Allogeneic blood transfusion results in the infusion into the recipient of large amounts of foreign antigens in both soluble and cell-associated forms. The persistence of these antigens in the circulation of the recipient may create conditions that allow the development of immune down-regulation. Evidence from a variety of sources indicates that allogeneic blood transfusion enhances the survival of renal allografts1 and may increase the recurrence rate of resected malignancies2and the incidence of postoperative bacterial infections,3-7 as well as reduce the recurrence rate of Crohn disease8 and/or activate infections with cytomegalovirus9 or human immunodeficiency virus.10 This clinical syndrome, the mechanisms of which remain to be defined, has been referred to in the transfusion medicine literature as allogeneic blood transfusion–associated immunomodulation (TRIM).11
Clinical evidence for the existence of TRIM has been available since 1973. In their seminal study, Opelz et al1 provided evidence, counterintuitive at the time, that recipients of allogeneic blood transfusion had improved renal allograft survival. Subsequent clinical studies and studies in experimental animals corroborated the results of Opelz et al,1 and allogeneic blood transfusions were used deliberately in the early 1980s to prevent rejection of renal allografts.12 The beneficial effect of TRIM was obscured following the introduction of immunosuppression with cyclosporine, but it was recently reported to persist even in renal allograft recipients receiving modern immunosuppressive therapy.13
On the basis of the immunomodulatory effect of allogeneic blood transfusion in renal allograft recipients, Gantt14 raised the question in 1981 whether the TRIM effect might also be associated with an increased risk of cancer recurrence in patients undergoing resection of a malignancy. Gantt's hypothesis was based on the premise that, if allogeneic blood transfusion down-regulated the host's immune surveillance mechanism that targets malignant cells, the receipt of allogeneic blood transfusion could enhance tumor growth. A subsequent hypothesis proposed that, if allogeneic blood transfusion causes immunosuppression, then recipients of perioperative allogeneic blood transfusion could be at increased risk for postoperative bacterial infection.
Since 1981, more than 150 clinical studies have examined the association of perioperative allogeneic blood transfusion with cancer recurrence and/or postoperative bacterial infection. Most of these are observational cohort studies comparing patients who had or did not have transfusion.15-20 In addition, 7 randomized controlled trials (RCTs) have compared the risk of cancer recurrence2,21,22 and/or postoperative infection3-7,21-23 between a treatment arm receiving standard7 or buffy-coat–reduced red blood cells (RBCs)2,4-6,21,22 or whole blood3 and a control arm receiving autologous or white blood cell (WBC)-reduced RBCs or whole blood.24,25 These studies are based on the assumption that the transfusion of autologous2,4,21,23 or WBC-reduced3,5-7,22 RBCs, or whole blood, is immunologically neutral. Both the earlier observational cohort studies and the recent RCTs have produced contradictory findings, and—because of the discrepancies among the published studies—the long-standing hypothesis of the potentially deleterious immunomodulatory effect of perioperative allogeneic blood transfusion remains unresolved.11,26 27
The mechanism(s) of the TRIM effect(s) also remains elusive, and it is possible that a large number of biologic mechanisms may underlie the effect(s).28-31 The infusion of foreign antigen in either a soluble31-36 or cell-associated37-43 form has been shown to induce immune suppression, anergy, and clonal deletion in studies in experimental animals. However, most studies evaluating proposed mechanisms have been done in rodents, and these findings may not be applicable to the human immune system.44 Caution should be exercised, therefore, when findings from experimental animals are extrapolated to humans. Moreover, different biologic mechanisms may be involved in each reported clinical manifestation of TRIM,1-10 and the clinical evidence supporting each of the aforementioned hypotheses1-10 should be examined on its own merits.
The specific constituent(s) of allogeneic blood that mediates the TRIM effect1-10 remains unclear. Allogeneic plasma,31-36 allogeneic WBCs,30,37-43 and substances that accumulate in blood components during storage39 have been implicated in the pathogenesis of TRIM. However, both the animal and human data suggest that the TRIM effects are most likely mediated by transfused allogeneic WBCs.45,46 Lee et al47 reported persistence of donor WBCs in humans for up to 1.5 years after an allogeneic blood transfusion. In murine and rabbit experimental models, Blajchman et al37,38 and Bordin et al39 demonstrated a tumor growth–promoting effect of allogeneic blood transfusion that appeared to be associated with transfusion of allogeneic WBCs. The findings of these experiments support the hypothesis that allogeneic WBCs actively induce immune suppression in transfusion recipients. In another relevant study, Kao40 induced immune suppression in mice receiving donor WBCs free of plasma and platelets. These and several other investigators41,42 also attributed an induction of TH2 cells in transfusion recipients to the allogeneic donor WBCs, showing that TH2 cells can produce immunosuppression in transfusion recipients by down-regulating the activity of TH1 cells. Mincheff et al43 implicated the antigen-presenting cells of the allogeneic donor in the induction of a state of anergy in the recipient and proposed that, during refrigeration, antigen-presenting cells lose their ability to deliver costimulation. These investigators hypothesized that after an allogeneic transfusion, the recipient's T cells are stimulated by allogeneic donor WBCs in the absence of costimulation and that this interaction induces a state of anergy in the recipient's T cells.
More than 100 observational cohort studies comparing patients with and without transfusion, as well as 2 RCTs comparing recipients of allogeneic and autologous RBCs,2,4,21,23 did not associate the TRIM effect with any particular blood constituent. Five recent RCTs3,5-7,22 were designed on the basis of the assumption that the allogeneic transfusion effect responsible for cancer recurrence and/or postoperative infection is mediated specifically by allogeneic WBCs. These studies produced contradictory findings, and the issue as to whether any deleterious TRIM effects are lessened or abrogated by WBC reduction of the transfused allogeneic cellular blood components remains unresolved.11,26,27,48Jensen et al attributed an 86%3 and a 71%5reduction in the incidence of postoperative infection to the use of WBC-reduced allogeneic whole blood3 or RBCs,5with the use of poststorage WBC reduction by filtration. In contrast, Houbiers et al22 found no difference in the incidence of postoperative infection between the recipients of WBC-reduced and buffy-coat–reduced RBCs with the use of prestorage WBC reduction by filtration. In the RCT by van de Watering et al,6 the use of WBC-reduced RBCs decreased the incidence of postoperative infection by 30% compared with that seen in patients having transfusion with buffy-coat–reduced RBCs. Moreover, there was no difference in the incidence of postoperative infection between the recipients of RBCs that were WBC reduced by filtration before or after storage.6
Recently, the United Kingdom, Ireland, and Portugal implemented universal WBC reduction of all transfused cellular blood components, based on the hypothesis that this practice would prevent the theoretical risk of transmission by transfusion of the agent of variant Creutzfeldt-Jakob disease (vCJD).49,50 France and Canada also implemented universal WBC reduction, primarily to enhance overall transfusion safety.50 Following these developments, public debate began regarding the appropriateness of introducing universal WBC reduction in the United States and elsewhere. The risk of transmission of vCJD by transfusion is considered only a theoretical possibility because an epidemic of bovine spongiform encephalopathy and cases of vCJD have occurred almost exclusively in the United Kingdom. Therefore, if a decision were made to implement universal WBC reduction in the United States and elsewhere, such a policy would probably not be introduced with an intent to prevent the transmission of vCJD, but to reap other potential benefits of WBC reduction. Most of the published evidence regarding such potential benefits pertains to the prevention of the deleterious TRIM effects, and the public debate regarding the implementation of universal WBC reduction is likely to focus mainly (although not exclusively) on an examination of the efficacy of WBC reduction in preventing these potential adverse effects of allogeneic blood transfusion.27,51 Furthermore, the recently described relation between WBC-containing allogeneic blood transfusion and increased postoperative mortality from causes other than postoperative infection,6 and the report by Hébert et al52 that a restrictive strategy of RBC transfusion may be superior to a liberal transfusion strategy when mortality is evaluated as an outcome in critically ill patients, may affect the debate over whether to implement universal WBC reduction.
Thirty-one physicians from academic blood banks in the United States recently voiced their strong opposition to the intent of the Food and Drug Administration (FDA) to mandate the implementation of universal WBC reduction in the United States.53 In a letter to the editor of Transfusion,53 they stated that “It is our view that published reports fail to document a substantial health benefit that would serve to justify WBC reduction of cellular blood components transfused to all patients. Accordingly, we feel that the currently available evidence regarding the deleterious effects of allogeneic blood transfusion is not sufficiently compelling to warrant universal WBC reduction for the prevention of these effects.” This present review examines the available evidence from: (1) the observational cohort studies that investigated the hypothesis that allogeneic blood transfusion provokes cancer recurrence and/or postoperative infection; (2) the findings of the RCTs that examined the possible deleterious TRIM effects associated with allogeneic blood transfusion in general or with transfusion of allogeneic WBCs in particular; and (3) the results of the recent RCTs that reported an association between allogeneic blood transfusion and increased mortality.6 52 To help readers process and understand the contradictory information, we include a section on the rationale, design, and analysis of the clinical studies that investigated these possible deleterious TRIM effects. Also, after reviewing the available evidence regarding the association of allogeneic transfusion with cancer recurrence or postoperative infection, we discuss the various arguments for and against a potential decision to implement universal WBC reduction of all transfused cellular blood components to prevent the deleterious effects of allogeneic transfusion-associated immunomodulation.
Design of clinical studies
Evidence in support of deleterious TRIM effects has been obtained from observational cohort studies, RCTs, meta-analyses of the observational cohort studies, and meta-analyses of the RCTs.54 The observational studies were either retrospective or prospective, and compared the risk of an adverse clinical outcome between cohorts of patients who did or did not receive transfusion and who differed with respect to baseline determinants of the need for transfusion and the risk of cancer recurrence or postoperative infection. These studies relied on multivariate statistical analyses to adjust for the effects of possible confounding factors. The RCTs were prospective clinical experiments comparing the risk of an adverse outcome between patients randomly allocated to receive different blood products, thus relying on randomization (ie, chance) to distribute all possible confounding factors equally between the treatment and control arms. The RCTs presented univariate (ie, unadjusted) analyses calculating the odds ratio (OR) of an adverse outcome in a treatment arm compared with that in a control arm, as shown in Table 1. Such analyses are valid if the counts in each of the 4 cells of the 2 × 2 contingency table (Table 1) are free of the effects of confounding factors—a condition usually satisfied when large numbers of patients are randomized. Meta-analyses of RCTs integrate the ORs from individual RCTs into a summary OR, which is also free of the effects of confounders and represents a measure of the “average” TRIM effect across the combined RCTs. Meta-analyses of observational studies extract 2 × 2 contingency table counts from each study, use these 4 counts to calculate an OR for each corresponding original report, and then also integrate the ORs from the individual studies into a summary OR. Unlike the summary ORs calculated by combining the results of RCTs, the summary ORs reported from meta-analyses of observational studies incorporate the effects of all known and unknown confounders of the association of transfusion with cancer recurrence and/or postoperative infection. Therefore, the summary ORs calculated by meta-analyses of the observational studies of the adverse TRIM effects should not be interpreted as a measure of the “average” TRIM effect across the combined studies. In only one of these meta-analyses18 was meta-regression used to adjust for the effects of 3 confounders on which information had been reported by most of the published observational studies.
Calculation of the odds ratio for studies investigating the relationship between allogeneic blood transfusion and cancer recurrence or postoperative infection
| Group . | Adverse outcome: cancer recurrence or postoperative infection . | Totals . | |
|---|---|---|---|
| Occurring . | Not occurring . | ||
| Exposed to allogeneic transfusion* | a | b | a + b |
| Not exposed to allogeneic transfusion† | c | d | c + d |
| Totals | a + c | b + d | n‡ |
| Group . | Adverse outcome: cancer recurrence or postoperative infection . | Totals . | |
|---|---|---|---|
| Occurring . | Not occurring . | ||
| Exposed to allogeneic transfusion* | a | b | a + b |
| Not exposed to allogeneic transfusion† | c | d | c + d |
| Totals | a + c | b + d | n‡ |
| Odds of an adverse outcome in those exposed to allogeneic transfusion: [a / (a + b)] / [b / (a + b)] = a / b | |||
| Odds of an adverse outcome in those not exposed to allogeneic transfusion: [c / (c + d)] / [d / (c + d)] = c / d | |||
| | |||
| Odds of an adverse outcome in those exposed to allogeneic transfusion: [a / (a + b)] / [b / (a + b)] = a / b | |||
| Odds of an adverse outcome in those not exposed to allogeneic transfusion: [c / (c + d)] / [d / (c + d)] = c / d | |||
| | |||
Treatment arm (patients prospectively randomized to receive transfusion of allogeneic WBCs) in RCTs, or treatment group (patients having transfusion) in observational cohort studies.
Control arm (patients prospectively randomized to receive transfusion of autologous or WBC-reduced allogeneic RBCs) in RCTs, or control group (patients not having transfusion) in observational cohort studies.
n = a + b + c + d; a, b, c, and d are the 4 counts of a 2 × 2 contingency table.54
When the results of studies retrieved for a meta-analysis are discrepant, or if the variation among the reported findings is greater than can be attributed to chance (a situation called “heterogeneity of effects”), a meta-analysis can offer insight into the reasons for the disagreements among the available studies. Provided that the results from the available studies are concordant, meta-analysis can be used to derive, through the application of a number of quantitative techniques, a measure of the effect of allogeneic blood transfusion based on a combination of the available data. This “average” TRIM effect is more precise (and more likely to attain statistical significance) than the TRIM effects reported from individual studies because it is based on a much larger sample (ie, the study population accrued when the study populations from all available reports are combined). Before the results of studies are integrated, however, the degree of agreement among reports must be assessed both conceptually and statistically. The Q test statistic quantifies the probability that the variation in reported results might have arisen by chance. Most analysts hold that there is sufficient agreement (or “homogeneity”) among studies to permit the undertaking of a meta-analytic synthesis of the results if P > .05 for the Q test statistic (ie, if there is a greater than 5% probability that the variation in reported results might have arisen by chance).55 In this review, we use meta-analysis both to investigate reasons for disagreements among studies (if P < .05 for the Q test statistic) and to integrate the results of the available studies for the calculation of an “average” TRIM effect across combined investigations (ifP > .05 for the Q test statistic). Where meta-analysis is used in this review, results of homogeneous studies are integrated using the random-effects method of DerSimonian and Laird.56
Patients allocated to the treatment arm of the reviewed RCTs3-7,22,23 were exposed to all the constituents of allogeneic blood and were at risk for developing adverse TRIM effects. Control-arm patients were presumed to be at reduced risk because they were not exposed to allogeneic WBCs3,5-7,22 or allogeneic WBCs and allogeneic plasma.4,23 Five4-6,22,23 of 6 RCTs3-6,22,23 conducted in Europe transfused buffy-coat–reduced RBCs to the treatment arm. In one RCT,3 whole blood was given, and in one RCT7conducted in the United States, standard RBCs (ie, not buffy coat reduced) were transfused. The buffy-coat reduction method is used widely in western Europe, but not elsewhere, for the preparation of blood components from whole blood. Components produced by the buffy-coat reduction method contain 60% to 80% fewer WBCs compared with cellular blood components produced by North American methods.57 Thus, the buffy-coat–reduced RBCs transfused by Houbiers et al22 and van de Watering et al6contained a mean of 0.8 × 109 WBCs per RBC unit; those administered by Jensen et al5 contained a mean of 1.2 × 109 WBCs per unit. The number of allogeneic WBCs contained in the average RBC transfusion dose given in 4 of the European RCTs4,5,22,23 (eg, in a median dose of 3 U of buffy-coat–reduced RBCs administered in the study of Houbiers et al22) was equivalent to the number of allogeneic WBCs contained in only 1 U of allogeneic RBCs produced in North America. Therefore, if the TRIM effect is mediated by allogeneic WBCs, the WBC dose given to patients in the treatment arm of most European RCTs4,5,22,23 may have been insufficient to provoke clinically significant TRIM effects. In a study of New Zealand White rabbits with established tumors, Blajchman30 reported a significant (P < .0001) decrease in the tumor growth–promoting effect of allogeneic blood transfusion in association with buffy-coat reduction of whole blood (Table2). The ameliorative effect of buffy-coat reduction was not complete, however, and the median number of pulmonary nodules seen in rabbits receiving buffy-coat–reduced whole blood was significantly (P = .0004) greater than that seen in control animals receiving no transfusion or in animals receiving WBC-reduced blood.30
Effect of buffy-coat reduction in decreasing allogeneic blood transfusion–induced tumor growth in New Zealand White rabbits with established tumors*
| Red cell transfusion received . | Sample size . | Number of pulmonary tumor nodules (median ± SE) . |
|---|---|---|
| Allogeneic, unmodified (stored) | 20 | 74.0 ± 8.7 |
| Allogeneic, buffy coat reduced before storage† | 19 | 34.0 ± 7.0 |
| Allogeneic, WBC reduced before storage‡ | 20 | 23.5 ± 1.8 |
| Control group (no transfusion) | 16 | 21.5 ± 2.5 |
| Red cell transfusion received . | Sample size . | Number of pulmonary tumor nodules (median ± SE) . |
|---|---|---|
| Allogeneic, unmodified (stored) | 20 | 74.0 ± 8.7 |
| Allogeneic, buffy coat reduced before storage† | 19 | 34.0 ± 7.0 |
| Allogeneic, WBC reduced before storage‡ | 20 | 23.5 ± 1.8 |
| Control group (no transfusion) | 16 | 21.5 ± 2.5 |
All 5 RCTs administering WBC-reduced RBCs5-7,22 or whole blood3 to the control arm transfused RBCs prepared by leukocyte filtration. Prestorage-filtered RBCs were prepared by passing, within 246 or 4822 hours of collection, a buffy-coat–reduced RBC unit through a leukocyte-reduction filter. Poststorage-filtered RBCs were prepared by passing a unit of buffy-coat–reduced RBCs5,6 or whole blood,3 stored for 6 or more days, through a leukocyte-reduction filter. The average WBC content of transfused WBC-reduced RBCs was less than 5 × 106 per unit, and was often as low as 1 × 106 per unit. The timing of the filtration procedure is important because biologic response modifiers released from WBC granules in a time-dependent manner during storage may mediate the adverse TRIM effects,39,48,56-60 and—if such TRIM effects really exist—only WBC-reduced RBCs filtered before storage can be expected to abrogate the adverse effects. Two RCTs administered autologous RBCs4 or whole blood23to the control arm. Autologous blood can be expected to abrogate the TRIM effects mediated by allogeneic plasma,31-36 or by intact, immunologically competent allogeneic WBCs, but not the TRIM effects mediated by biologic response modifiers released from WBC granules during storage.59
Observational studies
Observational studies have compared the incidence of cancer recurrence, death due to cancer recurrence, and/or overall mortality15-18 between patients undergoing cancer resection who did or did not receive transfusion; or the incidence of postoperative bacterial infection with or without transfusion in patients undergoing gastrointestinal surgery, orthopedic operations, cardiac surgery, or various other procedures. These studies tended to indicate that patients having transfusion (compared with those not having transfusion) had a higher incidence of cancer recurrence or death due to cancer recurrence as well as a shorter overall survival after a cancer resection operation; and almost always had a higher incidence of postoperative bacterial infection.16 These studies also indicated that patients having transfusion generally differed from those without transfusion in several prognostic factors, including clinical stage of the malignancy; size, histologic grade, and type of tumor; age; preoperative hemoglobin; duration and extent of surgery; amount of perioperative blood loss; the frequency of chronic systemic illness, such as congestive heart disease, lung disease, liver disease, kidney failure, or diabetes mellitus; and the presence of risk factors for postoperative urinary tract infection (UTI), pneumonia, or wound infection.18,19 61
These 2 sets of observations have led to different interpretations of the results of the studies that compared patients who did or did not receive transfusion. Some authors concluded that perioperative allogeneic blood transfusion had a direct deleterious effect on the recipient, causing an increase in the incidence of cancer recurrence and/or postoperative bacterial infection.16 Other investigators concluded that allogeneic blood transfusions were a surrogate marker for a variety of adverse prognostic factors and that the other variables that generated the need for perioperative transfusions determined the subsequent clinical outcome.19The latter school of thought reasoned that the adverse prognostic factors that were associated with both the need for perioperative transfusion and the cancer recurrence and/or postoperative bacterial infection were confounders of the relation between transfusion and these outcomes, and engendered a spurious association between allogeneic transfusion and cancer recurrence and/or postoperative infection.19
In many of the reported observational studies, the authors used multivariate regression analysis to adjust the effect of transfusion for the effects of confounding factors. Regression analysis can calculate an allogeneic blood transfusion effect that is independent of the effects of known and measurable confounders, provided that: (1) the investigators measure all known potentially confounding factors; (2) the variables that are associated with both transfusion and cancer recurrence and/or postoperative infection in a particular set of data are identified as true confounders; and (3) all true confounders are included in the final regression model on which the conclusions are based.19 In most published observational studies, however, important potential confounding factors were not considered by the investigators61; and the multivariate regression model on which the conclusions were based was built by a stepwise (as opposed to forced-entry) method, which did not ensure the inclusion of several measured confounders in the final model.19 For example, although postoperative UTI was the most frequent type of postoperative infection in most observational studies that reported an association between transfusion and postoperative infection, the number of patient days with an indwelling urinary catheter—the predominant risk factor for nosocomial UTI in the United States—was not considered as a potential confounder and was not measured in most studies.61
As a result, TRIM effects reported as “independent” by many teams of investigators were not truly free of the effects of known and measurable confounding factors, and the calculated TRIM effect decreased in magnitude (or became statistically insignificant) depending on which confounding factors were included in the final regression model.61 These caveats notwithstanding, allogeneic blood transfusion often emerged as the leading predictor of cancer recurrence and/or postoperative bacterial infection from multivariate analyses, and was reported to increase the risk of postoperative infection in patients having transfusion (compared with those not having transfusion) by up to 10-fold.16
Cancer recurrence
Chung et al,62 Vamvakas,17 and Brand and Houbiers18 used meta-analysis to integrate the findings from the univariate (ie, unadjusted; Table 1) analyses of the observational studies of the association of perioperative allogeneic blood transfusion with cancer recurrence, death due to cancer recurrence, or overall mortality. The most recent meta-analysis18 also included studies not published in English and considered 7 cancer sites; 100 observational studies were identified as eligible for analysis. There was agreement among the 3 meta-analyses with regard to the magnitude and statistical significance of the calculated summary ORs of the risk of an adverse clinical outcome in patients having transfusion (compared with those not having transfusion). Figure 1 shows the unadjusted summary OR of an adverse outcome for 7 cancer sites. As shown in Figure 1, when the unadjusted results were integrated from 28 reports on colorectal cancer, 14 reports on head and neck cancer, 10 reports on breast cancer, 8 reports on gastric cancer, 8 reports on lung cancer, 6 reports on cervical cancer, and 6 reports on prostate cancer, the summary OR of an adverse clinical outcome with transfusion (versus without transfusion) was statistically significant (P ≤ .05) for all sites except the cervix (P > .05). These unadjusted results contrast with the conclusions of most of the studies that presented a multivariate analysis. A statistically significant (P ≤ .05) TRIM effect adjusted for the effects of confounding factors was reported from only 11 studies of colorectal cancer,17 4 studies of head and neck cancer, 1 study of breast cancer, 2 studies of gastric cancer, 4 studies of lung cancer, and 2 studies of prostate cancer.18
Summary odds ratio of an adverse clinical outcome (ie, cancer recurrence, death due to cancer recurrence, or overall mortality) across published observational studies comparing patients having or not having transfusion.
The univariate (unadjusted) results of the available studies are integrated separately for each cancer site. The data on colorectal and prostate cancer (representing 28 and 6 studies, respectively) are shown as reported by Vamvakas.17 The data on head and neck (14 studies), breast (10 studies), gastric (8 studies), lung (8 studies), and cervical cancer (6 studies) are shown as reported by Brand and Houbiers.18 The references to the included primary studies can be found in the reports of these 2 meta-analyses.17 18Each summary OR is surrounded by its 95% CI. A summary OR of the null value (1) indicates that the risk of an adverse clinical outcome is the same, on average, with or without transfusion across the combined studies. When the 95% CI of the summary OR extends on both sides of the null value, the calculated summary TRIM effect is not statistically significant.
Summary odds ratio of an adverse clinical outcome (ie, cancer recurrence, death due to cancer recurrence, or overall mortality) across published observational studies comparing patients having or not having transfusion.
The univariate (unadjusted) results of the available studies are integrated separately for each cancer site. The data on colorectal and prostate cancer (representing 28 and 6 studies, respectively) are shown as reported by Vamvakas.17 The data on head and neck (14 studies), breast (10 studies), gastric (8 studies), lung (8 studies), and cervical cancer (6 studies) are shown as reported by Brand and Houbiers.18 The references to the included primary studies can be found in the reports of these 2 meta-analyses.17 18Each summary OR is surrounded by its 95% CI. A summary OR of the null value (1) indicates that the risk of an adverse clinical outcome is the same, on average, with or without transfusion across the combined studies. When the 95% CI of the summary OR extends on both sides of the null value, the calculated summary TRIM effect is not statistically significant.
On the basis of such a statistical combination of the unadjusted findings of the retrieved observational studies, Chung et al62 concluded that allogeneic blood transfusion given for a cancer resection operation had an adverse effect on subsequent prognosis; Vamvakas17 and Brand and Houbiers18reached the opposite conclusion. In the case of colorectal cancer studies, there was marked variation among the findings of the available reports, and the probability that such variation might have arisen by chance was smaller than 1 per 1000 (P < .001 for the Q test statistic). As already discussed, this situation precludes the calculation of a summary estimate of the allogeneic transfusion effect across the available studies by the techniques of meta-analysis.55
Brand and Houbiers18 separately analyzed the studies that reported on colorectal cancer recurrence (27 studies), death due to colorectal cancer recurrence (15 studies), or overall mortality following colorectal cancer resection (30 studies). When the unadjusted findings from these reports were integrated, there was still unexplained heterogeneity (P < .001 for the Q test statistic) in all 3 analyses. Meta-regression was used to adjust the summary OR of cancer recurrence for the effects of tumor location and clinical (Dukes) stage of tumor; the summary OR of death due to cancer recurrence for the effect of tumor location; and the summary OR of death from any cause for the effects of tumor location, clinical (Dukes) stage of tumor, and mean patient age. After adjustment for the effects of these confounding factors, no association was found between perioperative transfusion and cancer recurrence (P = .55) or death due to cancer recurrence (P = .19), but the relationship between perioperative transfusion and overall mortality persisted (P < .001). Brand and Houbiers18therefore reasoned that, because meta-regression could attribute the association of transfusion with cancer recurrence (or death due to cancer recurrence) to the effects of confounding factors, and because only a minority of the published observational studies had reported a significant adverse TRIM effect based on multivariate analyses, a link between perioperative transfusion and colorectal cancer recurrence could not be established. These investigators concluded that the observed effect of allogeneic transfusion on overall mortality might be due to an association of perioperative transfusion with causes of death other than cancer recurrence.18
It is also possible to ascribe the observed effect of allogeneic transfusion on mortality to a greater physiologic severity of illness in patients having transfusion than in those not having transfusion. Most observational studies presented data on the severity of disease in the included subjects; that is, they reported the clinical (Dukes) stage of tumor. In contrast, severity of illness incorporates both the severity of disease and the prevalence and severity of comorbidities, such as diabetes mellitus, congestive heart disease, lung disease, liver disease, or kidney failure. Such chronic systemic illnesses may have as much of an impact on overall mortality as the clinical (Dukes) stage of tumor, but the available observational studies did not present data on chronic systemic illness, making it impossible to adjust for the effect of illness severity in the meta-analysis. It is thus possible that the patients having perioperative transfusion survived for a shorter period after the cancer resection operation simply because they were sicker at the time of surgery than the patients who did not require transfusion.
The findings of the available observational studies on the association of perioperative transfusion with cancer recurrence may no longer be relevant to the management of contemporary cancer patients. Many of these studies (eg, all of the studies that reported on patients with gastric cancer) examined only overall survival; others did not report a multivariate analysis; still others included patients operated on before 1980 and over an extended period. For example, only 3 of the 14 studies of head and neck cancer retrieved for the meta-analysis of Brand and Houbiers18 fulfilled the selection criteria of reporting on cancer-specific complications rather than overall mortality, adjusting for the effects of confounding factors by multivariate analysis, and including patients operated on after 1980 and over a period of less than 5 years. Only 1 of these 3 studies reported a statistically significant adverse effect of allogeneic blood transfusion.18
Furthermore, the findings of the available observational studies of the association of perioperative transfusion with cancer recurrence may reflect the effect of publication bias,63-65 a consideration that may also apply to the discussion of the reported observational studies of the relationship between perioperative transfusion and postoperative infection. Studies reporting null results are less likely to be published than studies reporting statistically significant findings. Easterbrook et al63 documented a statistically significant 3.8-fold increase in the odds of publication for observational studies reporting significant findings, compared with studies with null results. Multivariate analysis showed that the better odds of publication could not be explained by the quality of the study design. On the contrary, there was a trend toward a greater number of significant results with poorer quality studies.63
Rather than comparing patients with or without transfusion, Ness et al66 prospectively compared the recurrence rate of prostate cancer in 309 recipients of autologous or allogeneic blood transfusion, and observed no clinical benefit from the use of autologous blood. The hazard ratio of allogeneic transfusion in a univariate Cox proportional hazard model of time to tumor recurrence was 0.87 (P > .05), and Ness et al66 commented that a beneficial TRIM effect could not be excluded. It is noteworthy that allogeneic blood transfusion is not associated with an adverse prognosis in patients with cervical cancer, even when all available univariate (unadjusted) results are combined (Figure 1). Because human papillomavirus (HPV) is implicated in the pathogenesis of cervical cancer, cytotoxic T cells directed against HPV antigens expressed on the surface of tumor cells could contribute to immunologic control of the growth of residual tumor cells following cancer resection. Accordingly, if allogeneic blood transfusion induced immune suppression, it could down-regulate these cytotoxic T-cell responses, promoting tumor cell growth. Transfusion would thus be expected to have a larger adverse effect in patients with cervical cancer than in patients with cancer from other sites (Figure 1) where the tumors are not virus induced and are probably subject to weaker immunologic control mechanisms. However, it has been observed that early in the development of cervical intraepithelial neoplasia, the expression of class I HLA molecules on malignant cells is specifically down-regulated. HPV-specific, HLA-restricted cytotoxic T cells (whose function may be suppressed by allogeneic blood transfusion) may not be effective against cervical cancer cells because of the reduction in class I HLA molecule expression on the tumor cells.67
Postoperative infection
Except for a handful of studies,61,68-70observational investigations of the association of allogeneic blood transfusion with postoperative bacterial infection almost uniformly have reported a relationship between perioperative transfusion and infection, which persisted after statistical adjustment for the effects of the confounding factors considered by the authors.16,19Postoperative infection often develops, however, because of a higher physiologic severity of illness and a higher prevalence of risk factors for postoperative infection at specific sites in patients having transfusion compared with those not having transfusion. As already discussed in the context of cancer recurrence, in most published observational studies of transfusion and postoperative infection, the reported allogeneic transfusion effect was adjusted for the effect of severity of disease (ie, the severity of the principal diagnosis), but the severity of the principal diagnosis differs from the severity of a patient's overall illness. In a study that compares the frequency of postoperative infection with or without transfusion in patients undergoing colorectal cancer resection, the presence and severity of comorbidities (eg, diabetes mellitus, congestive heart disease, lung disease, liver disease, or kidney failure) may be a more important determinant of postoperative infection than the severity of the principal diagnosis according to the clinical (Dukes) stage of tumor. Furthermore, the number of patient days with an indwelling urinary catheter may be the most important determinant of postoperative UTI; the number of days of endotracheal intubation and/or impaired consciousness may be a cardinal determinant of postoperative pneumonia; and so on.61
Until recently, observational studies reporting an association between allogeneic blood transfusion and postoperative infection did not adjust for the effects of severity of illness and/or risk factors for postoperative infection at specific sites. Some teams of investigators secured partial control for the effects of these variables by excluding UTIs from the definition of postoperative infection69,71; by limiting the outcome variable to postoperative wound infection72-74; or by adjusting for the effects of serum albumin,75,76 insertion of a urinary catheter,77,78 or presence of chronic systemic illness,70 or diabetes mellitus.79 However, adjustment for the effects of all these factors in combination has rarely been presented in the literature.
In a study of 492 patients undergoing colorectal cancer resection, Vamvakas et al61 calculated the probability of infection in association with perioperative blood transfusion with and without adjustment for the effects of chronic systemic illness, number of days with an indwelling urinary catheter, endotracheal intubation, and impaired consciousness. In an analysis that adjusted only for the effects of 18 confounding variables considered by previous authors and that adjusted for insertion of a urinary catheter (as opposed to number of patient days with an indwelling urinary catheter), these investigators61 detected a highly significant (P < .001) transfusion effect on the risk of postoperative infection at any site. However, when adjustment was also made for the effects of the aforementioned variables, the association between transfusion and postoperative infection at any site disappeared (P = .407); the only significant predictors of postoperative infection were the number of patient days with an indwelling urinary catheter, the presence of chronic systemic illness, the number of days of impaired consciousness, and the duration of anesthesia. Except for the duration of anesthesia, the 17 other confounding variables considered by previous authors proved to be insignificant predictors of postoperative infection in this analysis.
Observational studies of the association of allogeneic blood transfusion with postoperative infection in patients having orthopedic surgery secured partial adjustment for the effect of greater illness severity in the transfusion group by comparing subjects who received autologous or allogeneic blood transfusion. At least to some extent, patients who made preoperative autologous blood donations did so because they were in better health than subjects who did not make autologous donations. Also, autologous blood may have been transfused more liberally than allogeneic blood, after less surgical blood loss, and it is difficult to attribute any increase in the infection rate seen in the allogeneic (compared with the autologous) transfusion group to the allogeneic transfusion per se if adjustment is not made for the effects of chronic systemic illness and risk factors for postoperative infection at specific sites. Duffy and Neal20 conducted a meta-analysis of the univariate (ie, unadjusted) results of 5 observational studies68,70,80-82 and 2 RCTs4,83comparing the postoperative infection rates of patients having transfusion with similar volumes of autologous or allogeneic blood. Patients receiving no transfusion or a mixture of autologous and allogeneic RBCs in these studies4,68,70,80-83 were excluded from the meta-analysis. Because it is uncommon for more than 3 predonated units of autologous blood to be available, subjects receiving 4 or more units of allogeneic RBCs were also excluded. The studies of Busch et al21,23 and Triulzi et al84were excluded because of insufficient published information. The summary OR across the 7 studies was 2.4 (95% confidence interval [CI], 1.6-3.6; P < .0001). Figure2 updates the meta-analysis of Duffy and Neal20 by including the study of Innerhofer et al.78 The 8 available studies were homogeneous (P = .50 for the Q test statistic), and the summary OR of postoperative infection in the allogeneic (compared with the autologous) transfusion group was 2.1 (95% CI, 1.3-3.4;P < .001).
Summary odds ratio of postoperative bacterial infection derived from the univariate (unadjusted) results of observational68,70,78,80,82 and experimental4,83 clinical studies comparing recipients of similar volumes of allogeneic or autologous blood.20
For each study, the figure shows the OR of postoperative bacterial infection in recipients of allogeneic (compared with autologous) blood. The data from the experimental studies4 83 depicted here represent observational comparisons restricted to patients receiving similar volumes of allogeneic or autologous blood (see text). Each OR is surrounded by its 95% CI. If the 95% CI of the OR includes the null value of 1, the TRIM effect is not statistically significant. The data are plotted on a logarithmic scale extending from 0.1 to 1000.
Summary odds ratio of postoperative bacterial infection derived from the univariate (unadjusted) results of observational68,70,78,80,82 and experimental4,83 clinical studies comparing recipients of similar volumes of allogeneic or autologous blood.20
For each study, the figure shows the OR of postoperative bacterial infection in recipients of allogeneic (compared with autologous) blood. The data from the experimental studies4 83 depicted here represent observational comparisons restricted to patients receiving similar volumes of allogeneic or autologous blood (see text). Each OR is surrounded by its 95% CI. If the 95% CI of the OR includes the null value of 1, the TRIM effect is not statistically significant. The data are plotted on a logarithmic scale extending from 0.1 to 1000.
Recently, Carson et al71 conducted a retrospective cohort study of 9598 consecutive patients with hip fracture who underwent surgical repair between 1983 and 1993 at 20 hospitals across the United States. The primary outcome variable was serious bacterial infection, defined as bacteremia, pneumonia, deep wound infection, or septic arthritis/osteomyelitis. Information was collected on numerous variables, including comorbid conditions such as the determinants of the Charlson Comorbidity Index, but the method used for building the statistical models was not described. The adjusted relative risk of serious postoperative infection with transfusion (versus without transfusion) was 1.43 (95% CI, 1.16-1.78; P = .001). Chang et al74 analyzed a database of 1349 patients undergoing elective colorectal surgery for any disease of the colon or rectum at 11 university hospitals across Canada. To better adjust for the effects of factors confounding the association of transfusion with postoperative infection, these investigators limited the outcome variable to postoperative wound infection. Ten prognostic variables were found to be associated with both transfusion and postoperative wound infection, and the final regression model adjusted for 4 of these identified confounders. Allogeneic blood transfusion was reported to be a significant independent predictor of postoperative wound infection (OR = 1.18; 95% CI, 1.05-1.33; P = .007). Vamvakas and Carven60 reported a retrospective cohort study of 416 consecutive patients admitted to one hospital for coronary artery bypass graft (CABG) operations. The outcome variable was limited to postoperative wound infection or pneumonia, and adjustment was made for the effects of chronic systemic illness and specific risk factors for wound infection or pneumonia. Statistical models were built by the forced-entry method, and the adjusted risk of postoperative wound infection or pneumonia increased by 6% per unit of allogeneic RBCs and/or platelets transfused (P = .0284), or by 43% for a patient receiving the mean transfusion dose of 7.2 U of RBCs and/or platelets.
Randomized controlled trials
Cancer recurrence
The 3 RCTs that compared the incidence of cancer recurrence between recipients of buffy-coat–reduced allogeneic RBCs and recipients of autologous whole blood21 or RBCs2or WBC-reduced, buffy-coat–reduced allogeneic RBCs22 were medically and statistically homogeneous. All 3 studies enrolled patients undergoing colorectal cancer resection. The proportion of patients having transfusion varied from 58%21 to 64%22 among the studies, and the proportion of patients developing recurrent cancer varied from 23%2 to 25.5%.22 There was also agreement among the findings of these RCTs (Figure 3), as the noted variation in reported results was sufficiently modest to be attributed to chance (P > .10 for the Q test statistic).55 Accordingly, the findings of the studies were combined in 2 meta-analyses,24,25 and the summary OR of cancer recurrence in the allogeneic transfusion (compared with the control) group across the 3 studies was 1.04 (95% CI, 0.81-1.35;P > .05) (Figure 3). The summary OR of death due to cancer recurrence was 0.98 (95% CI, 0.76-1.26;P > .05).24
Summary odds ratio of cancer recurrence24derived from randomized controlled trials investigating the association of perioperative allogeneic blood transfusion with cancer recurrence in patients undergoing elective colorectal cancer resection.2,21 22
For each RCT, the figure shows the OR of cancer recurrence in recipients of buffy-coat–reduced allogeneic RBCs as compared with recipients of autologous whole blood21 or RBCs2or recipients of WBC-reduced allogeneic RBCs.22 Each OR is surrounded by its 95% CI. If the 95% CI of the OR includes the null value of 1, the TRIM effect is not statistically significant. The data are plotted on a logarithmic scale extending from 0.1 to 10.
Summary odds ratio of cancer recurrence24derived from randomized controlled trials investigating the association of perioperative allogeneic blood transfusion with cancer recurrence in patients undergoing elective colorectal cancer resection.2,21 22
For each RCT, the figure shows the OR of cancer recurrence in recipients of buffy-coat–reduced allogeneic RBCs as compared with recipients of autologous whole blood21 or RBCs2or recipients of WBC-reduced allogeneic RBCs.22 Each OR is surrounded by its 95% CI. If the 95% CI of the OR includes the null value of 1, the TRIM effect is not statistically significant. The data are plotted on a logarithmic scale extending from 0.1 to 10.
Busch et al21 reported on 423 of 510 randomized patients; Houbiers et al22 reported on 697 of 1021 randomized subjects; and Heiss et al2 reported on 100 of 120 randomized patients. In addition to the patients who did not have transfusion (42%, 36%, and 40% of the study samples, respectively), 28%, 10%, and 33%, respectively, of the subjects randomized to receive autologous or WBC-reduced allogeneic RBCs also received buffy-coat–reduced allogeneic RBCs. Many violations of the experimental protocol occurred in the studies of Busch et al21 and Heiss et al2 because of a design problem that needs to be discussed. Patients randomly allocated to the autologous transfusion arm in these studies donated 2 U of whole blood before surgery, and, if they needed transfusion of more than 2 U of RBCs perioperatively, they were given buffy-coat–reduced RBCs. In addition, because of the preoperative autologous blood donations, these patients presented to the operating room with a lower hematocrit than patients from the allogeneic transfusion arm. Therefore, autologous transfusion recipients probably received transfusions sooner, after less surgical blood loss, than did patients allocated to the allogeneic transfusion arm. It is thus possible that patients from the autologous arm having transfusion with a particular number of RBC units may have had less invasive surgery than patients from the allogeneic transfusion arm given the same number of RBCs; this design problem may have led to overestimation of any adverse effect of allogeneic blood transfusion, as discussed by Heiss et al.2 4
It is impossible, for ethical reasons, to perform RCTs in which patients are randomly allocated not to receive blood transfusion or to always receive transfusion; only patients prospectively randomized to receive different blood products—should the need for transfusion arise perioperatively—can be compared in RCTs. Ideally, the blood components administered to patients enrolled in RCTs should differ in only one factor, which reflects a biologic mechanism presumed to underlie the immunosuppressive effect of allogeneic blood transfusion. If the TRIM effect is assumed to be mediated by allogeneic WBCs, recipients of standard or WBC-reduced RBCs should be compared. However, only one RCT22 investigating the association between allogeneic transfusion and cancer recurrence has transfused WBC-reduced RBCs to patients from the control arm.
Because of the high frequency of patients who did not have transfusion and the protocol violations in the 3 RCTs investigating the association of allogeneic transfusion with cancer recurrence,2,21,22 a deleterious TRIM effect on colorectal cancer recurrence could be evaluated in an effective sample of only 696 patients across the 3 studies.18 Therefore, the meta-analysis of the 3 RCTs24 did not have adequate statistical power to rule out the possibility of an adverse TRIM effect smaller than a 33% increase in the risk of cancer recurrence among the recipients of buffy-coat–reduced allogeneic RBCs, compared with the recipients of autologous or WBC-reduced allogeneic RBCs. Moreover, as already discussed, if allogeneic WBCs are assumed to mediate the deleterious TRIM effect(s), then the problem of limited statistical power is compounded by the problem of limited exposure to allogeneic WBCs in these RCTs in which buffy-coat–reduced RBCs were transfused to the treatment arm.2,21 22 The question that is pertinent to clinical transfusion practice in North America—that is, whether transfusion of standard allogeneic RBCs increases the risk of cancer recurrence—has not yet been addressed by RCTs.
Furthermore, the setting of colorectal cancer resection may be inappropriate for the detection of a deleterious TRIM effect on cancer recurrence. Allogeneic transfusion–associated immunomodulation can be expected to increase the recurrence rate of a resected malignancy if the growth of residual cancer cells is indeed controlled by immunologic mechanisms. The existence of a specific immune response to colorectal cancer cells has not been proven.18 Although it is possible to generate cytotoxic T cells in vitro that recognize antigens expressed by colorectal cancer cells, the relevance of these cytotoxic cells in tumor growth may be limited because of loss of the expression of HLA molecules and adhesion molecules on colorectal cancer cells.85,86 If further RCTs are to be conducted, it may be preferable to concentrate on tumors known or presumed to be virus-induced and/or tumors that occur with increased frequency in patients receiving high doses of immunosuppressive drugs for the prevention of organ allograft rejection (ie, skin cancers, lymphoma, cervical carcinoma, and Kaposi sarcoma, as well as vulvar, perineal, and renal tumors).87
The reports of the 3 RCTs2,21,22 also presented observational comparisons of the incidence of cancer recurrence between patients having or not having transfusion in each study. The results of these analyses were conflicting.18 In the study of Houbiers et al,22 blood transfusion was not associated with cancer recurrence, and there was a statistically significant association between blood transfusion and mortality from causes other than cancer. Busch et al21 observed a 10% increase in the risk of cancer recurrence with transfusion (versus without transfusion) that could be attributed to local tumor recurrences (as opposed to distant metastases); the authors ascribed this increase in risk to the factors that generate a need for transfusion, as opposed to a TRIM effect.88 Heiss et al2 observed an increased risk of cancer recurrence in recipients of allogeneic (or autologous and allogeneic) RBCs compared with patients not having transfusion or recipients of autologous RBCs. However, their study sample was small (n = 100), and these 4 groups of patients were unbalanced with regard to the levels of potential confounding factors.
Postoperative infection
The 7 RCTs that compared the incidence of postoperative infection between recipients of buffy-coat–reduced4-6,22,23 or standard7 allogeneic RBCs or whole blood3 and recipients of autologous or WBC-reduced, buffy-coat–reduced allogeneic RBCs or whole blood were medically and statistically heterogeneous (Table 3). When all 7 studies were considered together, the probability that the disagreements among the findings of these studies might have arisen by chance was smaller than 1 per 10 000 (P < .0001 for the Q test statistic) (Figure 4). Two studies3,5reported a significant (P < .05) TRIM effect, 2 studies4,6 reported a marginally significant (P < .10) effect, and 3 studies7,22,23 did not detect an effect. More important, the variation in reported results ranged from a 7.3-fold increase in the risk of infection3(Figure 4) to no TRIM effect.7,22 23 Table4 stratifies the 7 RCTs according to various study characteristics and then combines the results of RCTs that share each particular design attribute. The purpose of this meta-analysis is to examine whether specific differences in the design of the studies may have been responsible for the disagreements among the results. A study design attribute is considered to account for the disagreements among the studies if the integrated results from RCTs that have the same level of that attribute (eg, multicenter study organization) are statistically homogeneous and do not show an association between allogeneic transfusion and postoperative infection; and if the combined findings from RCTs that have other levels of the attribute (eg, single-center study organization) are also statistically homogeneous and demonstrate a statistically significant relationship between allogeneic transfusion and postoperative infection.
Randomized controlled trials investigating the association between perioperative allogeneic blood transfusion and postoperative bacterial infection
| Study . | Total sample size3-150 . | Design . | RBC product given to the treatment arm . | RBC product given to the control arm . | Overall percentage having transfusion3-151 . | Overall percentage developing postoperative infection3-151 . |
|---|---|---|---|---|---|---|
| Jensen et al3 | 197 | ?Single blind | Allogeneic whole blood | WBC-reduced allogeneic whole blood (filtered after storage) | 52.8 | 8.1 |
| Heiss et al4 | 120 | Unblinded | BC-reduced allogeneic RBCs | Autologous RBCs | 75.0 | 20.0 |
| Busch et al23 | 470 | Unblinded | BC-reduced allogeneic RBCs | Autologous whole blood | 59.0 | 26.0 |
| Houbiers et al22 | 697 | Single blind3-152 | BC-reduced allogeneic RBCs | WBC-reduced, BC-reduced allogeneic RBCs (filtered before storage) | 64.0 | 33.4 |
| Jensen et al5 | 589 | Single blind3-152 | BC-reduced allogeneic RBCs | WBC-reduced, BC-reduced allogeneic RBCs (filtered after storage) | 44.1 | 17.5 |
| van de Watering et al6 | 909 | Single blind3-152 | BC-reduced allogeneic RBCs | WBC-reduced, BC-reduced allogeneic RBCs (filtered before [n = 302] or after [n = 302] storage) | 94.8 | 19.3 |
| Tartter et al7 | 221 | Unblinded | Allogeneic RBCs | WBC-reduced allogeneic RBCs | 26.7 | 16.7 |
| Study . | Total sample size3-150 . | Design . | RBC product given to the treatment arm . | RBC product given to the control arm . | Overall percentage having transfusion3-151 . | Overall percentage developing postoperative infection3-151 . |
|---|---|---|---|---|---|---|
| Jensen et al3 | 197 | ?Single blind | Allogeneic whole blood | WBC-reduced allogeneic whole blood (filtered after storage) | 52.8 | 8.1 |
| Heiss et al4 | 120 | Unblinded | BC-reduced allogeneic RBCs | Autologous RBCs | 75.0 | 20.0 |
| Busch et al23 | 470 | Unblinded | BC-reduced allogeneic RBCs | Autologous whole blood | 59.0 | 26.0 |
| Houbiers et al22 | 697 | Single blind3-152 | BC-reduced allogeneic RBCs | WBC-reduced, BC-reduced allogeneic RBCs (filtered before storage) | 64.0 | 33.4 |
| Jensen et al5 | 589 | Single blind3-152 | BC-reduced allogeneic RBCs | WBC-reduced, BC-reduced allogeneic RBCs (filtered after storage) | 44.1 | 17.5 |
| van de Watering et al6 | 909 | Single blind3-152 | BC-reduced allogeneic RBCs | WBC-reduced, BC-reduced allogeneic RBCs (filtered before [n = 302] or after [n = 302] storage) | 94.8 | 19.3 |
| Tartter et al7 | 221 | Unblinded | Allogeneic RBCs | WBC-reduced allogeneic RBCs | 26.7 | 16.7 |
RBC indicates red blood cell; WBC, white blood cell; BC, buffy coat.
Used in the analysis of postoperative bacterial infection after patient exclusions and withdrawals.
In both the treatment and control arms combined.
Investigators making diagnoses of postoperative infection were blinded to the treatment allocations.
Randomized controlled trials investigating the association of perioperative allogeneic blood transfusion with postoperative bacterial infection in patients undergoing abdominal3-5,7,22,23 or open heart6 surgery.
For each RCT, the figure shows the OR of postoperative infection in recipients of buffy-coat–reduced4-6,22,23 or standard7 allogeneic RBCs or whole blood3 as compared with recipients of autologous or WBC-reduced allogeneic RBCs or whole blood (Table 3). For the studies of Jensen et al3,5 and Tartter et al,7 the OR is shown not as reported by the authors, but as recalculated according to an intention-to-treat analysis.54 For the study of van de Watering et al,6 the depicted OR represents a comparison between 2 groups, that is, the recipients of buffy-coat–reduced RBCs and the recipients of WBC-reduced RBCs filtered before or after storage. Each OR is surrounded by its 95% CI. If the 95% CI of the OR includes the null value of 1, the TRIM effect is not statistically significant. The data are plotted on a logarithmic scale extending from 0.1 to 100.
Randomized controlled trials investigating the association of perioperative allogeneic blood transfusion with postoperative bacterial infection in patients undergoing abdominal3-5,7,22,23 or open heart6 surgery.
For each RCT, the figure shows the OR of postoperative infection in recipients of buffy-coat–reduced4-6,22,23 or standard7 allogeneic RBCs or whole blood3 as compared with recipients of autologous or WBC-reduced allogeneic RBCs or whole blood (Table 3). For the studies of Jensen et al3,5 and Tartter et al,7 the OR is shown not as reported by the authors, but as recalculated according to an intention-to-treat analysis.54 For the study of van de Watering et al,6 the depicted OR represents a comparison between 2 groups, that is, the recipients of buffy-coat–reduced RBCs and the recipients of WBC-reduced RBCs filtered before or after storage. Each OR is surrounded by its 95% CI. If the 95% CI of the OR includes the null value of 1, the TRIM effect is not statistically significant. The data are plotted on a logarithmic scale extending from 0.1 to 100.
Possible sources of variation in the findings of the available randomized controlled trials investigating the association between perioperative allogeneic blood transfusion and postoperative bacterial infection: integration of the findings of studies sharing a particular design characteristic by a meta-analysis
| Study attribute . | Categories of study attribute . | Number of studies . | Q test statistic: Pvalue . | Summary odds ratio4-150 . | |
|---|---|---|---|---|---|
| Point estimate . | 95% confidence interval . | ||||
| Study organization | Single center3-7 | 5 | > .05 | 2.294-151 | 1.35-3.874-151 |
| Multicenter22 23 | 2 | .95 | 0.88 | 0.69-1.12 | |
| Surgical setting | Open heart surgery6 | 1 | N/A‡ | 1.424-153 | 1.00-1.994-153 |
| Elective abdominal surgery3-5,7,22 23 | 6 | < .001 | N/A4-155 | N/A4-155 | |
| Homogeneity of patients having | Colorectal cancer4,22 23 | 3 | > .10 | 0.96 | 0.67-1.37 |
| elective abdominal surgery: diagnosis | Any disease of the colorectum35 or gastrointestinal tract7 | 3 | > .25 | 2.954-151 | 1.73-5.054-151 |
| RBC product transfused to the | Allogeneic whole blood3 | 1 | N/A‡ | 7.324-154 | 1.62-33.124-154 |
| treatment arm | Allogeneic RBCs7 | 1 | N/A‡ | 1.854-154 | 0.89-3.854-154 |
| Buffy-coat–reduced RBCs4-6,22 23 | 5 | < .001 | N/A4-155 | N/A4-155 | |
| RBC product transfused to the | Autologous RBCs4 or whole blood23 | 2 | > .05 | 1.35 | 0.45-4.08 |
| control arm | WBC-reduced allogeneic RBCs5-7 22 or whole blood3 | 5 | < .001 | N/A4-155 | N/A4-155 |
| WBC-reduced allogeneic RBCs5-7 22 | 4 | < .001 | N/A4-155 | N/A4-155 | |
| Fresh-filtered, WBC-reduced allogeneic RBCs6 22 | 2 | < .05 | N/A4-155 | N/A4-155 | |
| WBC-reduced allogeneic RBCs or whole blood filtered after storage3,5 6 | 3 | < .01 | N/A4-155 | N/A4-155 | |
| Overall | Exceeding 20%22 23 | 2 | .95 | 0.88 | 0.69-1.12 |
| postoperative infection rate# | 20% or lower3-7 | 5 | > .05 | 2.294-151 | 1.35-3.874-151 |
| Overall percentage | Less than 50%57 | 2 | > .10 | 2.734-151 | 1.48-5.044-151 |
| of patients having | 50% to 67%3,22 23 | 3 | > .10 | 0.96 | 0.62-1.49 |
| transfusion# | More than 67%46 | 2 | > .25 | 1.534-151 | 1.00-2.334-151 |
| Reported by Jensen | Jensen et al35 | 2 | .50 | 3.68 | 2.11-6.43 |
| et al or by other teams of investigators | Other teams4,6,7,22 23 | 5 | > .05 | 1.17 | 0.84-1.62 |
| Study attribute . | Categories of study attribute . | Number of studies . | Q test statistic: Pvalue . | Summary odds ratio4-150 . | |
|---|---|---|---|---|---|
| Point estimate . | 95% confidence interval . | ||||
| Study organization | Single center3-7 | 5 | > .05 | 2.294-151 | 1.35-3.874-151 |
| Multicenter22 23 | 2 | .95 | 0.88 | 0.69-1.12 | |
| Surgical setting | Open heart surgery6 | 1 | N/A‡ | 1.424-153 | 1.00-1.994-153 |
| Elective abdominal surgery3-5,7,22 23 | 6 | < .001 | N/A4-155 | N/A4-155 | |
| Homogeneity of patients having | Colorectal cancer4,22 23 | 3 | > .10 | 0.96 | 0.67-1.37 |
| elective abdominal surgery: diagnosis | Any disease of the colorectum35 or gastrointestinal tract7 | 3 | > .25 | 2.954-151 | 1.73-5.054-151 |
| RBC product transfused to the | Allogeneic whole blood3 | 1 | N/A‡ | 7.324-154 | 1.62-33.124-154 |
| treatment arm | Allogeneic RBCs7 | 1 | N/A‡ | 1.854-154 | 0.89-3.854-154 |
| Buffy-coat–reduced RBCs4-6,22 23 | 5 | < .001 | N/A4-155 | N/A4-155 | |
| RBC product transfused to the | Autologous RBCs4 or whole blood23 | 2 | > .05 | 1.35 | 0.45-4.08 |
| control arm | WBC-reduced allogeneic RBCs5-7 22 or whole blood3 | 5 | < .001 | N/A4-155 | N/A4-155 |
| WBC-reduced allogeneic RBCs5-7 22 | 4 | < .001 | N/A4-155 | N/A4-155 | |
| Fresh-filtered, WBC-reduced allogeneic RBCs6 22 | 2 | < .05 | N/A4-155 | N/A4-155 | |
| WBC-reduced allogeneic RBCs or whole blood filtered after storage3,5 6 | 3 | < .01 | N/A4-155 | N/A4-155 | |
| Overall | Exceeding 20%22 23 | 2 | .95 | 0.88 | 0.69-1.12 |
| postoperative infection rate# | 20% or lower3-7 | 5 | > .05 | 2.294-151 | 1.35-3.874-151 |
| Overall percentage | Less than 50%57 | 2 | > .10 | 2.734-151 | 1.48-5.044-151 |
| of patients having | 50% to 67%3,22 23 | 3 | > .10 | 0.96 | 0.62-1.49 |
| transfusion# | More than 67%46 | 2 | > .25 | 1.534-151 | 1.00-2.334-151 |
| Reported by Jensen | Jensen et al35 | 2 | .50 | 3.68 | 2.11-6.43 |
| et al or by other teams of investigators | Other teams4,6,7,22 23 | 5 | > .05 | 1.17 | 0.84-1.62 |
RBC(s) indicates red blood cell(s); WBC, white blood cell; N/A, not applicable.
Calculated by the method of DerSimonian and Laird.56
Statistically significant (P ≤ .05) summary odds ratio.
Not applicable because only one study was available for meta-analysis.
Odds ratio of postoperative infection in patients receiving buffy-coat–reduced RBCs, as compared with recipients of WBC-reduced, buffy-coat–reduced RBCs filtered before or after storage.
P < .05 for the Q test statistic; accordingly, the results of the studies could not be integrated by a meta-analysis, and N/A is shown under the summary odds ratio.
Odds ratio shown not as reported by the authors, but as recalculated according to an intention-to-treat analysis.
#In the entire study population regardless of randomization arm.
Five study characteristics could account individually for the disagreements among the 7 RCTs.3-7,22,23 These are: (1) study organization (ie, single center versus multicenter), (2) homogeneity of enrolled patients having elective abdominal surgery, (3) postoperative infection rate recorded in the entire study population, (4) proportion of patients having transfusion, and (5) study authorship. The RBC product transfused to the treatment or control arm could not account for the disagreements among the studies (Table 4). Single-center RCTs,3-7 studies enrolling a mixed patient population with any disease of the colorectum or gastrointestinal tract,3,5,7 studies recording an overall infection rate of 20% or less,3-7 trials in which less than 50% or more than 67% of the enrolled patients received perioperative transfusion,4-7 and RCTs reported by the team of Jensen et al3,5 were all homogeneous when considered together (P > .05 for the Q test statistic) and indicated a beneficial effect of the transfusion of autologous or WBC-reduced allogeneic RBCs. Multicenter RCTs,22,23 studies enrolling a homogeneous patient population having colorectal cancer resection,4,22,23 studies recording an overall infection rate of greater than 20%,22,23 trials in which 50% to 67% of the patients had transfusion,3,22,23 and RCTs reported by teams of investigators other than Jensen et al4,6,7,22 23 were also homogeneous when considered together (P > .05 for the Q test statistic) and indicated no difference in the incidence of postoperative infection between the treatment and control arms (Table 4).
Only the 2 multicenter RCTs22,23 reported an overall infection rate exceeding 20%; the overall infection rate was 20% or less in the 5 single-center studies. The results of the 2 multicenter RCTs of Busch et al23 and Houbiers et al22 may thus differ from the findings of the other studies because of the lower statistical power of multicenter (compared with single-center) studies to detect a treatment effect.89-91 In addition, Busch et al23 and Houbiers et al22 may have used more lenient criteria for the diagnosis of postoperative infection (or may have included more patients with risk factors for postoperative infection) compared with the other studies. Because the reports of all 7 RCTs presented only very limited information about the prevalence of risk factors for postoperative infection in the treatment and control arms of patients, the latter possibility cannot be investigated without access to the raw data. With regard to the former possibility, multicenter RCTs often have reduced statistical power compared with single-center studies because of the influence of the “center effect” and their greater susceptibility to the effects of random error.89-92 The “center effect” refers to differences among the participating medical centers in variables that can influence the postoperative infection rate, but are not captured (or standardized) by the common research protocol. The “center effect” can be removed from the results of a multicenter RCT if a statistical analysis stratified by participating hospital is conducted, but such an analysis was not presented in the reports of Busch et al23and Houbiers et al.22 Random error refers to missing data, incorrect data, interobserver variability, and the like; both random error and the “center effect” bias the estimate of a treatment effect toward the null.92 The “center effect” may be especially influential when the rates of postoperative infection in abdominal surgery are studied; a survey of 20 surgical departments across Israel reported a variation in postoperative infection rates of 0% to 65%.93
Jensen et al3,5 reported an implausibly large allogeneic blood transfusion effect and observed an extremely low postoperative infection rate among the patients who received WBC-reduced RBCs. In their 1996 study, Jensen et al5 detected 0 postoperative wound infections and intra-abdominal abscesses among 118 patients sick enough to need perioperative transfusion with WBC-reduced RBCs. If this finding were not due to the effect(s) of bias and confounding, it would implicate the TRIM effect as the sole cause of postoperative wound infections, because the incidence of postoperative wound infections and intra-abdominal abscesses among the 142 patients who had transfusion with buffy-coat–reduced RBCs in that trial was 12%.5Jensen et al3,5 did not present information on some important potential confounding variables (eg, Dukes stage of tumor and tumor fixation to adjacent organs), allowing one to speculate that the observed large TRIM effect (Figure 4) could be due to the effects of uncontrolled confounding factors.24,25 In addition, selection bias and observation bias are possible in RCTs despite the randomized design and the blinding of investigators, respectively.94,95 In the RCTs of Jensen et al,3,5 sicker patients may have been systematically allocated to the treatment arm, and/or investigators who became aware of the treatment allocations may have been inclined to diagnose postoperative infection more often in patients from the treatment arm. Both Vamvakas24 and McAlister et al25 excluded the RCTs of Jensen et al3,5 from their meta-analyses24,25 because of concerns about bias and residual, uncontrolled confounding factors in those studies.3 5
Jensen et al3,5 published 2 of the 3 studies3,5,7 that enrolled a mixed population of patients having abdominal surgery for either benign or malignant disease. In addition, 222,23 of the 34,22,23 studies that enrolled a homogeneous population of patients having colorectal cancer resection were multicenter. Furthermore, 222,23 of the 33,22,23 studies in which 50% to 67% of the enrolled patients had transfusion involved multiple centers. The influence of these 2 study attributes (ie, homogeneity of the enrolled patient population and overall percentage of transfusion) on the reported results is probably due to the distribution of the 2 multicenter RCTs22,23 and the 2 trials by Jensen et al3 5among the categories formed when these 2 study attributes are considered (Table 4).
To explain the disagreements among the 7 RCTs (Figure 4), Blajchman11 and Vamvakas and Blajchman96proposed a meta-analysis of the 7 studies using individual patient data (IPD).97 The proposed project96 would require recoding of the raw data collected prospectively by the authors of the 7 RCTs with a common patient data form, as well as the collection of additional data through a retrospective review of the medical records of the patients who were enrolled in the 7 studies.3-7,22,23 The additional information needed to explain the disagreements among the studies relates primarily to the prevalence of risk factors for postoperative infection, as well as the criteria used to reach each diagnosis of postoperative infection in the 7 RCTs. If information on these variables were obtained, it would highlight any differences in severity of illness or application of the diagnostic criteria for postoperative infection between the treatment and control arms of the studies, thereby allowing meta-analysts to assess the possible effects of bias and confounding in the 7 RCTs. In addition, the variation in the postoperative infection rate among the studies (Table 1) could be removed, or attributed to a differential distribution of risk factors for postoperative infection among the RCTs; and the data from the 2 multicenter RCTs22,23 could be stratified by participating hospital to remove the influence of the “center effect.” If these postulated sources of variation accounted for the disagreements among the studies, a summary estimate of the TRIM effect (or of the benefit from the use of autologous and/or WBC-reduced RBCs) across the 7 RCTs could be calculated by the techniques of meta-analysis.97
Because the results of an IPD meta-analysis of all published RCTs3-7,22,23 are not available, the results of the study of van de Watering et al6 may be the most reliable existing data regarding the potential benefit from the use of WBC-reduced blood components for the prevention of postoperative infection.27 The study of van de Watering et al6 was well designed and analyzed and enrolled a large number of patients, almost all of whom received transfusion. Furthermore, observers who were unaware of patient assignment made the diagnoses of postoperative infection on the basis of the detailed criteria of the Centers for Disease Control and Prevention.98 All of these conditions together were not met by any of the other RCTs shown in Table 3.
Van de Watering et al6 randomized 914 patients scheduled to have CABG surgery, cardiac valve surgery, or a combination of the 2 to one of 3 treatment arms: buffy-coat–reduced allogeneic RBCs; WBC-reduced, buffy-coat–reduced allogeneic RBCs filtered before storage; and WBC-reduced, buffy-coat–reduced allogeneic RBCs filtered after storage. The intention-to-treat analysis compared the incidence of postoperative infection among these 3 arms and found no significant difference (P = .13); the incidence of postoperative infection was 23%, 16.9%, and 17.9%, respectively, in each arm. However, when the prestorage and poststorage WBC-reduced arms were combined, recipients of buffy-coat–reduced RBCs had a higher incidence of postoperative infection than patients receiving WBC-reduced, buffy-coat–reduced RBCs, and this difference was marginally significant (P = .06; Figure 4).
Postoperative mortality
In addition to a possible association between allogeneic transfusion and postoperative infection (Figure 4), van de Watering et al6 detected an unexpected association between WBC-containing allogeneic blood transfusion and postoperative mortality from causes other than postoperative infection. Twenty-four of 306 patients (7.8%) having transfusion with buffy-coat–reduced RBCs died, compared with 11 of 305 patients (3.6%) receiving buffy-coat–reduced RBCs that were WBC reduced before storage and 10 of 303 patients (3.3%) receiving buffy-coat–reduced RBCs that were WBC reduced after storage (P = .015). This overall difference in 60-day mortality was due to a highly significant (P = .001) difference among the 3 randomization arms in the proportion of patients who died of noncardiac causes, that is, multiorgan failure or dehiscence of their aortic bypass anastomosis (3.3% of patients receiving buffy-coat–reduced RBCs, as compared with 0.3% of patients receiving buffy-coat–reduced RBCs that were WBC reduced either before or after storage). The proportions of patients who died of cardiac causes (ie, myocardial infarction, heart failure, or arrhythmia) did not differ among the 3 arms (P = .53).
Furthermore, in an analysis of the effect of the volume of blood infused among the 866 patients who had transfusion, no difference in mortality between recipients of buffy-coat–reduced or WBC-reduced RBCs was observed in the subgroup of subjects receiving 1 to 3 U of blood (P = .82). In this subgroup of 355 patients, mortality was 1.6% for the recipients of buffy-coat–reduced units, as compared with 1.8% for the recipients of WBC-reduced units filtered after storage and 0.9% for the recipients of WBC-reduced units filtered before storage. In contrast, in the subgroup of 511 patients who received 4 U or more of RBCs, there was a statistically significant (P = .014) association between mortality and WBC-containing allogeneic blood transfusion across the 3 arms. When the recipients of WBC-reduced RBCs filtered before or after storage were combined, the postoperative mortality rate of recipients of buffy-coat–reduced RBCs was 12.5%, as compared with 5.1% for the recipients of WBC-reduced, buffy-coat–reduced RBCs (P = .005).
Van de Watering et al6,99 pointed out that their study had not been designed to investigate postoperative mortality as an outcome variable. For this reason, prognostic factors for mortality had not been measured during the trial, and randomization might (or might not) have distributed such prognostic variables equally among the 3 arms. Accordingly, using multivariate regression analysis, van de Watering et al6 adjusted the observed difference across the randomization arms for the effects of measured prognostic factors for mortality. All variables that demonstrated a univariate association (P < .05) with postoperative mortality were entered into this multivariate regression model. Randomization to the buffy-coat–reduced RBC arm was associated with significantly increased mortality (P = .012) after adjustment for the effects of type of operation, age, history of myocardial infarction, previous open heart surgery, preoperative platelet count, and gender. When the number of RBC units transfused was entered into the regression model, it was the most significant (P < .001) predictor of postoperative mortality, while randomization to the buffy-coat– reduced RBC arm still exercised a significant (P = .009) effect on mortality.
Van de Watering et al6 concluded that when 4 U or more of allogeneic buffy-coat–reduced RBCs are transfused in cardiac surgery, WBC reduction of the transfused units results in significantly decreased mortality. However, recognizing that this finding was novel and unexpected, and that a recommendation for using only WBC-reduced blood components in cardiac surgery would significantly increase the cost of transfusion, these investigators urged that further research be conducted to replicate their finding before such a recommendation was made.6
Van de Watering et al postulated that their study6 was more likely than previous RCTs3-5,7,22,23 to detect differences among the randomization arms because the transfusion dose administered to their cardiac surgery patients was substantially higher than that given to patients having colorectal surgery in the previously published trials. Only 48 (5.2%) of these cardiac surgery patients6received no RBC transfusion; 777 (85.0%) received 1 to 10 U of RBCs each (mean, 5.5 U; median, 4.0 U) and 89 (9.7%) received more than 10 U each (mean, 16.9 U; median, 14.0 U). To further investigate a possible relation between WBC-containing allogeneic blood transfusion and an increased risk of death or multiorgan failure in patients receiving high transfusion doses perioperatively, investigators from the Leiden University Medical Center initiated an RCT enrolling subjects admitted for resection of aortic aneurysm or gastrointestinal malignancy. The purpose of the new study is to determine whether the previously detected6 associations between WBC-containing allogeneic blood transfusion and increased risk of death or multiorgan failure represent transfusion-associated complications (TAC) or transfusion-induced complications (TICS); thus, the acronym TACTICS is used to refer to this new study.99 To optimize the design of this large, double-blind, multicenter trial, Brand et al100 conducted a pilot study enrolling 73 patients with aortic aneurysm and 84 patients with abdominal malignancy. No statistically significant differences were observed in the pilot study. Ten percent of the recipients of buffy-coat–reduced RBCs died, as compared with 7.8% of the recipients of WBC-reduced RBCs; approximately 20% of the patients in either randomization arm developed multiorgan failure.100
Information on 30-day mortality was available for 1806 patients enrolled in the 3 RCTs conducted at the Leiden University Medical Center (ie, the RCTs of Houbiers et al22 and van de Watering et al6 and the pilot component of TACTICS), and an interim analysis based on the accumulated experience from these patients has been presented.99 100 In the univariate analysis, an increased mortality risk in recipients of buffy-coat–reduced (compared with WBC-reduced) RBCs was observed only among recipients of 4 U or more of RBCs. In a multivariate regression analysis, randomization to the WBC-reduced (as compared with the buffy-coat–reduced) arm was associated with a statistically significant decrease in postoperative mortality after adjustment for the effects of age, gender, and transfusion dose. However, because prognostic factors for mortality had not been considered in the design of the 3 combined studies, there may exist other prognostic variables that could be unequally distributed between the buffy-coat–reduced and WBC-reduced arms and that could account for the observed difference in mortality between the arms.
The association between WBC-containing allogeneic blood transfusion and increased mortality6 may have limited applicability because data obtained from patients undergoing cardiac surgery may not be generalizable to other clinical settings. The extracorporeal circuit used in cardiac surgery induces a diffuse inflammatory response that may predispose to postoperative infection or other surgical complications.101 It is possible that WBC reduction of transfused blood components may be capable of suppressing the diffuse inflammatory response to the extracorporeal circuit and may thus indirectly reduce the incidence of postoperative infection or the incidence of other postoperative complications.101 If this were the mechanism of the beneficial effect observed by van de Watering et al,6 the same benefit from the use of WBC-reduced blood components would probably not be expected to occur in other surgical settings.
The mechanism of the reported association6 between WBC-containing allogeneic blood transfusion and increased postoperative mortality from causes other than postoperative infection is unclear. If such a relationship truly exists, its mechanism may (or may not) be related to transfusion-induced immunomodulation. It is possible that, among recipients of multiple transfusions, allogeneic transfusion may exercise other deleterious effects that have not yet been recognized or defined, probably because of the confounding effect of the association between transfusion and illness severity (which becomes extremely strong in patients receiving multiple transfusions). Associations between allogeneic transfusion and various adverse clinical outcomes (eg, prolonged mechanical ventilation,102,103 impaired wound healing,104 or multiorgan failure105-107) have been reported from retrospective cohort studies, but it remains uncertain whether these associations reflect true relationships because it has been impossible to separate the effects of the allogeneic transfusions from the effects of confounding factors.
Hébert et al52 reported that a restrictive strategy of (non–WBC-reduced) RBC transfusion may be superior to a liberal transfusion strategy in critically ill patients with normovolemia. Thirty-day mortality was 18.7% in patients having transfusion when their hemoglobin concentration fell to 7 g/dL, as compared with 23.3% in subjects having transfusion when their hemoglobin concentration fell to 10 g/dL (P = .11). In subgroup analyses, patients who were less acutely ill (with an Acute Physiology and Chronic Health Evaluation II score of ≤ 20) and patients who were younger than 55 years experienced a statistically significant survival benefit by having been allocated to the restrictive-strategy arm of the study. Among the less acutely ill patients, 30-day mortality was 8.7% in the restrictive-strategy arm and 16.1% in the liberal-strategy arm (P = .03). Among the patients who were younger than 55 years, 30-day mortality was 5.7% with the restrictive strategy and 13.0% with the liberal strategy (P = .02).
As with the finding of a reduced 60-day mortality rate among the recipients of WBC-reduced RBCs reported by van de Watering et al,6 the finding of a trend (P = .11) toward reduced 30-day mortality in the restrictive-strategy arm of the RCT of Hébert et al52 was unexpected. There were no differences between the arms in the rates of cardiac events, infectious complications, multiorgan failure of more than 3 organs, or multiorgan failure in the 48 hours before death. The harm caused by the liberal use of transfusions in critically ill patients was thus difficult to explain.108 It is possible that the overall trend observed by Hébert et al52 was due to chance because a statistically significant difference between the restrictive- and liberal-strategy arms could be demonstrated only in subgroup analyses. Alternatively, it is possible that allogeneic transfusion may exercise other deleterious effects that have not yet been recognized or defined. More research is needed to elucidate the possible biologic mechanism(s) that may underlie such other adverse effects of allogeneic transfusion.27
Conclusions
A causal relationship between allogeneic blood transfusion and cancer recurrence or postoperative infection has not been proven by double-blind RCTs in accordance with the tenets of evidence-based medicine.109 The available RCTs2,21,22 provide no indication that perioperative allogeneic blood transfusion causes an increase in cancer-related complications, whether cancer recurrence or death due to cancer recurrence is considered the outcome variable. The OR of an adverse outcome in the allogeneic blood transfusion (compared with the control) arm is approximately 1. In the case of postoperative infection, the results of the available RCTs3-7,22 23 are highly contradictory, and they cannot be combined by the techniques of meta-analysis to generate an estimate of the TRIM effect based on all available data.
Observational studies comparing recipients of similar volumes of autologous or allogeneic blood indicate an increased risk of postoperative infection in association with allogeneic transfusion (Figure 2). However, it is impossible to determine from the published results whether some portion of this excess risk seen in association with allogeneic transfusion would persist if the effects of selection bias, observation bias, and confounding factors were to be removed completely. Observational studies comparing patients with and without transfusion, and using multivariate analysis to adjust for the effects of confounding factors, almost uniformly support the hypothesis of an increased risk of postoperative infection in the transfusion group.71,72,74-77,79 However, in most of these studies, the reported allogeneic transfusion effects were not adjusted for the effects of illness severity and risk factors for postoperative infection at specific sites.19 61
Reported RCTs investigating the association of perioperative allogeneic blood transfusion with postoperative infection were either unblinded or single-blind (Table 1), and no double-blind RCTs have been reported. Because the diagnosis of nosocomial infections is subjective, observation bias is an important concern, and it may represent an alternative explanation for the findings of some studies and for disagreements among the reported RCTs.110 Furthermore, the reports of the available RCTs3-7,22,23 did not present sufficient information about illness severity or the distribution of risk factors for postoperative infection in the treatment versus control arms.96 In the absence of this information, the possible contributions of selection bias, observation bias, and confounding factors to the reported results cannot be evaluated, and therefore cannot be excluded.
A possible explanation for the disagreements among the 7 RCTs3-7,22,23 may be that the adverse TRIM effect is small: for example, a 5% to 10% increase in the risk of postoperative infection in association with allogeneic blood transfusion. Such a small effect would not be detected consistently, and its detection would be highly dependent on the size and the design of each study. To detect a 10% difference in the risk of postoperative infection between treatment and control arms of equal size, 3000 patients would have to be randomized to each arm if the overall infection rate in the entire study population were 35%.24 A study population of 20 000 patients would be required if the overall infection rate were approximately 20% and if almost half of the enrolled patients did not receive transfusion. Therefore, the available data from the 3203 patients enrolled in the 7 published RCTs3-7,21,22 are grossly insufficient for the investigation of an adverse TRIM effect of such small magnitude. In fact, it is unlikely that a definitive RCT capable of detecting such a small effect will ever be conducted. To reach a conclusion about the existence and magnitude of any increase in the risk of postoperative infection that is caused by allogeneic transfusion, decision makers will probably have to rely on cumulative meta-analyses of the data from all available RCTs, and the excessive variation in the findings of the published reports3-7,21,22will have to be accounted for to conduct such meta-analyses.11 96
As shown in Table 4, it is impossible to reconcile the findings of all the available RCTs3-7,22,23 by relying only on the published information. The crux of the disagreements involves the radically different results reported by the team of Jensen et al3,5 and the 2 multicenter RCTs.22,23 To permit a meta-analytic synthesis of the published findings, either the studies of Jensen et al3,5 or the 2 multicenter RCTs22,23 have to be excluded from the analysis.92 In the absence of objective evidence of bias or confounding, any exclusions are arbitrary, and they bias the conclusions of the meta-analysis either in the direction of a null result or in the direction of a large (and likely spurious) deleterious TRIM effect (Table 4). A reanalysis of the raw data on the individual patients enrolled in the 7 RCTs3-7,22,23 and collection of additional information on these subjects are needed11 96 to reconcile the published results and to reach a conclusion about the existence of a deleterious TRIM effect based on all available data from clinical experimental studies.
The question currently debated at the national level is the issue of universal WBC reduction. If TRIM really causes deleterious effects in recipients of blood products, these likely affect many (or potentially all) patients having transfusion. Therefore, it would be logical to implement measures to prevent these immunomodulatory effects of allogeneic blood transfusion. Because most evidence from studies in experimental animals implicates allogeneic WBCs in the production of the deleterious TRIM effects,30,37-43 and because universal WBC reduction of all transfused cellular blood components has been implemented in some western European countries and in Canada,50 51 policy makers in the United States and elsewhere are currently considering the question of implementing universal WBC reduction for the purpose of preventing the various possible deleterious TRIM effects.
As shown in Table 4, when the 5 RCTs that transfused WBC-reduced allogeneic RBCs5-7,22 or whole blood3 to the control arm were considered together, the results were heterogeneous (P < .001 for the Q test statistic) and could not be combined. Therefore, the existing evidence regarding the efficacy of WBC reduction in preventing the deleterious TRIM effects is conflicting. If standards similar to those used by the FDA's new-drug evaluation process were applied to transfusion safety, the only appropriate recommendation at this time would be that further double-blind RCTs be conducted to establish the existence of any adverse TRIM effects mediated by transfused allogeneic WBCs, as well as the efficacy of WBC reduction in preventing these effects.
Decisions made in the 1990s,111-113 however, indicate a willingness on the part of policy makers to use a substantially different threshold for judging the efficacy of measures that affect transfusion safety, compared with the criteria used for the evaluation of other therapeutic interventions. Because of these precedents,111-113 the question now is whether universal WBC reduction should be implemented in the United States and elsewhere at approximately the same time that this practice is introduced in western Europe and Canada.
Should universal WBC reduction be introduced now, or should we wait for definitive, large, double-blind RCTs to establish both the existence of deleterious TRIM effects and the efficacy of WBC reduction in abrogating these effects? Table 5 looks at this question from a public policy (as opposed to an evidence-based medicine109) perspective and summarizes the arguments for and against a policy to implement universal WBC reduction for the prevention of the adverse TRIM effects, based on the current state of knowledge.26,27 All “pro” arguments are logical, and they are countered by equally reasonable “con” arguments. Therefore, any decision at this time to implement (or not to implement) universal WBC reduction in the United States for the purpose of preventing the purported deleterious TRIM effects will have to be arbitrary. One can argue that a policy decision should be made on the basis of the existing evidence because better evidence is unlikely to be forthcoming.26 Alternatively, one can argue that no policy decision should be made until it is possible to make such a decision based on sound scientific evidence.27
Arguments for (pro) and against (con) a policy to implement universal white cell reduction of all transfused cellular blood components for the purpose of preventing postoperative infection caused by the purported deleterious immunomodulatory effects of allogeneic blood transfusion
| Pro26 27 . | Con27 . |
|---|---|
| 1. An increase in the incidence of postoperative infection attributable to an immunomodulatory effect of allogeneic WBCs is supported by at least one single-blind and correctly designed and analyzed RCT conducted by van de Watering et al.6 | 1a. The RCT of van de Watering et al6 did not detect a TRIM effect in the intention-to-treat analysis; only when the 2 control (WBC-reduced) arms were combined was a marginally significant (P = .06) difference demonstrated between the recipients of buffy-coat–reduced and WBC-reduced RBCs. 1b. The RCT of van de Watering et al6 enrolled patients having open heart surgery, and an allogeneic blood transfusion effect detected in the setting of cardiac surgery may not apply to other surgical settings. |
| 2. Four3,5-7 of 5 RCTs3,5-7 22investigating the relationship between WBC-containing (versus non–WBC-containing) allogeneic blood transfusion and postoperative infection show at least a trend toward an increase in the incidence of postoperative bacterial infection in association with the transfusion of allogeneic WBCs. | 2. The 2 RCTs by Jensen et al35 reported an implausibly large allogeneic blood transfusion effect, and, if these 2 studies35 were excluded from the analysis, the OR of postoperative infection in the allogeneic transfusion (compared with the control) arm across the remaining RCTs6,7 22 would no longer be statistically significant and would be sufficiently modest to be attributed to chance.5-150 |
| 3. It is unlikely that further RCTs examining the relationship between allogeneic blood transfusion and postoperative infection will be reported in the foreseeable future. Even if more studies are conducted in the future, it is unlikely that such future RCTs will be large enough to be definitive. | 3. If a policy decision is made to implement universal WBC reduction based on the evidence that is currently available, it will be impossible to rescind this policy in the future if further studies establish that universal WBC reduction does not decrease the incidence of postoperative infection. |
| 4. In the future, research into the purported deleterious immunomodulatory effects of allogeneic blood transfusion can continue, by means of observational studies comparing the incidence of postoperative infection between patients having transfusion before or after the implementation of universal WBC reduction. | 4. Such future observational studies are bound to have at least some of the flaws of their predecessors,19 making it impossible to attribute any observed difference between patients having transfusion before or after the implementation of universal WBC reduction to the receipt of WBC-containing allogeneic blood transfusion. |
| 5. The receipt of WBC-containing allogeneic blood transfusion is associated with increased postoperative mortality in the RCT of van de Watering et al.6 | 5a. The RCT of van de Watering et al6 was not designed to investigate mortality as an outcome variable. 5b. The association between WBC-containing allogeneic blood transfusion and increased postoperative mortality reported by van de Watering et al6 has not been corroborated by another RCT. |
| Pro26 27 . | Con27 . |
|---|---|
| 1. An increase in the incidence of postoperative infection attributable to an immunomodulatory effect of allogeneic WBCs is supported by at least one single-blind and correctly designed and analyzed RCT conducted by van de Watering et al.6 | 1a. The RCT of van de Watering et al6 did not detect a TRIM effect in the intention-to-treat analysis; only when the 2 control (WBC-reduced) arms were combined was a marginally significant (P = .06) difference demonstrated between the recipients of buffy-coat–reduced and WBC-reduced RBCs. 1b. The RCT of van de Watering et al6 enrolled patients having open heart surgery, and an allogeneic blood transfusion effect detected in the setting of cardiac surgery may not apply to other surgical settings. |
| 2. Four3,5-7 of 5 RCTs3,5-7 22investigating the relationship between WBC-containing (versus non–WBC-containing) allogeneic blood transfusion and postoperative infection show at least a trend toward an increase in the incidence of postoperative bacterial infection in association with the transfusion of allogeneic WBCs. | 2. The 2 RCTs by Jensen et al35 reported an implausibly large allogeneic blood transfusion effect, and, if these 2 studies35 were excluded from the analysis, the OR of postoperative infection in the allogeneic transfusion (compared with the control) arm across the remaining RCTs6,7 22 would no longer be statistically significant and would be sufficiently modest to be attributed to chance.5-150 |
| 3. It is unlikely that further RCTs examining the relationship between allogeneic blood transfusion and postoperative infection will be reported in the foreseeable future. Even if more studies are conducted in the future, it is unlikely that such future RCTs will be large enough to be definitive. | 3. If a policy decision is made to implement universal WBC reduction based on the evidence that is currently available, it will be impossible to rescind this policy in the future if further studies establish that universal WBC reduction does not decrease the incidence of postoperative infection. |
| 4. In the future, research into the purported deleterious immunomodulatory effects of allogeneic blood transfusion can continue, by means of observational studies comparing the incidence of postoperative infection between patients having transfusion before or after the implementation of universal WBC reduction. | 4. Such future observational studies are bound to have at least some of the flaws of their predecessors,19 making it impossible to attribute any observed difference between patients having transfusion before or after the implementation of universal WBC reduction to the receipt of WBC-containing allogeneic blood transfusion. |
| 5. The receipt of WBC-containing allogeneic blood transfusion is associated with increased postoperative mortality in the RCT of van de Watering et al.6 | 5a. The RCT of van de Watering et al6 was not designed to investigate mortality as an outcome variable. 5b. The association between WBC-containing allogeneic blood transfusion and increased postoperative mortality reported by van de Watering et al6 has not been corroborated by another RCT. |
WBC indicates white blood cell; RCT, randomized controlled trial; TRIM, allogeneic blood transfusion–associated immunomodulation; RBC, red blood cell; OR, odds ratio.
The OR of postoperative infection in recipients of buffy-coat–reduced6,22 or standard7 RBCs, as compared with recipients of WBC-reduced RBCs, was 1.20 (95% confidence interval for the OR, 0.78-1.85; P > .05). These 3 studies6,7 22 are sufficiently homogeneous to permit meta-analysis (P > .05 for the Q test statistic).
Regardless of the content of the public policy debate, should universal WBC reduction be implemented, the question as to whether WBC-containing allogeneic blood transfusion causes deleterious TRIM effects will remain. Therefore, should a decision to introduce universal WBC reduction be made in the near future, research into the purported deleterious TRIM effects should continue. Double-blind RCTs should be conducted in countries that do not implement universal WBC reduction; observational studies comparing the incidence of postoperative infection and/or cancer recurrence before and after the implementation of universal WBC reduction should be conducted in jurisdictions that convert to a 100% WBC-reduced blood supply.26 27
In summary, definitive evidence regarding the existence of a deleterious immunomodulatory effect of allogeneic blood transfusion, causing an increased incidence of cancer recurrence and/or postoperative bacterial infection in humans, has not been presented. However, the existing data from the various clinical studies and studies in experimental animals, as well as the data on possible beneficial immunomodulatory allogeneic transfusion effects,12,114 justify a high degree of suspicion that an adverse TRIM effect probably does exist. This effect may be small, perhaps representing less than a 10% increase in the risk of postoperative infection. Because of the small magnitude of the effect and the strong relationship between the need for transfusion and illness severity, it may have been difficult to document the existence of the TRIM effect after adjusting appropriately for the effects of confounding factors. However, a risk as small as a 10% increase in the risk of postoperative infection in association with WBC-containing allogeneic blood transfusion—if it really exists—represents a clinically important complication of transfusion that ought to be prevented, if possible, in all patients by introducing universal WBC reduction. A decision whether to implement such a policy in the United States on the basis of the currently available evidence will probably be made soon, and will likely be based, in part, on political and societal considerations.27,50 51 Depending on which policy decision is made, appropriately designed clinical studies should be conducted to either establish the existence of any deleterious immunomodulatory effect(s) of allogeneic blood transfusion or document that the TRIM effect(s) is not clinically significant.
References
Author notes
Eleftherios C. Vamvakas, Blood Bank, RRG 17, New York University Medical Center, 400 East 34th St, New York, NY 10016; e-mail: stephen.vamvakas@med.nyu.edu.

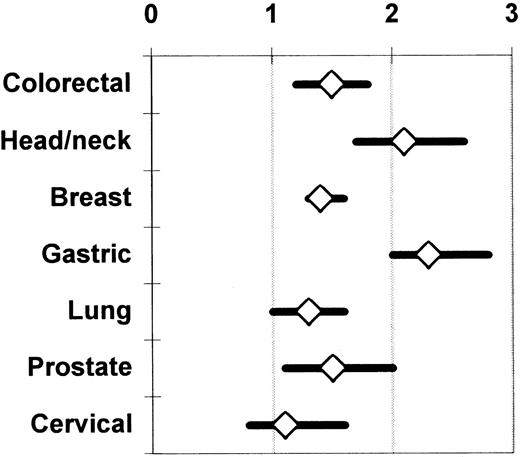
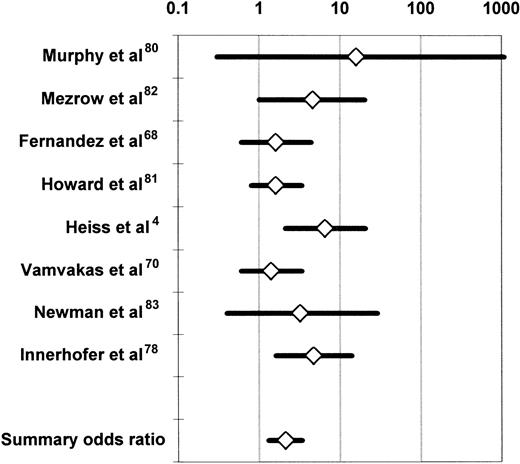
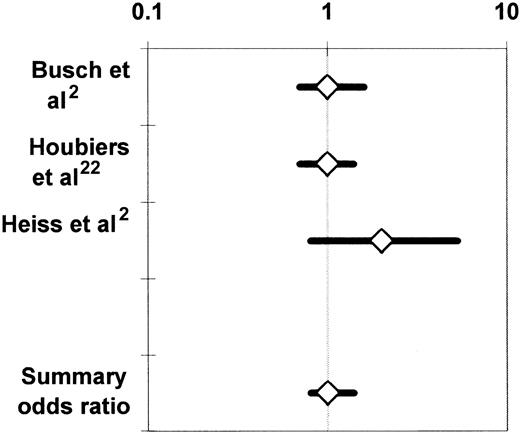
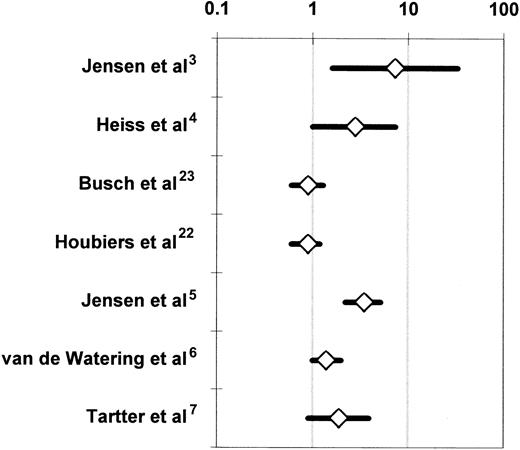
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal