Vascular endothelial growth factor (VEGF) induces both angiogenesis and an increase in vascular permeability, 2 processes that are considered to be important for both tumor growth and the delivery of drugs to the site of tumors. This study demonstrates that transmembrane expression of tumor necrosis factor (tmTNF) is up-regulated in the endothelium of a murine methylcholanthrene (meth A)-induced sarcoma in comparison to the adjacent normal dermal vasculature and is also present on cultivated human endothelial cells. It is further shown that tmTNF is required for VEGF-mediated endothelial hyperpermeability in vitro and in vivo. This permissive activity of TNF appears to be selective, because anti-TNF antibodies ablated the VEGF-induced permeability but not proliferation of cultivated human endothelial cells. Furthermore, tnfgene–deficient mice show no obvious defects in vascularization and develop normally but failed to respond to administration of VEGF with an increase in vascular permeability. Subsequent studies indicated that the tmTNF and VEGF signaling pathways converge at the level of a secondary messenger, the “stress-activated protein kinase-2” (SAPK-2)/p38: (1) up-regulated endothelial expression of tmTNF resulted in the continuous activation of SAPK-2/p38 in vitro, and (2) an inhibitor of SAPK-2/p38 activation abolished the vascular permeability activity of VEGF in vivo. In conclusion, the study's finding that continuous autocrine signaling by tmTNF sensitizes endothelial cells to respond to VEGF by increasing their vascular permeability provides new therapeutic concepts for manipulating vascular hyperpermeability.
Introduction
Vascular endothelial growth factor (VEGF) is essential for both vasculogenesis and angiogenesis1 but was first identified as a tumor-produced vascular permeability factor (VPF) because of its potent activity in the Miles assay of vascular permeability.2 Accordingly, VEGF causes the accumulation of fluids in dermal and ascitic tumors.3,4 Furthermore, VEGF-induced vascular permeability has been suggested to be involved in vasculopathies of diseases different than cancer, including rheumatoid arthritis, acquired immunodeficiency syndrome, and allergic inflammation.5-7 In animal experiments it has been shown that the rapid induction of VEGF-mediated vascular permeability correlates with the formation of endothelial vesiculovacuolar organelles (VVOs) and fenestrations.8 An increase of vascular permeability has 2 important consequences for potential therapeutic interventions in tumors: First, it facilitates drug delivery9 and, secondly, it contributes to the formation of hemorrhagic necroses, which are induced by the administration of the soluble form of tumor necrosis factor-α (TNF), both in experimental tumor models and in isolated limb perfusions in patients.10-12
In addition to its ability to induce tumor necrosis, TNF is a potent inflammatory cytokine that is involved in various immunologic and pathophysiologic reactions.13 It is predominantly produced by activated macrophages and lymphocytes as a 26-kd transmembrane protein, which can be proteolytically cleaved to the soluble 17-kd form.14 Several reports describe pathophysiologic changes in transgenic animals in which transmembrane TNF (tmTNF) expression was artificially up-regulated.15 However, little is known about the role of endogenously expressed tmTNF.
This study demonstrates that tmTNF is present in the endothelium of methylcholanthrene (meth A) sarcomas and that the expression of tmTNF in endothelial cells in vitro is linked to an autocrine continuous activation of these cells. We also show that neutralization of TNF ablates vascular permeability, induced by VEGF in endothelial cells, in vitro and in vivo, which suggests that the ability of VEGF to induce vascular permeability is facilitated by endogenously expressed TNF.
Material and methods
Immunohistochemistry
Tumor tissue from mouse bearing meth A sarcoma16was perfusion-fixed with 4% paraformaldehyde and embedded in paraffin. Sections of tumors and other tissues were stained with antibody using indirect immunohistochemistry. Polyclonal antimouse TNF (Genzyme Virotech, Rüsselheim, Germany) antibodies (1:100 diluted serum) were visualized with Cy5-conjugated goat antirabbit antibodies (diluted 1:1000; Dianova, Hamburg, Germany). Cryostat sections from the same type of tumor were fixed with cold acetone and stained with TNF receptor-2–human immunoglobin G (TNFR2-huIgG) fusion protein17 18 (10 μg/mL) and visualized using rabbit antihuman IgG antibody (diluted 1:100; Cappel, Durham, NC), biotinylated goat antirabbit IgG antibody (diluted 1:250; Boehringer, Mannheim, Germany), and fluorescein isothiocynate (FITC)-labeled streptavidin (diluted 1:1000; Zymed, San Francisco, CA). Rat antimouse CD31 (1 μg/mL; Pharmingen, San Diego, CA) was visualized with tetrarhodamine isothiocyanate–conjugated goat antirat IgG (diluted 1:25; Zymed).
Cell culture and transfection of endothelial cells
Human umbilical cord vein endothelial cells (HUVECs) were prepared and cultivated as described previously.19Spontaneously immortalized endothelial cells were characterized by the presence of the VEGF receptor-2 (VEGFR-2) and von Willebrand factor using antibodies and immunofluorescence (data not shown) as described recently.20,21 To stably introduce tmTNF in endothelial cells, immortalized HUVECs (HUEs) were transfected with a noncleavable tmTNF mutant (kindly provided by T. Calarco) huTNF (1-12) in pcDNA3ZEO or with empty vector as control using the SuperFect reagent (Qiagen, Hilden, Germany). Cells were selected for resistance to zeocin, and clones were isolated, transferred into 96-well plates, and analyzed by fluorescence-activated cell sorter (FACS) analysis with the TNF-specific antibody T1.17
Cytofluorometric analysis and acid wash
For FACS analysis, confluent HUVECs were detached with 5 mM ethylenediaminetetraacetic acid in Hank's balanced salt solution in 25 mM HEPES and incubated with primary unconjugated monoclonal antibody (mAb) T1 for 30 minutes at 4°C and 10 μg/mL as final concentration, washed twice with FACS buffer (phosphate-buffered saline [PBS] supplemented with 1% bovine serum albumin [BSA] and 0.1% NaN3), and then incubated with PE-conjugated goat antimouse IgG (Dianova) for 30 minutes at 4°C. Flow cytometry analysis was performed on a FACStar (Becton Dickinson Immunocytochemistry Systems, Mountain View, CA). Isotype-matched control antibodies were used to determine the background staining. To distinguish tmTNF from receptor-bound TNF, cells were incubated for 5 minutes on ice with isotonic solution with the pH adjusted to 2.3 by the addition of hydrochloric acid to glycine (100 mM) buffer.22 Cells treated or untreated were detached, and preparation and flow cytometry was performed as described above.
IL-6 determination
For interleukin (IL)-6 assays, cells were seeded in 96-well microtiter plates overnight. After washing the cells with PBS, they were cultured for an additional 24 hours in the absence or presence of anti-TNF mAb T1 and control IgG, respectively. Supernatants were removed and cleared by centrifugation for 10 minutes at 15 000 rpm. IL-6 concentrations were determined using a commercially available IL-6 enzyme-linked immunosorbent assay (ELISA) kit according the manufacturer's recommendations (Pharmingen).
Tissue factor activity test
Tissue factor activity was assessed as described previously.23 Briefly, clones of immortalized HUVECs, transfected with either tmTNF or control vector, were grown to confluence in 35-mm dishes. Assays were carried out with whole cells obtained in suspension following scraping. Clotting times were measured after addition of human plasma and recalcification. Tissue factor activity equivalents were determined by the use of a standard curve with recombinant tissue factor.
NF-κB and p38 MAP kinase determination
To determine nuclear factor (NF)-κB activity by the luciferase reporter assay, cells were transfected with a minimal promoter containing a 3–NF-κB element-driven firefly luciferase reporter plasmid using the SuperFect reagent. After one day of recovery, the cells were treated as indicated, washed, and harvested in lysis buffer (Promega, Madison, WI); 45-μL aliquots of cell lysates were mixed with 100 μL luciferase assay reagent (Promega), and the luciferase activity was determined using a Lumat LB9501 (Berthold, Wildbad, Germany). For normalization of transfection efficacy, a second, β-galactosidase–encoding cytomegalovirus promoter was used. For analysis of p38 mitogen-activated protein (MAP) kinase phosphorylation cells were treated as indicated, lysed in 120 μL sodium dodecyl sulfate loading buffer, boiled, and electrophoresed under reducing conditions. After transfer onto nitrocellulose membranes, Western blots were performed according the instructions of the manufacturer by the use of specific antibodies capable of recognizing the phosphorylated (pp38) or unphosphorylated (p38) p38 MAP kinase forms (BioLabs, Boston, MA).
Endothelial permeability and proliferation
Hydraulic conductivity by VEGF was determined according to Suttorp et al.24 Briefly, 3H2O was applied together with recombinant human VEGF (rhVEGF; 1 ng/mL, produced as described19 from baculovirus-infected insect cells) to the upper chamber, and the transflux of tracer was determined by continuously sampling from the lower chamber. For experiments with neutralizing antibodies, cells were preincubated for 20 minutes at 37°C prior to the addition of reagents. For determination of endothelial proliferation, HUVECs were seeded in 24-well plates (6000 cells per well) and subsequently cultivated for 3 days with or without VEGF (10 ng/mL) in the presence or absence of antibodies. Cells were then detached by treatment with trypsin, and cell numbers were counted in a Casy cell counter (Schärfe-System, Reutlingen, Germany).
Modified Miles assay
Miles assay was modified according to Senger et al2: Briefly, mice were injected intravenously with 2.5 mg FITC-labeled BSA (Sigma, Munich, Germany) in 100 μL PBS. After 5 minutes, mice were anesthetized and VEGF (0.2 μg in 20 μL PBS) or solvent (PBS) was injected into the left or right ear, respectively. Six minutes later, the animals were killed, and the extravasated fluorescent dye was measured using a fluorescence photometer. In some experiments mice were pretreated for 30 minutes (prior to VEGF administration) intraperitoneally with polyclonal anti-TNF serum (100 μL; Genzyme), the TNF-α proteinase inhibitor (TAPI; 500 μg per animal), the stress-activated protein kinase-2 (SAPK-2)/p38 inhibitor SB 203580 (10 μg per mouse; Upstate Biotechnology, Lake Placid, NY), or solvent (PBS or dimethyl sulfoxide). Alternatively, Evans blue dye (1.2 mg in 100 μL PBS) was used instead of FITC-labeled BSA, and extravasation of the dye was documented by photography.
Results
TNF is up-regulated in the endothelial lining of tumor blood vessels
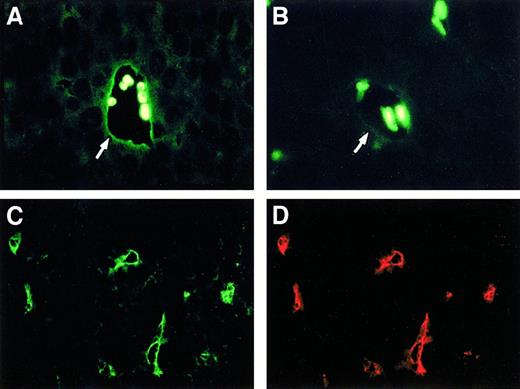
Based on the reasoning that endothelial cells produce TNF following stimulation25 and that the tumor vasculature is in a preactivated state,26 we analyzed sections from transplanted meth A sarcomas for endogenous TNF expression. We found strong labeling for TNF in the endothelial lining of the tumor vasculature using either anti-TNF antibodies (Figure1A, arrow) or a TNFR2-IgG fusion protein (Figure 1C). Control antibodies and an unrelated IgG fusion protein did not stain the tumor vasculature (data not shown). Specific staining of the endothelium was demonstrated by costaining with an anti–platelet endothelial cell adhesion molecule antibody (Figure 1D). Normal dermal vasculature, distal from the tumor, also reacted weakly with TNF-specific antibodies (Figure 1B, arrow), whereas vessels from other organs (lung and liver) showed no detectable staining (data not shown). Of note, the TNFR2-IgG fusion protein recognizes the transmembrane form of TNF (tmTNF) but not receptor-bound soluble TNF.17
Immunohistologic analysis of endothelial TNF expression.
(A) TNF staining on the endothelial lining (arrow) of the tumor-associated vasculature of transplanted meth A sarcomas. A strong autofluorescent reaction of some trapped red blood cells is also visible. (B) Weak staining of blood vessels (arrow) for TNF can be observed in paraffin-embedded dermal tissue distant from the tumor. (C,D) Double staining of tmTNF and CD31 as an endothelial cell marker in frozen sections of transplanted meth A sarcomas using a TNFR2-IgG fusion protein (C) and a rat antimouse CD31-specific mAb (D).
Immunohistologic analysis of endothelial TNF expression.
(A) TNF staining on the endothelial lining (arrow) of the tumor-associated vasculature of transplanted meth A sarcomas. A strong autofluorescent reaction of some trapped red blood cells is also visible. (B) Weak staining of blood vessels (arrow) for TNF can be observed in paraffin-embedded dermal tissue distant from the tumor. (C,D) Double staining of tmTNF and CD31 as an endothelial cell marker in frozen sections of transplanted meth A sarcomas using a TNFR2-IgG fusion protein (C) and a rat antimouse CD31-specific mAb (D).
Human endothelial cells can express the transmembrane form of TNF leading to activation
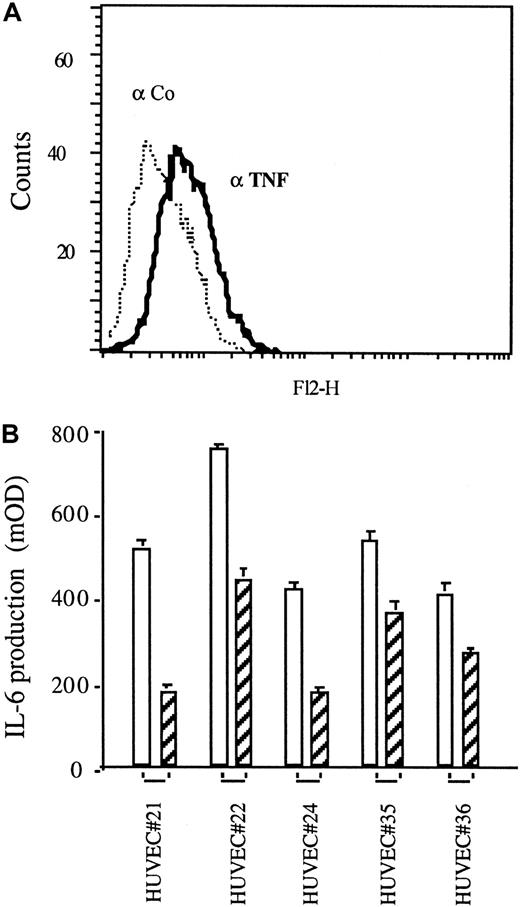
Because tmTNF is strongly up-regulated on the tumor vasculature but also expressed on normal dermal vasculature, we asked whether TNF is expressed and active on cultivated endothelial cells. We found a low constitutive expression of TNF on the surface of some, but not all, preparations of endothelial cells derived from HUVECs, as demonstrated by FACS analysis (Figure 2A) using a specific anti-TNF mAb T1. This observation was also made with human microvascular and human aortic endothelial cells (data not shown). In several preparations of HUVECs (6 of 17) we found a low constitutive TNF-dependent production of IL-6, as demonstrated using TNF-specific neutralizing antibodies (Figure 2B, hatched bars). This finding indicates a constitutive TNF-dependent autocrine stimulation of these cells, similar to what we have found in TNF-transfected cell lines.27
Autocrine activation of TNF-expressing HUVECs.
(A) TNF expression of HUVECs revealed by FACS analysis using TNF-specific mAb T1 (αTNF, solid line) versus an isotype-matched control antibody (Co, dotted line). (B) IL-6 production in the supernatants of HUVECs as determined by ELISA in the absence (empty bars) or presence (hatched bars) of the TNF-specific mAb T1 (30 μg/mL each). Shown are 5 examples of IL-6–expressing HUVECs generated from different cord donors (No. 21-36).
Autocrine activation of TNF-expressing HUVECs.
(A) TNF expression of HUVECs revealed by FACS analysis using TNF-specific mAb T1 (αTNF, solid line) versus an isotype-matched control antibody (Co, dotted line). (B) IL-6 production in the supernatants of HUVECs as determined by ELISA in the absence (empty bars) or presence (hatched bars) of the TNF-specific mAb T1 (30 μg/mL each). Shown are 5 examples of IL-6–expressing HUVECs generated from different cord donors (No. 21-36).
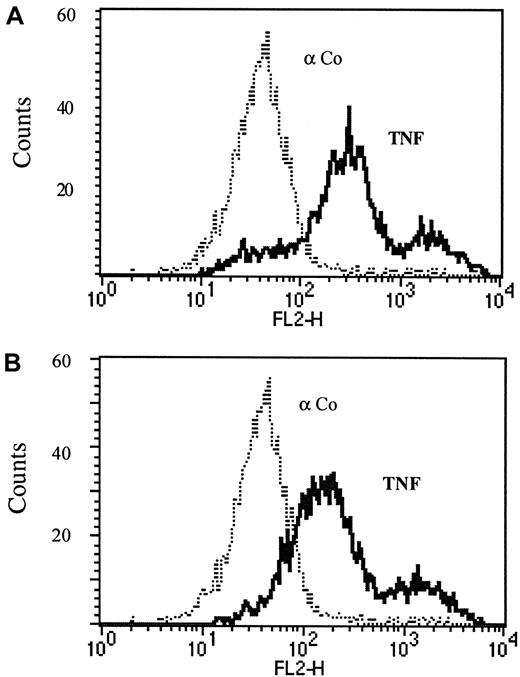
The observed endothelial TNF expression in vitro apparently comprises tmTNF because the T1 antibody does not recognize soluble TNF receptor–bound TNF.17 Furthermore, no soluble TNF was detectable in the supernatants of VEGF-stimulated HUVECs when assessed either by the use of an ELISA (detection limit 10 pg/mL; R&D Systems, Minneapolis, MN) or a standard cytotoxicity assay using L929 cells (detection limit 1 pg/mL 28). In addition, we performed acid washes of endothelial cells to confirm that the antigen detected is not soluble TNF bound to the receptors. After acid wash, a method described to remove membrane- and receptor-bound soluble proteins,22 the antibody could still detect TNF on the surface of endothelial cells, indicating that this TNF is membrane-integrated and not simply attached to the cells (Figure3A,B).
Endothelial surface TNF is in the transmembrane form.
Cytofluorimetric analysis of immortalized HUVECs without (A) or with (B) acid wash was performed as described in the text. FACS analysis was performed using TNF-specific mAb T1 (αTNF, solid line) versus an isotype-matched control antibody (Co, dotted line).
Endothelial surface TNF is in the transmembrane form.
Cytofluorimetric analysis of immortalized HUVECs without (A) or with (B) acid wash was performed as described in the text. FACS analysis was performed using TNF-specific mAb T1 (αTNF, solid line) versus an isotype-matched control antibody (Co, dotted line).
TNF is required for VEGF-induced permeability of endothelial cell monolayers but not for VEGF-induced endothelial cell proliferation
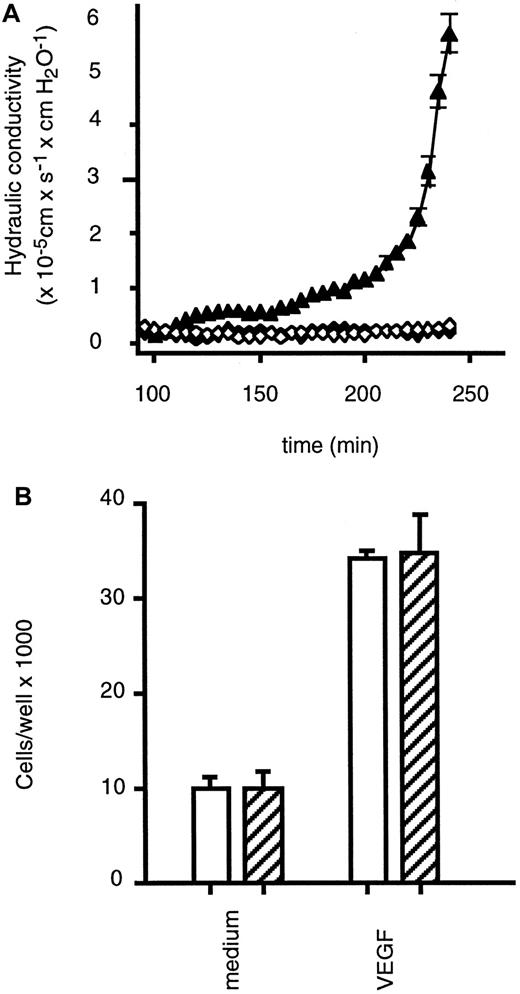
In light of the herein provided evidence for constitutive endothelial preactivation by endogenous tmTNF and the previously demonstrated synergistic interaction between soluble TNF and VEGF,29 we assessed whether endothelial TNF is involved in VEGF-induced permeability and proliferation of HUVECs. A neutralizing antibody against TNF completely inhibited the VEGF-induced increase in permeability of an endothelial monolayer (Figure4A) as determined by measuring the hydraulic conductivity of HUVEC monolayers grown on polycarbonate filter membranes.30 We did not find a change of the constitutive, low expression of tmTNF on HUVECs upon VEGF treatment in the course of the experiment. Furthermore, HUVECs stimulated with VEGF in the presence of neutralizing TNF-specific antibodies have not become refractory to the induction of permeability, because addition ofStaphylococcus aureus α toxin, a well-established permeability-increasing agent,24 resulted in a rapid increase in the water filtration rate (data not shown).
Effects of TNF-neutralizing antibodies on VEGF-induced endothelial cell permeability and proliferation.
(A) HUVECs were preincubated in the presence (closed diamonds) or absence (closed triangles) of the neutralizing TNF-specific mAb T1 (10 μg/mL) prior to treatment with VEGF (1 ng/mL) or left untreated (open diamonds), and hydraulic conductivity was determined as described in the text. (B) HUVECs were cultivated for 3 days with or without VEGF (10 ng/mL) in the presence (hatched bars) or absence (empty bars) of the neutralizing TNF-specific antibody T1 (30 μg/mL), and cells were counted. Data are presented as means (± SD) of 3 separate experiments.
Effects of TNF-neutralizing antibodies on VEGF-induced endothelial cell permeability and proliferation.
(A) HUVECs were preincubated in the presence (closed diamonds) or absence (closed triangles) of the neutralizing TNF-specific mAb T1 (10 μg/mL) prior to treatment with VEGF (1 ng/mL) or left untreated (open diamonds), and hydraulic conductivity was determined as described in the text. (B) HUVECs were cultivated for 3 days with or without VEGF (10 ng/mL) in the presence (hatched bars) or absence (empty bars) of the neutralizing TNF-specific antibody T1 (30 μg/mL), and cells were counted. Data are presented as means (± SD) of 3 separate experiments.
In contrast to the effects on endothelial permeability, the neutralizing antibodies against TNF did not affect the stimulation of endothelial proliferation by VEGF (Figure 4B). This indicates that in endothelial cells, VEGF-mediated permeability, but not VEGF-mediated proliferation, is dependent on the permissive role of TNF.
TNF is permissive for VEGF-induced vascular permeability in vivo
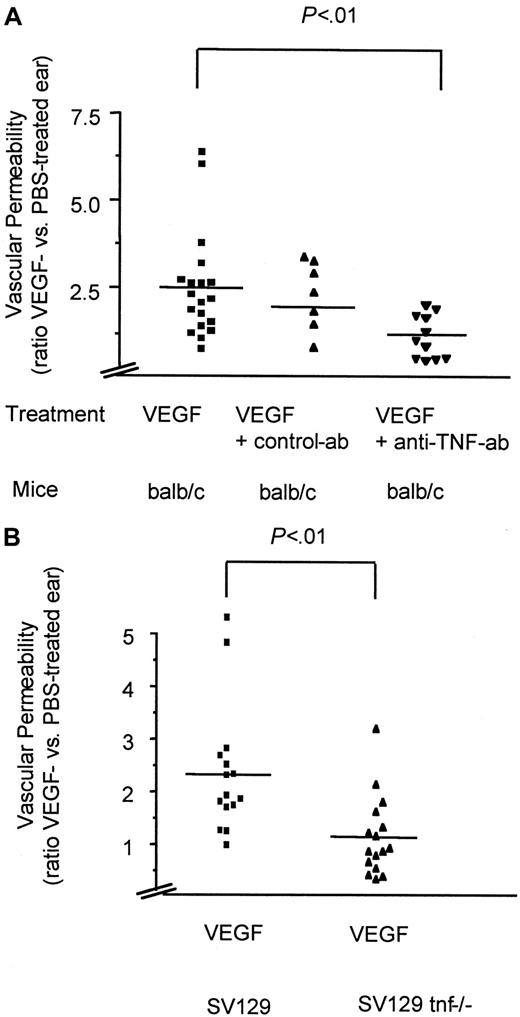
To examine whether VEGF-induced endothelial permeability in vivo depends on the activity of TNF, we used a modified Miles assay of vascular permeability.2 In this assay an intravenous injection of FITC-conjugated albumin is followed by an intradermal injection of 0.2 μg VEGF into the ear. After 6 minutes the extravasation of the fluorophore is quantitatively assessed by dye extraction and measurement in a fluorescence reader. The ratios between the values derived from the VEGF- versus the solvent- (PBS) treated ears correspond to the permeability effect of VEGF. Pretreatment with TNF-neutralizing antibodies blocked the ability of VEGF to induce permeability (Figure 5A), whereas control antibodies had no effect (Figure 5A). Intriguingly, TNF-deficient mice31 also showed a significant defect in the ability to respond to the permeability-increasing activity of VEGF in comparison to wild-type mice of the same strain (Figure 5B). These results were also directly illustrated using intravenous injection of Evans blue instead of FITC-albumin prior to VEGF treatment of TNF-deficient versus wild-type mice (Figure 6A,B).
TNF is essential for VEGF-induced vascular permeability in vivo.
VEGF-induced vascular permeability in BALB/c mice with or without pretreatment with anti-TNF antibodies (A) in TNF gene–deficient (tnf−/−) and the corresponding wild-type mice (SV129) (B). Shown are scatter diagrams with each symbol representing the ratio between the VEGF- and PBS-treated ear of one tested animal. Depicted are the mean (line) and the significance (P values) as calculated from an unpaired t test using a commercial program (InStat 2.01, GraphPad, San Diego, CA).
TNF is essential for VEGF-induced vascular permeability in vivo.
VEGF-induced vascular permeability in BALB/c mice with or without pretreatment with anti-TNF antibodies (A) in TNF gene–deficient (tnf−/−) and the corresponding wild-type mice (SV129) (B). Shown are scatter diagrams with each symbol representing the ratio between the VEGF- and PBS-treated ear of one tested animal. Depicted are the mean (line) and the significance (P values) as calculated from an unpaired t test using a commercial program (InStat 2.01, GraphPad, San Diego, CA).
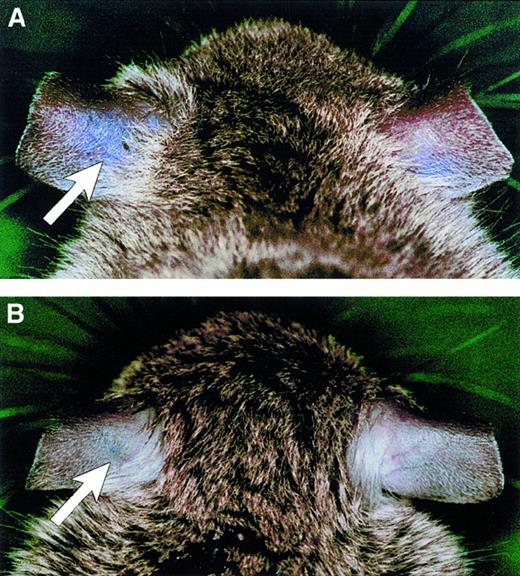
Ablation of vascular permeability in TNF gene–deficient (tnf−/−) mice.
VEGF-induced vascular permeability was determined in wild-type (A) and TNF-deficient mice (B) after intravenous injection of Evans blue and subsequent dermal injection of VEGF into the left ear (arrows) and of PBS into the right ear.
Ablation of vascular permeability in TNF gene–deficient (tnf−/−) mice.
VEGF-induced vascular permeability was determined in wild-type (A) and TNF-deficient mice (B) after intravenous injection of Evans blue and subsequent dermal injection of VEGF into the left ear (arrows) and of PBS into the right ear.
TAPI, an inhibitor of TNF-release, does not abolish VEGF-induced vascular permeability in vivo
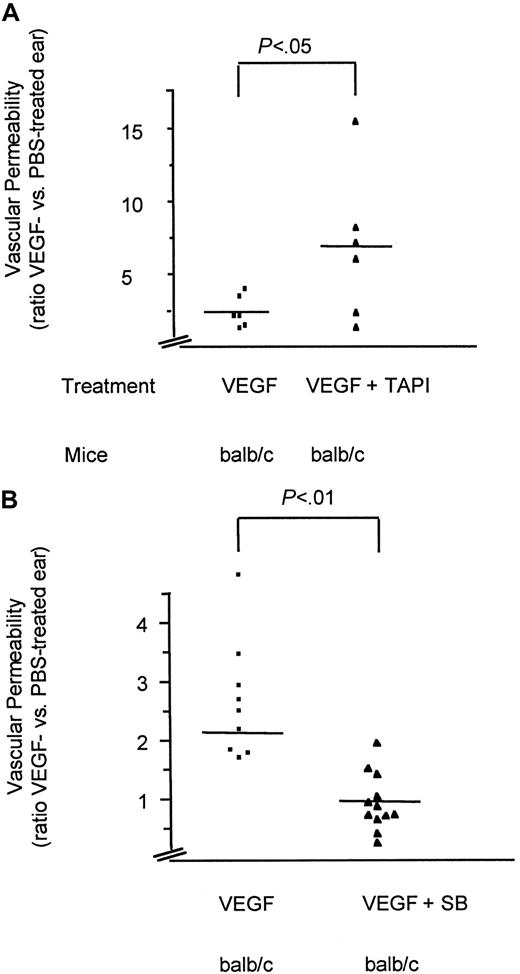
To test the possibility that VEGF-induced permeability in vivo is mediated by the generation of soluble TNF, we applied TAPI, a hydroxamic acid–based inhibitor of the TNF-converting enzyme.32 Pretreatment of the animals with this inhibitor at concentrations that have been shown to prevent release of TNF in vivo32 did not reduce but rather increased the effect of VEGF on vascular permeability (Figure7A). These data indicate that the generation of the soluble form of TNF is not essential for this VEGF effect but that the transmembrane form of TNF has an obligatory role in VEGF-induced vascular permeability in vivo.
Analysis of VEGF-induced vascular permeability in vivo.
VEGF-induced vascular permeability in BALB/c mice pretreated (30 minutes) with TAPI or solvent (A) or the SAPK-2/p38 specific inhibitor SB203580 (VEGF plus SB) or solvent (VEGF) (B). Shown are scatter diagrams with each symbol representing the ratio between the VEGF- and PBS-treated ear of one tested animal. Depicted are the mean (line) and the significance (P values) as calculated from an unpaired t test using a commercial program (InStat 2.01). Note the different y-axis scales. Although variations between the values of individual animals were observed, probably due to injuries of small capillaries during injections, the differences between the individual groups were highly significant.
Analysis of VEGF-induced vascular permeability in vivo.
VEGF-induced vascular permeability in BALB/c mice pretreated (30 minutes) with TAPI or solvent (A) or the SAPK-2/p38 specific inhibitor SB203580 (VEGF plus SB) or solvent (VEGF) (B). Shown are scatter diagrams with each symbol representing the ratio between the VEGF- and PBS-treated ear of one tested animal. Depicted are the mean (line) and the significance (P values) as calculated from an unpaired t test using a commercial program (InStat 2.01). Note the different y-axis scales. Although variations between the values of individual animals were observed, probably due to injuries of small capillaries during injections, the differences between the individual groups were highly significant.
A specific inhibitor of SAPK-2/p38 activation inhibits VEGF-induced vascular permeability
Our in vitro data demonstrated that tmTNF expression in endothelial cells leads to continuous activation as assessed by IL-6. Because the IL-6 induction is NF-κB– and SAPK-2/p38–dependent,27 we have investigated the possibility that activated SAPK-2/p38 is required for the increase of permeability by VEGF. Therefore, we pretreated mice with an inhibitor of SAPK-2/p38 prior to assessment in the Miles assay. As shown in Figure 7B, inhibition of SAPK-2/p38 abolished VEGF-mediated increase in vascular permeability to the same extent as did neutralization of TNF or the use of tnf−/− animals.
The transmembrane form of TNF causes continuous activation of intracellular signaling in endothelial cells
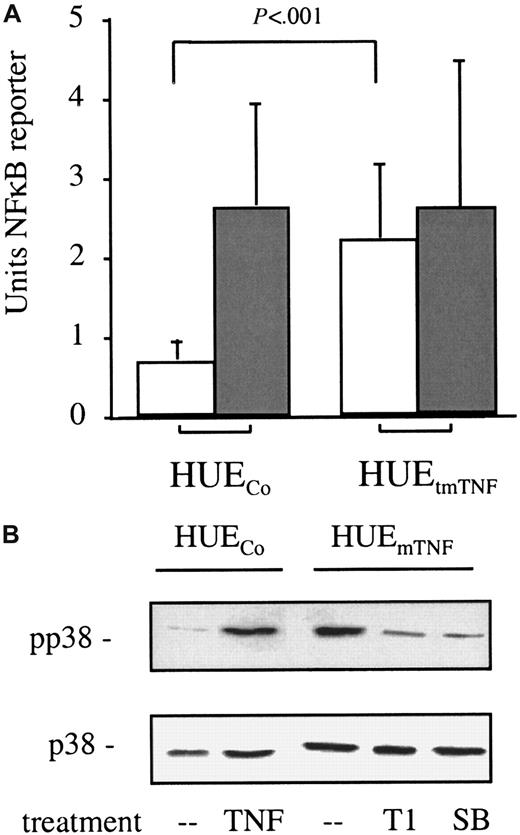
Our observations that endothelial cells in vitro can secrete IL-6 and that this production can be reduced by pretreatment with anti-TNF antibody suggests that endothelial transmembrane expression leads to continuous activation of signaling molecules such as SAPK-2/p38 and thus primes the endothelium to respond to VEGF as a VPF in vivo. To test this hypothesis, we analyzed the principal capacity of endothelial tmTNF to cause continuous activation of intracellular signaling molecules. Because activation of NF-κB and SAPK-2/p38—2 signaling molecules that mediate TNF-induced IL-6 production in HeLa cells27—was difficult to assess in primary HUVECs because of their given heterogeneity, we employed spontaneously immortalized HUVECs (HUEs) and introduced an uncleavable mutant of tmTNF in these cells (HUEtmTNF). Testing several clones of these cells for NF-κB activation, we found a continuous activation of NF-κB in tmTNF-overexpressing but not in control HUE clones, as shown by transient reporter gene assays (Figure 8, empty bars). In addition, control clones but not the tmTNF-expressing clones could be further stimulated by application of soluble TNF (Figure 8A, shaded bars). Furthermore, Western blot analysis revealed that in tmTNF-overexpressing clones SAPK-2/p38 is activated to an extent comparable to soluble TNF-treated control clones (Figure 8). This activation was blocked by preincubation of the tmTNF-expressing cells with the neutralizing anti-TNF antibody T1 or the specific SAPK-2/p38 inhibitor SB203580 (Figure 8B). Together, these data demonstrate that endogenous overexpression of tmTNF continuously activates endothelial cells.
Constitutive NF-κB and SAPK-2/p38 activation by immortalized HUVECs expressing tmTNF.
(A) Constitutive NF-κB activation in immortalized HUVECs expressing tmTNF (HUEtmTNF) but not in control transfected cells (HUECo) as assessed by NF-κB reporter assays (empty bars). The effect of soluble TNF (10 ng/mL) on NF-κB activation is also depicted (shaded bars). Shown are the means and the SD of a minimum of 3 independent experiments (with 5 clones in case of the HUEtmTNF and 4 clones in case of the HUECo). (B) Continuous SAPK-2/p38 activation in tmTNF-expressing HUE cells as determined by Western blot analysis. The effects of pretreatment (6 hours) with either 30 μg/mL TNF-specific mAb T1 (T1) or the SAPK-2/p38–specific inhibitor SB203580 (SB) on HUEtmTNFcells and of 10 ng/mL soluble TNF (20 minutes) on HUECocells on phosphorylation (pp38, upper panel) and on protein expression (p38, lower panel) of SAPK-2/p38 using the appropriate antibodies (as described in the text) are shown.
Constitutive NF-κB and SAPK-2/p38 activation by immortalized HUVECs expressing tmTNF.
(A) Constitutive NF-κB activation in immortalized HUVECs expressing tmTNF (HUEtmTNF) but not in control transfected cells (HUECo) as assessed by NF-κB reporter assays (empty bars). The effect of soluble TNF (10 ng/mL) on NF-κB activation is also depicted (shaded bars). Shown are the means and the SD of a minimum of 3 independent experiments (with 5 clones in case of the HUEtmTNF and 4 clones in case of the HUECo). (B) Continuous SAPK-2/p38 activation in tmTNF-expressing HUE cells as determined by Western blot analysis. The effects of pretreatment (6 hours) with either 30 μg/mL TNF-specific mAb T1 (T1) or the SAPK-2/p38–specific inhibitor SB203580 (SB) on HUEtmTNFcells and of 10 ng/mL soluble TNF (20 minutes) on HUECocells on phosphorylation (pp38, upper panel) and on protein expression (p38, lower panel) of SAPK-2/p38 using the appropriate antibodies (as described in the text) are shown.
The transmembrane form of TNF causes increased production of tissue factor
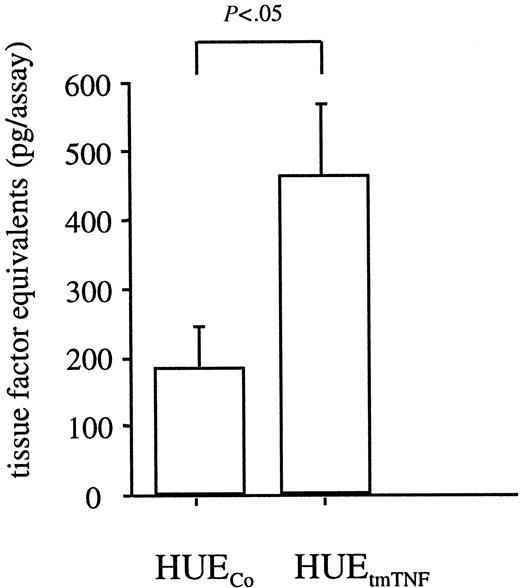
Next, we wanted to know whether the continuous activation of signaling molecules in tmTNF-overexpressing cells also causes increased production of TNF-inducible genes. Because the immortalized HUVECs have lost the ability to synthesize IL-6, we assessed tissue factor, which is also induced by TNF in endothelial cells.33 We found increased tissue factor production in TNF-transfected clones versus control-transfected clones (Figure 9), suggesting that tmTNF overexpression in endothelial cells can account for an activated and procoagulant state.
Constitutive tissue factor production in tmTNF-expressing endothelial cells.
Tissue factor production in immortalized HUVECs expressing tmTNF (HUEtmTNF) versus control transfected cells (HUECo) was assessed as described in the text. Shown are the means and the SD of a minimum of 3 independent experiments (with at least 5 clones in case of the HUEtmTNF and 3 clones in case of the HUECo).
Constitutive tissue factor production in tmTNF-expressing endothelial cells.
Tissue factor production in immortalized HUVECs expressing tmTNF (HUEtmTNF) versus control transfected cells (HUECo) was assessed as described in the text. Shown are the means and the SD of a minimum of 3 independent experiments (with at least 5 clones in case of the HUEtmTNF and 3 clones in case of the HUECo).
Discussion
This study demonstrates a novel principle for a permissive cytokine crosstalk because the presence of endothelial tmTNF evokes a cellular response by cooperating with a second, unrelated soluble cytokine, VEGF. Our data support the hypothesis that TNF preactivates endothelial cells to a “VEGF responsive status” by continuous signaling, leading to constitutive and continuous SAPK-2/p38 and NF-κB activation (Figure 8A,B). Furthermore, our finding that inhibition of the SAPK-2/p38 ablated a VEGF-mediated increase in vascular permeability—to the same extent as did neutralizing of TNF or the use of tnf−/− animals—suggests that also in endothelial cells in vivo there is continuous SAPK-2/p38 activation and that this activation is a prerequisite for VEGF to act as a VPF.
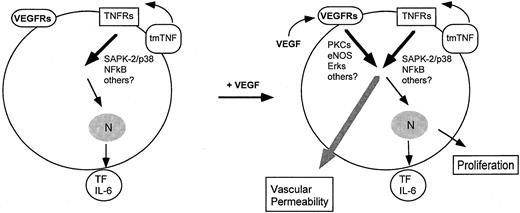
Although our observation of continuous SAPK-2/p38 activation is derived from TNF overexpression studies in vitro, it suggests a plausible mechanism to explain the TNF-dependent IL-6 production34in endothelial cells in vitro and the VEGF-induced permeability in vitro and in vivo. Because we have demonstrated that in vivo extremely short pretreatment (30 minutes) with either SAPK-2/p38 inhibitor (SB203580) or TNF antibodies is sufficient to abolish induction of vascular permeability in response to a further short treatment (6 minutes) with VEGF, a direct cytosolic effect is more likely to be involved than transcriptional activity. In this context, SAPK-2/p38 activation has been described to induce transcription-independent effects such as the induction of actin reorganization and cellular motility.35 However, additional signaling pathways may be involved in VEGF-induced permeability changes. For example, other TNF signaling molecules such as SAPK-1/JNK have not been analyzed in this context. Further, an essential role of nitric oxide (NO) signaling for VEGF-mediated vascular permeability, both in vitro and in vivo, is well documented.36-39 We suppose here that VEGF-induced NO formation, leading to further downstream signaling events such as the activation of extracellular signal–regulated kinase 1/2 (ERK1/2), cooperates with continuous SAPK-2/p38 and NF-κB signaling in TNF-expressing endothelium to initiate cellular events leading to hyperpermeability (Figure 10).
Convergence of continuous TNF and VEGF signaling pathways leading to vascular permeability.
Shown are candidate pathways for tmTNF signaling (as shown in this study) and for VEGF signaling (as described in the text) leading to vascular permeability.
Convergence of continuous TNF and VEGF signaling pathways leading to vascular permeability.
Shown are candidate pathways for tmTNF signaling (as shown in this study) and for VEGF signaling (as described in the text) leading to vascular permeability.
Our study is the first to demonstrate that the induction of endothelial permeability, an important (patho)physiologic process attributed to VEGF, essentially relies on the function of TNF. Although administration of soluble TNF by itself can induce endothelial permeability in vivo, this occurs with slower kinetics and is dependent on the recruitment of leukocytes.40 It has been suggested that the VEGF-induced formation of VVOs and fenestration in the endothelium contribute to the rapid induction of VEGF-mediated vascular permeability in vivo.8 In vitro, we have shown recently that VEGF-mediated endothelial permeability occurs with a delay (150 minutes) as compared with the response in vivo, although the same cells were able to secrete P-selectin within 15 minutes.30 This different kinetic of in vivo and in vitro permeability induction can be explained by the delay in VVO formation observed in vitro41 (unpublished observation, 1998). Again, the molecular mechanisms of VEGF- and TNF-induced permeability seem to be different. Soluble TNF had no consistent effect on endothelial permeability within the time frame studied (maximum 3 hours) in our test system. This may be due to the fact that TNF decreases endothelial monolayer barrier function by rearranging the cytoskeleton and forming intracellular gaps,42 whereas VEGF-induced permeability was observed in the absence of gap formation.30
The delineation of the molecular mechanisms leading to vascular permeability in vivo is difficult. For example, stimulus-dependent changes in the endothelium surface as well as flow effects are confounding parameters that cannot to be separated from barrier function in the Miles assay.43 The principal ability of VEGF to induce vascular permeability, however, has been demonstrated in a more sophisticated system using isolated perfused microvessels.44 In fact, flow changes appear to be important because, in a rabbit skin model of vascular permeability, VEGF would only increase plasma leakage when prostaglandin E2 was coapplied.45 Although we cannot totally exclude the possibility that the role of TNF in vivo as measured by the Miles assay may also imply additional parameters, this assay was a helpful tool to establish the role of several mediators and modulators in VEGF-induced permeability. These mediators include the known vasoactive substances prostacyclin and NO,46 but also family members of Src kinases because mice deficient in pp60c-src or pp62c-yes displayed refractory to VEGF-induced vascular permeability.47 Most recently, angiopoietin-1 emerged as a molecule that, when overexpressed in vivo, protects the vasculature against plasma leakage induced by VEGF.48 49In conclusion, our data, demonstrating an essential role for endothelial TNF expression and continuous SAPK-2/p38 activation in vascular permeability evoked by VEGF, provide the basis for further studies in the light of the findings listed above.
Our suggestion that vascular permeability is caused by 2 pathways, one dependent on the rapid induction of NO formation and the other one on constitutive SAPK-2/p38 activation, may help to identify mechanisms that dissect VEGF activities leading to angiogenesis from those initiating vascular hyperpermeability. NO formation has been demonstrated to be essential for VEGF-mediated angiogenesis in vivo. In vitro, NO-dependent ERK1/2 phosphorylation has been delineated as an essential element in VEGF-induced endothelial proliferation.38,50-54 In contrast, SAPK-2/p38 activation causes inhibition rather than stimulation of endothelial proliferation.55 Furthermore, our own results demonstrate that only VEGF-induced permeability, but not increased endothelial cell proliferation, essentially depends on TNF (Figure 4B). The notion that TNF-dependent signaling events, such as permanent SAPK-2/p38 activation, are permissive for VEGF-induced vascular permeability but not for angiogenesis is in line with results from mice that lack the genes for VEGF or TNF. Mice heterozygous for vegf gene deficiency suffer from a lethal impairment of embryonic vessel formation and capillary sprouting, whereas homozygous tnfgene–deficient mice develop normally with no obvious vascular afflictions, indicating that physiologic angiogenesis is independent of TNF.31 56
Our finding that in unstimulated HUVECs low levels of TNF surface protein are expressed is supported by mRNA expression data from other groups,57,58 which demonstrate low endothelial TNF expression in comparison to induced TNF expression in stimulated cells. Furthermore, in vivo, additional evidence for up-regulated endothelial TNF expression in pathologic situations such as atheroma59and multiple sclerosis60,61 was provided. Unstimulated HUVECs express the tmTNF as demonstrated by Western blot analysis62 and by ELISA of membrane fractions.63 Until now, it was unclear whether these observations were of any physiologic significance. This study provides several lines of evidence for the hypothesis that the transmembrane form of TNF rather than soluble TNF mediates the VEGF-initiated induction of permeability in endothelial cells. For instance, expression of the transmembrane form of TNF can be demonstrated in endothelial cells both in vitro (Figure 2A) and in vivo (Figure 1). The reagents, which we used to detect TNF expression (TNF receptor–IgG fusion protein and human TNF-specific mAb T1) do not interact with soluble TNF when bound to cellular TNF receptors.17 In addition, no soluble TNF was detectable in the supernatants of VEGF-stimulated and unstimulated HUVECs by the use of immunologic or functional assays. Finally, pretreatment of the animals with the inhibitor of the TNF-converting enzyme, TAPI, did not reduce the effect of VEGF on vascular permeability (Figure 7). Although this inhibitor is not specific for the cleavage of TNF in vitro and in vivo,14 it was shown to block the effects of soluble TNF in the model of lethal endotoxemia in D-galactosamine–sensitized animals.32 The finding that the inhibition of TNF cleavage can lead to an accumulation of tmTNF molecules on the cell membrane32 would be in line with our observation that TAPI pretreatment did not reduce but rather increased the VEGF-induced vascular permeability.
Based on our findings that TNF is up-regulated in the endothelium of the meth A sarcoma and that VEGF-induced permeability in vivo can be inhibited by treatment with anti-TNF antibodies, we hypothesize that hyperpermeability of tumor vessels is dependent on the coordinate overexpression of endothelial tmTNF and tumor-secreted soluble VEGF (Figure 10). The permissive function of tmTNF for VEGF-induced permeability but not for VEGF-mediated angiogenesis is of potential significance for the treatment of solid tumors, perhaps by combining antiangiogenesis and chemotherapy. Such therapy would require conditions in which angiogenesis is reduced, but vascular permeability is maintained or even increased, to achieve optimal drug delivery. Therefore, our conclusion that only the VEGF-induced signaling, which causes permeability but not proliferation, is dependent on TNF provides the basis for the development of novel drugs, which either can increase selectivity of antiangiogenic processes or can enhance the local delivery of cytostatic drugs to the tumor. Such drugs may be developed based on the herein reported evidence that endogenous TNF-mediated signaling is essential for VEGF-mediated permeability but not for angiogenesis. Alternatively, therapeutic angiogenesis with VEGF, which is limited by VEGF-induced vascular permeability,64 should benefit from the application of either anti-TNF antibodies or SAPK-2/p38 inhibitors.
We are grateful to R. Black (Immunex, Seattle, WA) for a generous gift of TAPI, D. Moosmayer for TNF receptor–IgG fusion protein, and T. Calarco (Chiron, Emeryville, CA) for providing us with the clone for uncleavable human TNF. We greatly appreciate the excellent technical assistance of C. Fangmann, S. Merfeld, G. Zimmermann, and E. Richards. We would also like to thank C. Mitchell for critically reading the manuscript and giving valuable suggestions.
Supported by the European Community (Biomed 2: BMH4-CT98-3277 to M.C.), the Deutsche Forschungsgemeinschaft (SFB 547/B2 to N.S., 547/C5 to M.C. and W.R.), and the Engelhorn Stiftung (fellowship to M.G.).
M.G. and W.R. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Matthias Clauss, Max-Planck-Institute, Park Str 1, 61231 Bad Nauheim, FRG; e-mail: m.clauss@kerckhoff.mpg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal