000 38C13 tumor cells and followed for survival. Results are representative of 4 individual experiments.
Survival following vaccination with 38C13 Id adenoviruses versus Id-KLH protein.
Groups of 10 mice were vaccinated with a single IM injection of 108 PFU Ad-IdhuCR (▴) or Ad-IdmuCR (▿) or 2 biweekly injections of PBS (○) or 38C13 Id-KLH plus QS21 adjuvant (■). Three weeks following vaccinations, mice were injected SQ with 1000 38C13 tumor cells and followed for survival. Results are representative of 4 individual experiments.
Therapeutic effects of 38C13 adenovirus vaccination in mice bearing pre-existing tumor
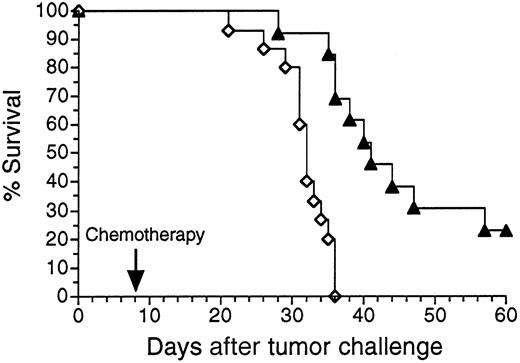
To determine whether Id adenovirus vaccines could protect against growth of pre-existing tumor, mice were first injected with a SQ inoculum of 38C13 tumor cells. Later the same day animals were vaccinated with either Ad-IdhuCR or Ad-CTRLhuCR. Eight to 10 days after tumor inoculation, mice were treated with cyclophosphamide to cytoreduce the growing tumor while allowing time for maturation of the humoral anti-Id response. Mice receiving the control adenovirus vaccine all died within 36 days of tumor challenge, whereas in mice receiving Ad-IdhuCR, survival was significantly prolonged compared to control mice (P < .003), and 23% of animals were without clinical evidence of tumor at day 60 (Figure4). Significant improvements in survival were also observed even when vaccination was performed up to 3 days following tumor inoculation (P < .05; data not shown), although long-term survival was not achieved.
Survival of mice bearing pre-existing tumor following treatment with 38C13 Id adenovirus plus chemotherapy.
Groups of mice were injected with 1000 38C13 tumor cells SQ. Later that same day, animals were vaccinated with either Ad-IdhuCR (▴) (n = 13) or Ad-CTRLhuCR (⋄) (n = 15). Eight days later mice received a single dose of cyclophosphamide and were followed for survival. Results are representative of 2 experiments.
Survival of mice bearing pre-existing tumor following treatment with 38C13 Id adenovirus plus chemotherapy.
Groups of mice were injected with 1000 38C13 tumor cells SQ. Later that same day, animals were vaccinated with either Ad-IdhuCR (▴) (n = 13) or Ad-CTRLhuCR (⋄) (n = 15). Eight days later mice received a single dose of cyclophosphamide and were followed for survival. Results are representative of 2 experiments.
Role of effector T cells in tumor protection following 38C13 Id adenovirus vaccination
We next sought to determine the contribution of effector T cells to tumor protection in mice following 38C13 Id adenovirus vaccination. Groups of 15 mice vaccinated with Ad-IdhuCR were treated with mAbs to deplete effector CD4+ or CD8+ T cells or with isotype-matched control mAbs prior to and following tumor challenge, as described in “Materials and methods” (Figure5). Survival of mice vaccinated with Ad-CTRLhuCR and treated with control mAbs is shown as a negative control. Depletion of each T-cell subset from the peripheral blood of vaccinated mice was confirmed by flow cytometry using non–cross-blocking anti-CD4 and anti-CD8 mAbs prior to tumor challenge (data not shown). Depletions were maintained throughout the experiment by continued weekly administration of the depleting mAbs. Control mAbs had no significant effect on the relevant T-cell populations. The survival of mice depleted of CD4+ and CD8+ T cells after vaccination with Ad-IdhuCR was equivalent to that of mice receiving control mAbs (P = .150).
Effects of in vivo depletion of T cells on survival following vaccination with Id-encoding adenoviruses.
Groups of 15 mice were vaccinated with Ad-IdhuCR or Ad-CTRLhuCR, then challenged 3 weeks later (day 0) with 1000 38C13 tumor cells SQ and followed for survival. On days −6, −5, −4, and 0 and weekly thereafter, mice receiving Ad-IdhuCR were treated with CD4+ and CD8+ T-cell depleting mAbs GK1.5 and HB129 or isotype control mAbs. Animals vaccinated with Ad-CTRLhuCR also received control mAbs. ▵ indicates Ad-IdhuCR plus anti-CD4/CD8; ▴ indicates Ad-IdhuCR plus control mAbs; ⋄ indicates Ad-CTRLhuCR plus control mAbs.
Effects of in vivo depletion of T cells on survival following vaccination with Id-encoding adenoviruses.
Groups of 15 mice were vaccinated with Ad-IdhuCR or Ad-CTRLhuCR, then challenged 3 weeks later (day 0) with 1000 38C13 tumor cells SQ and followed for survival. On days −6, −5, −4, and 0 and weekly thereafter, mice receiving Ad-IdhuCR were treated with CD4+ and CD8+ T-cell depleting mAbs GK1.5 and HB129 or isotype control mAbs. Animals vaccinated with Ad-CTRLhuCR also received control mAbs. ▵ indicates Ad-IdhuCR plus anti-CD4/CD8; ▴ indicates Ad-IdhuCR plus control mAbs; ⋄ indicates Ad-CTRLhuCR plus control mAbs.
To further rule out CD8+ T cells as effectors of tumor immunity following vaccination with Ad-IdhuCR, we performed tumor challenge in β2M knockout mice, which are severely deficient in CD8+ cytotoxic T-lymphocyte activity.32 Groups of wild type C3H or C3H β2M−/− mice were vaccinated as above with Ad-IdhuCR, then challenged 3 weeks later with 1000 38C13 lymphoma cells SQ and followed for survival (Figure6). As a negative control for tumor protection, wild type mice received the irrelevant virus Ad-C6VL-TCR. In both wild type and β2M−/− strains, vaccination with Ad-IdhuCR protected 50% of mice from the lethal tumor inoculum (P = .786).
Effects of 38C13 adenovirus vaccination on survival of tumor-challenged wild type or β2M-deficient mice.
Groups of wild type C3H mice were vaccinated with 108 PFU Ad-IdhuCR (▴) (n = 10) or Ad-C6VL-TCR (▵) (n = 10) as a control. The β2M-deficient C3H mice were also vaccinated with Ad-IdhuCR (○) (n = 8). Three weeks later, mice were injected SQ with 1000 38C13 tumor cells and followed for survival.
Effects of 38C13 adenovirus vaccination on survival of tumor-challenged wild type or β2M-deficient mice.
Groups of wild type C3H mice were vaccinated with 108 PFU Ad-IdhuCR (▴) (n = 10) or Ad-C6VL-TCR (▵) (n = 10) as a control. The β2M-deficient C3H mice were also vaccinated with Ad-IdhuCR (○) (n = 8). Three weeks later, mice were injected SQ with 1000 38C13 tumor cells and followed for survival.
Effects of prior exposure to adenovirus on the induction of anti-Id immunity following Id adenovirus vaccination
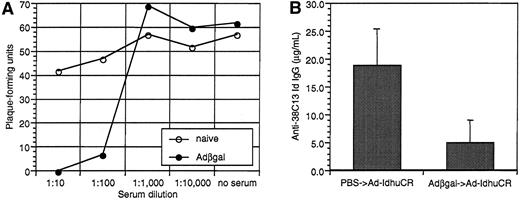
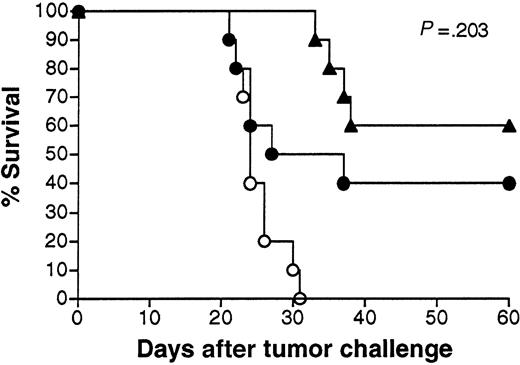
To simulate the clinical situation in which individuals can have pre-existing anti-adenovirus immunity secondary to natural infection, we vaccinated animals that were pre-exposed to an irrelevant adenovirus (Adβgal). As expected, mice injected with Adβgal developed significant titers of neutralizing anti-adenovirus antibodies (Figure7A). A test aliquot of virus was 100% and 89% neutralized in the presence of pooled immune serum at dilutions of 1:10 and 1:100, respectively. Mice pre-exposed to Adβgal developed significantly lower levels of anti-Id antibodies than PBS-treated mice following Id adenovirus vaccination (5.0 ± 4.1 μg/mL vs 18.6 ± 6.6 μg/mL, respectively) (Figure 7B). Two of the 10 mice pre-exposed to adenovirus had no detectable serum anti-Id antibodies. Nonetheless, both Adβgal and PBS pretreated mice were significantly protected from tumor challenge (P = .036 andP < .0001 vs controls, respectively) (Figure8). Moreover, while the proportion of long-term survivors was lower in mice pre-exposed to irrelevant adenovirus (40% vs 60%), this difference was not statistically significant (P = .203, as determined by the log-rank test). Three of the mice dying early in the Adβgal pretreated group had the lowest anti-Id antibody titers among the group (0, 0, and 1.0 μg/mL).
Effects of pre-exposure to irrelevant adenovirus on serum neutralizing anti-adenovirus activity and the subsequent humoral anti-Id response to Id-adenovirus vaccination.
Groups of 10 mice were injected with Adβgal or PBS. Sera were collected 3 weeks later, and both groups were then vaccinated with 108 PFU Ad-IdhuCR. Nineteen days later, mice were bled again before undergoing tumor challenge 2 days later. (A) Neutralizing activity of sera from Adβgal or PBS-treated mice. Pooled sera from each group (n = 10) were serially-diluted and incubated with 50 PFU Ad-BCL1huCR. Adenovirus-serum mixture or PBS was then added to 293 cell monolayers, agar-overlayed, and incubated at 37°C until viral plaques were enumerated 6 and 8 days later. (B) Humoral anti-Id response after vaccination with Ad-IdhuCR in mice pretreated with Adβgal or PBS. Sera collected 2 days prior to tumor challenge were analyzed for anti-38C13 Id IgG antibodies by ELISA. Bars represent mean determinations in 10 animals, with error bars showing the mean ± SD.
Effects of pre-exposure to irrelevant adenovirus on serum neutralizing anti-adenovirus activity and the subsequent humoral anti-Id response to Id-adenovirus vaccination.
Groups of 10 mice were injected with Adβgal or PBS. Sera were collected 3 weeks later, and both groups were then vaccinated with 108 PFU Ad-IdhuCR. Nineteen days later, mice were bled again before undergoing tumor challenge 2 days later. (A) Neutralizing activity of sera from Adβgal or PBS-treated mice. Pooled sera from each group (n = 10) were serially-diluted and incubated with 50 PFU Ad-BCL1huCR. Adenovirus-serum mixture or PBS was then added to 293 cell monolayers, agar-overlayed, and incubated at 37°C until viral plaques were enumerated 6 and 8 days later. (B) Humoral anti-Id response after vaccination with Ad-IdhuCR in mice pretreated with Adβgal or PBS. Sera collected 2 days prior to tumor challenge were analyzed for anti-38C13 Id IgG antibodies by ELISA. Bars represent mean determinations in 10 animals, with error bars showing the mean ± SD.
Survival following vaccination with 38C13 Id adenovirus with or without prior exposure to irrelevant adenovirus.
Groups of 10 mice were injected with Adβgal or PBS, vaccinated 3 weeks later with 108 PFU Ad-IdhuCR, then challenged 3 weeks later with 1000 38C13 tumor cells SQ, and followed for survival. Survival of unvaccinated PBS-treated mice is shown for comparison.P indicates comparison between Ad-IdhuCR–vaccinated mice pretreated with Adβgal or PBS. ▴ indicates PBS and Ad-IdhuCR; ● indicates Adβgal and Ad-IdhuCR; ○ indicates PBS control.
Survival following vaccination with 38C13 Id adenovirus with or without prior exposure to irrelevant adenovirus.
Groups of 10 mice were injected with Adβgal or PBS, vaccinated 3 weeks later with 108 PFU Ad-IdhuCR, then challenged 3 weeks later with 1000 38C13 tumor cells SQ, and followed for survival. Survival of unvaccinated PBS-treated mice is shown for comparison.P indicates comparison between Ad-IdhuCR–vaccinated mice pretreated with Adβgal or PBS. ▴ indicates PBS and Ad-IdhuCR; ● indicates Adβgal and Ad-IdhuCR; ○ indicates PBS control.
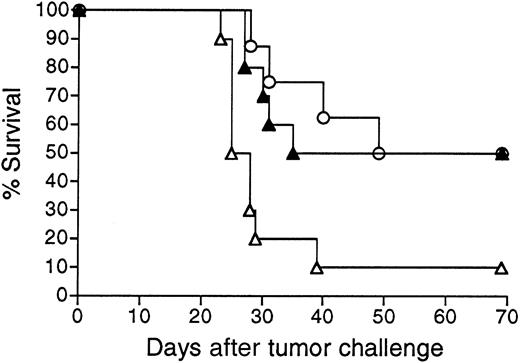
Adenovirus encoding BCL1 Id provides protective immunity against BCL1 tumor challenge
We next sought to determine whether Id-encoding adenoviral vaccination could be applied to another B-cell tumor. An adenoviral vector was constructed expressing the Id sequences of the widely studied murine B-cell lymphoma/leukemia BCL1 of BALB/c origin.3,11 34 Vector Ad-BCL1huCR was analogous to the 38C13 Id vector Ad-IdhuCR, with tumor VH and VLsequences joined to human constant region sequences. Vaccination of animals with Ad-BCL1huCR elicited substantial titers of BCL1-specific anti-Id antibodies in BALB/c mice (data not shown) and protected a majority of animals from challenge with BCL1 tumor cells (Figure9). While BCL1 Id-KLH vaccination significantly prolonged the survival of mice (P = .035 compared to PBS), long-term survival (more than 90 days) was not observed. In contrast to the 38C13 model in which Id adenovirus had no advantage over Id-KLH vaccination, tumor protection following Ad-BCL1huCR vaccination was markedly superior to that obtained using vaccination with BCL1 Id-KLH (P = .0006) (Figure9).
Survival following vaccination with BCL1 Id adenovirus versus BCL1 Id-KLH protein.
Groups of 10 BALB/c mice were given a single IM injection of 108 PFU Ad-BCL1huCR (▴) or 38C13 virus Ad-IdhuCR (▵) versus 2 biweekly SQ injections of PBS (○) or BCL1 Id-KLH protein (BCL1-KLH) plus QS21 adjuvant (▪). Three weeks later, mice were injected IP with 5 × 104 BCL1 tumor cells and followed for survival. Results are representative of 2 individual experiments.
Survival following vaccination with BCL1 Id adenovirus versus BCL1 Id-KLH protein.
Groups of 10 BALB/c mice were given a single IM injection of 108 PFU Ad-BCL1huCR (▴) or 38C13 virus Ad-IdhuCR (▵) versus 2 biweekly SQ injections of PBS (○) or BCL1 Id-KLH protein (BCL1-KLH) plus QS21 adjuvant (▪). Three weeks later, mice were injected IP with 5 × 104 BCL1 tumor cells and followed for survival. Results are representative of 2 individual experiments.
Discussion
A variety of vaccination approaches are currently being explored for inducing therapeutic anti-Id immunity against B-cell lymphomas. These include vaccination with tumor–myeloma cell hybridoma-derived4,14 or recombinant10,48 Id proteins, Id-pulsed dendritic-cell vaccination,5,15 and DNA vaccination.16 17 An ideal strategy for this customized immunotherapeutic approach would be one in which individual vaccines could be rapidly and easily prepared and Id determinants were presented to the immune system in a highly immunogenic form. Recombinant adenoviruses, which can be quickly assembled using PCR-amplified Id genes and are potently immunogenic, fulfill these criteria. We have now evaluated this approach in 2 murine B-cell lymphoma models and found that a single injection of recombinant Id adenovirus can induce substantial serum titers of Id-specific antibodies. Following vaccination, protection from subsequent tumor challenge is either equivalent to or superior to that attained with Id protein vaccination. When used in conjunction with chemotherapy, vaccination can also prolong survival in mice bearing pre-existing tumor. Mechanistic studies in one model have demonstrated that protection is not dependent upon effector T cells.
In developing Id vaccines for B-cell lymphomas, induction of a humoral response is desirable given the proven antitumor effects of anti-Id antibodies.1,3,42,49 Vaccination with 38C13 Id-encoding adenovirus Ad-IdhuCR induced substantial titers of Id-specific antibodies after only a single injection (Figure 2A). Induction of the humoral response was dependent on the inclusion of foreign (human) constant regions in the Id vector, as has been previously noted in studies of vaccination with recombinant Id proteins45 and DNA.17 In this arrangement the foreign determinants plus the weakly immunogenic self tumor antigen (Id) constitute a hapten-carrier system50 analogous to that formed by conjugation of Id to KLH.2 The predominant isotype of anti-Id antibodies detected after 38C13 Id adenovirus vaccination was IgG2a (Figure 2B), which is the murine isotype characteristic of a Th1 response47 and possessing the most efficient antibody-dependent cellular cytotoxicity effector functions in the 38C13 tumor model.51
Protection from tumor challenge following Id adenovirus vaccination was compared to that afforded by the standard approach to Id vaccination, that is, multiple injections of Id protein coupled to KLH together with adjuvant. In 4 independent experiments, a single IM injection of Id adenovirus imparted protection equivalent to Id-KLH plus adjuvant (Figure 3). In animals bearing pre-existing 38C13 lymphoma, treatment with Id adenovirus in combination with chemotherapy also induced significant resistance to tumor growth (Figure 4). Protection, like anti-Id antibody induction, also required human constant regions in the vector. In the BCL1 tumor model, while BCL1 Id-KLH plus QS21 significantly prolonged survival after tumor challenge, BCL1 Id adenovirus vaccination was found to be highly superior to BCL1 Id-KLH in 2 separate experiments (Figure 7). Most previous studies of adenovirus tumor antigen vaccination have used artificial tumor antigen systems to demonstrate protective29,30 and therapeutic30 31 antitumor immunity. However, our findings demonstrate that protective and therapeutic immunity against a clinically relevant, self-derived tumor antigen can be efficiently elicited by adenoviral vaccination.
The mechanism of tumor protection following Id adenoviral vaccination was investigated in the 38C13 lymphoma model. Depletion of both CD4+ and CD8+ effector T cells after establishment of the immune response and just prior to tumor challenge did not impair the protective response induced by vaccination (Figure 5). In addition, β2M knockout mice, which are severely deficient in CD8+ cytotoxic T-lymphocyte activity, were protected to the same degree as wild type mice following immunization with the Id-encoding adenovirus. This again indicates that CD8+ T cells were not required for the full level of protection afforded by this particular vaccine (Figure 6). Because T cells were not found to contribute to the tumor protection following 38C13 Id adenovirus vaccination, anti-Id antibodies are likely to be necessary effectors, as has been previously observed in other vaccine studies in this model.42,44,46 This does not rule out the recruitment of anti-Id T cells, however, as any antitumor effects of these cells could have been masked by the effects of anti-Id antibodies, which by themselves are sufficient to provide tumor protection in this model.42,44 The lack of a role for CD8+ T cells in mediating tumor protection in this model may reflect a relative paucity of class I MHC-restricted CTL epitopes in the 38C13 Id.44,46 However, as other tumor Id proteins do contain epitopes recognizable by CTL,7-10 and as adenoviral vaccination readily stimulates CTL responses,23,25,27-29,31 adenoviral vaccination against other tumor Ids might be expected to elicit anti-Id CTLs. This has recently been demonstrated in an analogous study of TCR vaccination against a murine T-cell lymphoma, in which an adenovirus encoding the tumor-specific TCR variable region (TCR-Id) elicited tumor protection that was dependent on CD8+ T cells.38
An important finding of our study was that pre-exposure to an irrelevant adenovirus does not preclude successful Id adenovirus vaccination. Substantial adenovirus-neutralizing activity was found in sera of mice pretreated with Adβgal (Figure 7A). In response to Id adenovirus vaccination, these mice developed lower and more highly variable levels of anti-Id antibodies compared with adenovirus-naive animals (Figure 7B). The greater variation in serum anti-Id titers between individual mice receiving Adβgal might reflect differing titers of neutralizing anti-adenovirus antibodies. However, prior exposure to adenovirus did not significantly impair the protective antitumor response to Id adenovirus vaccination (Figure 8). Thus, pre-exisiting anti-adenovirus immunity appears to represent at worst a relative, but not an absolute, barrier to the successful use of adenovirus vaccination in this model. These results are in agreement with those of Chen et al,31 who found that animals pre-immune to adenovirus could still mount a potent CTL response against a model tumor antigen encoded by a subsequently administered adenovirus. Comparable results have also been obtained in other recombinant viral vaccine models.52
Id-encoding adenoviral vaccination should be readily applicable to humans with B-cell malignancies. Custom-made adenoviruses containing PCR-amplified tumor Id genes could be produced at high titers under clinical grade conditions, thereby eliminating the need for tumor-cell hybridization procedures or protein purification. The safety of adenoviral vaccination in humans using SQ and IM routes has been demonstrated by Rosenberg and colleagues,53 who performed a phase I study of MART-1 and gp100 recombinant adenovirus immunization in patients with metastatic melanoma. No significant toxicity was observed at doses up to 1011 PFU administered IM. In addition, CTL responses to MART-1 and clinical activity were observed. In this study the majority of patients had detectable but highly variable neutralizing anti-adenovirus serum antibody titers. However, no correlation was found between high neutralizing titers and development of immune reactivity to MART-1. Notably, one patient with a serum neutralizing titer of 400 experienced regression of metastatic melanoma lasting over 31 months. Thus, even in humans, pre-existing adenovirus immunity appears to represent only a relative barrier to successful vaccination using free recombinant adenovirus. Numerous gene therapy trials have also documented the safety and efficiency of recombinant adenoviruses as gene transfer vectors in humans. Local transgene expression has been routinely observed following injection of engineered adenoviruses into tissues,54-56 with accompanying immune response induction to the transgene product in some cases.56
The generation of recombinant adenoviruses opens up several options for further augmentation of anti-Id immune responses induced by primary Id adenovirus vaccination. While neutralizing antivirus immunity may increase after one or more vaccinations with recombinant adenovirus and impair the immunogenicity of subsequent doses, alternative means of boosting immune responses would include vaccination with virus-infected dendritic cells, recombinant protein, or plasmid DNA. Immunization with adenovirus-infected dendritic cells57,58 allows endogenous production of the transgene product by potent antigen presenting cells, thereby favoring induction of CTLs. This would complement the humoral responses induced by primary adenovirus vaccination. Adenovirus-infected dendritic cells have been used by a number of investigators to successfully overcome even high levels of neutralizing anti-adenovirus antibodies that might interfere with free virus vaccination.57-59 Indeed, we have observed that mice vaccinated with Id adenovirus-infected dendritic cells can achieve the same titers of anti-Id antibodies as naive mice in the setting of prior exposure to adenovirus (J.M.T. and R.L., unpublished observations, January 2000). Id DNA sequences used to construct adenoviruses can also be administered in naked plasmid DNA form along with co-injected plasmids encoding cytokines17 60 to further tailor the anti-Id response. Thus, Id-encoding adenoviruses could be used in a variety of vaccine strategies that could augment those currently employed for inducing anti-Id immunity. Our present results, together with the proven clinical activity of Id vaccination in B-cell lymphomas, provide a rationale for future investigations of Id adenovirus vaccination in patients with B-cell malignancies.
We thank Drs Yifan Dai, Inder Verma, and Frank Graham for generously providing adenoviral and transfer plasmid vectors. Drs Dai and Verma's assistance with virus construction is also deeply appreciated. Additional thanks to Debbie Czerwinski for expert assistance with flow cytometry.
C.B.C. was a Cure for Lymphoma Foundation Fellow. J.M.T. was a Fellow of the Cancer Research Institute and recipient of the Young Investigator Award from the American Society of Clinical Oncology. S.L.L. is supported by a Predoctoral Fellowship Award from the Howard Hughes Medical Institute. R.L. is a Clinical Research Professor of the American Cancer Society.
Supported by grants CA33399 and CA34233 from the National Institutes of Health–US Public Health Service (NIH-USPHS) and by NIH immunology training grant 5T32 AI 07290 (A.D.S.), Bethesda, MD.
J.M.T. and C.B.C. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ronald Levy, Department of Medicine, Division of Oncology, Stanford University Medical Center, Room M207, Stanford, CA 94305.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal