Abstract
A congenital dysfibrinogenemia, fibrinogenNieuwegein, was discovered in a young man without any thromboembolic complications or bleeding. A homozygous insertion of a single nucleotide (C) in codon Aα 453 (Pro) introduced a stop codon at position 454, which resulted in the deletion of the carboxyl-terminal segment Aα 454-610. The ensuing unpaired cysteine at Aα 442 generated fibrinogen-albumin complexes of different molecular weights. The molecular abnormalities of fibrinogenNieuwegein led to a delayed clotting and a fibrin network with a low turbidity. Electron microscopy confirmed that thin fibrin bundles were organized in a fine network. The use of fibrinogenNieuwegein-derived fibrin (fibrinNieuwegein) in an in vitro angiogenesis model resulted in a strong reduction of tube formation. The ingrowth of human microvascular endothelial cells (hMVEC) was independent of αvβ3, indicating that the reduced ingrowth is not due to the absence of the RGD-adhesion site at position Aα 572-574. Rather, the altered structure of fibrinNieuwegeinis the cause, since partial normalization of the fibrin network by lowering the pH during polymerization resulted in an increased tube formation. Whereas factor XIIIa further decreased the ingrowth of hMVEC in fibrinNieuwegein, tissue transglutaminase (TG), which is released in areas of vessel injury, did not. This is in line with the absence of the cross-linking site for TG in the α-chains of fibrinogenNieuwegein. In conclusion, this newly discovered congenital dysfibrinogenemia has a delayed clotting time and leads to the formation of an altered fibrin structure, which could not be cross-linked by TG and which is less supportive for ingrowth of endothelial cells.
Introduction
Fibrinogen is a 340-kd glycoprotein that is present in the blood at a concentration of approximately 3 mg/mL.1It is composed of 6 polypeptide chains (α, β, γ)2that are linked together by disulfide bonds and are organized in a symmetrical dimeric fashion.2 During blood coagulation thrombin converts fibrinogen to fibrin monomers, which associate into staggered, overlapping, 2-stranded fibrils. These 2-stranded fibrils laterally associate to form fibrin bundles.3 The final step in the formation of a 3-dimensional fibrin network is the cross-linking of the fibrin subunits by formation of covalent intermolecular isopeptide bonds. This reaction is catalyzed by factor XIIIa, which in the presence of Ca++ links α- and γ-chains of 2 adjacent fibrinogen molecules,4,5 while cell-derived tissue transglutaminase (TG) mainly links 2 α-chains.6 Apart from its role in hemostasis, the fibrin formed also provides a temporary matrix for cell invasion during the subsequent healing process. Formation of new blood vessels, or angiogenesis, is an important component of tissue repair after injury.7,8 Endothelial cells degrade the fibrin matrix locally, migrate into it, proliferate, and form capillary tubes.9 This process not only requires proteases for cell detachment, invasion and lumen formation, but also new cell-matrix interactions for reattachment of the invading cells. Different receptors, including integrins, are involved in endothelial cell-matrix interactions during migration and subsequent reattachment.10
The structure of the fibrin network present in the fibrinous exudate is an important determinant in the extent of capillary tube formation.11-13 The architecture of the fibrin network and its fibrinolytic sensitivity are determined by the rate of polymerization and its extent of cross-linking.14-17 The carboxyl-terminal region of the Aα-chain of fibrinogen determines the thickness of bundles formed, and as such the structure of the fibrin network.18-21 Thin fibrin bundles will associate into a tight and rigid network, whereas thick bundles associate into a porous and malleable network.22
Several endothelial cell receptors interact with different domains in the fibrinogen molecule.23-25 Integrins are heterodimers, composed of an α- and a β-subunit, which bind to the RGD sequence present in extracellular matrix proteins.26 The αvβ3-integrin is a promiscuous receptor, binding a wide variety of extracellular matrix proteins as well as fibrinogen.27 The RGD-sequence at position A(572-574) of fibrinogen is involved in adhesion of the cell surface-localized endothelial αvβ3-integrin receptor.28 The expression of this receptor on endothelial cells is tightly regulated: it is only minimally expressed on resting endothelial cells, but highly up-regulated during angiogenesis in vivo.29,30 Inhibitors of αvβ3-integrin31 32 can block angiogenesis in various conditions, suggesting an important role for this receptor.
Congenital dysfibrinogenemias, fibrinogens with structural or functional defects, have proven not only to be valuable tools to study structure-function relationships in the fibrinogen molecule,33 but also to investigate interactions of various cells with fibrinogen and fibrin.34 In the present study, we characterized a new congenital dysfibrinogenemia, fibrinogenNieuwegein, with a truncated Aα-chain, lacking the adhesion site for endothelial αvβ3-integrin on the Aα-chain as well as the cross-linking site for TG. The effects of these alterations on endothelial capillary tube formation were studied in an in vitro angiogenesis model using human microvascular endothelial cells (hMVEC) overlaid on fibrin matrices.
Materials and methods
Coagulationstudies
Blood was collected by venipuncture and anticoagulated with 1/10 volume of 0.106 M trisodium citrate. Platelet-free plasma was prepared by centrifugation for 30 minutes at 4°C at 2300g. The concentration of fibrinogen in plasma was determined by a Rocket electrophoresis using Dade-Behring antiserum. Coagulation time was determined by the Clauss thrombin-method.35
Purification of fibrinogen
Fibrinogen was purified from the plasma using β-alanine as described by Straughn and Wagner.36 β-Alanine was added to plasma in a final concentration of 0.5 M and kept on ice for 3 minutes. After removal of the precipitate by centrifugation at 9000g for 15 minutes, the concentration of β-alanine in the supernatant was raised to 1.5 M, and the mixture was kept on ice for another 30 minutes. Subsequently, fibrinogen was collected by centrifugation at 9000g for 15 minutes. Residual fibrinogen was precipitated from the plasma by raising the β-alanine concentration to 3 M and collected by centrifugation after 30 minutes of incubation on ice. The fibrinogen pellets were dissolved in phosphate-buffered saline (PBS, pH 7.4) and dialyzed against PBS. The concentration of the fibrinogen solution was adjusted to 3 mg/mL for subsequent use.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of plasma and immunoblot analysis
Fibrin monomers were prepared by clotting plasma with Reptilase. After washing of the clot with PBS, fibrin monomers were dissolved in 0.025 M acetic acid. Fibrin monomers were reduced with 7% wt/vol β-mercaptoethanol and subjected to electrophoresis on a 0.1% sodium dodecyl sulfate (SDS), 10% wt/vol polyacrylamide gel (PAGE) (Biorad, Hercules, CA) according to Laemmli.37 For Western blotting, plasma or fibrin monomers were subjected to SDS-PAGE (Biorad) using 5% wt/vol or 4% to 16% wt/vol gradient gels, and proteins were semidry blotted onto a nitrocellulose filter. The blots were incubated with monoclonal antibodies (mAbs) against the amino-terminus (Y18)38 and the carboxy-terminus of the Aα-chain of fibrinogen (G8),39 a rabbit-antihuman-fibrinogen,40 or a rabbit-antihuman albumin (Nordic, Tilburg, The Netherlands). The antibodies were coupled to horseradish peroxidase and used in dilutions of 1:1000, 1:10 000, 1:3000, and 1:1000, respectively.
Fibrin structure
Turbidity of the fibrin network.
Human fibrin gels were prepared in 96-well plates by addition of 1 μL 10 U/mL thrombin (Leo Pharmaceutical Products, Weesp, The Netherlands) to 100 μL 3 mg/mL fibrinogen dialyzed against PBS. After 4 hours of polymerization, the turbidity of fibrin gels, which is dependent on fiber thickness,41 was measured at 340 nm by using a Titertek reader (Titertek Multiscan, Flows Labs, McLean, VA).
Scanning electron microscopy of fibrin.
Plasma was clotted on a formvar-coated 200-mesh nickel grid by the addition of 1 U/mL thrombin. After clotting, fibrin was washed, fixed with 2% glutaraldehyde, dehydrated with a serial dilution of ethanol, transferred in 100% acetone, and dried using a Polaron critical point drying apparatus. The samples were coated with gold and examined in a Hitachi scanning electron microscope.
DNA isolation and DNA sequencing
Genomic DNA was isolated from white blood cells42and the DNA encoding the carboxy-terminal part of the Aα-chain from amino acid 391 to 625, corresponding to exon V, was amplified by polymerase chain reaction (PCR), using as forward oligonucleotides α6A (TGGGGCACATTTGAAGAGGTGTCA) and as reverse αCD2 (GGAACTTACAGTCGACCACAAAAACAGACC). The 829-bp PCR product offibrinogen Aα-gene was sequenced by Baseclear (Leiden, The Netherlands).
Cell culture
Human foreskin microvascular endothelial cells were isolated, cultured, and characterized as previously described.43Cells were cultured until confluence at 5% CO2/95% air on fibronectin-coated dishes in M199 supplemented with 2 mMl-glutamine, 20 mM HEPES (pH 7.3) (Biowitthaker, Verviers, Belgium), 10% heat-inactivated human serum (serum pooled from 10 donors, obtained from a local blood bank), 10% heat-inactivated newborn calf serum (GIBCO BRL, Paisley, Scotland), 150 μg/mL crude endothelial cell growth factor supplement (ECGFs) (prepared from bovine brain as described by Maciag and coworkers44), 5 U/mL heparin (Leo Pharmaceutical Products), 100 IU/mL penicillin, and 100 μg/mL streptomycin (Biowitthaker). Subsequently, the endothelial cells were detached by treatment with trypsin/EDTA and transferred to fibronectin-coated dishes with a split ratio of 1:3. Confluent endothelial cells were used at passage 11.
In vitro angiogenesis
Preparation of fibrin gels.
Human fibrin gels were prepared in 96-well plates by addition of 2 μL 100 U/mL thrombin to 100 μL 3 mg/mL fibrinogen in PBS, pH 7.4. Bovine TG (Sigma Chemicals, St Louis, MO), human TG (purified from red blood cells according to Lobitz and coworkers45), or factor XIIIa (a kind gift from Dr H. Boeder and Dr P. Kappes, Centeon Pharma, Marburg, Germany) was added in different concentrations together with 5 mM CaCl2. After 4 hours of polymerization, thrombin was inactivated by equilibrating the gels for 12 hours with 0.2 mL M199 containing 10% human serum and 10% newborn calf serum. Inhibiting mAbs or peptides were added during this equilibration period to allow diffusion into the fibrin matrix. All experiments were performed with duplicate wells.
Altering the pH prior to polymerization by adding HCl (to pH 7.0) or NaOH (to pH 7.8) changes the structure of the formed fibrin network.46
In vitro angiogenesis model.
Endothelial cells were detached from the fibronectin-coated dishes with trypsin/EDTA and seeded on the fibrin matrices in a confluent density. After 24 hours the medium was replaced with medium containing different test compounds. Every 48 hours the medium was changed and collected, for a total of 4 to 6 days. Accumulation of fibrin degradation products in the conditioned media was assayed by a specific fibrin degradation enzyme-linked immunosorbent assay (ELISA; Organon Teknika, Boxtel, The Netherlands). The formation of tubular structures of endothelial cells by invasion in the underlying matrix was analyzed by phase contrast microscopy. Quantification of the length of the formed structures was performed by a computer equipped with Optimas image analysis software and a monochrome CCD camera (MX5) connected to it.47Cross-sectional analysis of the formed structures revealed that they reflected tubular structures or invaginations in the underlying fibrin matrix.12 47
Immunohistochemical analysis of the formed capillary-like tubular structures
Fibrin matrices were fixed in 4% P-formaldehyde in PBS, pH 7.4 for 2 hours at 4°C. After fixation the fibrin matrices were washed overnight with 5% sucrose in PBS and embedded in Tissue-Tek (Sakura, Zoeterwoude, The Netherlands) and snap-frozen in precooled 2-methyl butane and stored at −80°C. For immunohistochemical analysis sections 7 μm thick were made throughout the whole specimen and the sections were fixed on the slide by 4%P-formaldehyde in PBS. Incubation with the primary antibody (CD-51, a mAb against the αv-chain was used at a concentration of 1:100) was in dakodiluent (Dako, Carpinteria, CA) for 1 hour at room temperature. As secondary antibody antimouse-Cy3 (Sigma Chemicals) was used in a 1:100 dilution for a 1-hour incubation. After washing the sections were mounted with Vectashield (Vector Laboratories, Burlingame, CA).
Adhesion of endothelial cells to fibrin
Fibrin matrices were prepared in a 96-well plate. After clotting the matrices were equilibrated with M199 with 1% wt/vol human serum albumin (HSA) (CLB, Amsterdam, The Netherlands) with or without the addition of anti-αvβ3-antibodies (LM609, Chemicon, Temecula, CA) or c-RGD peptides (c-RGD, ie, cyclo(-Arg-Gly-Asp-D-Phe-Val) (Bachem United Kingdom, Saffron Walden, United Kingdom), for a period of 12 hours. The hMVEC-forming confluent monolayers were detached by trypsin/EDTA, sedimented by centrifugation at 250g for 5 minutes, and resuspended in 1% wt/vol HSA in M199 in a concentration of 50 000 cells/mL. A volume of 150 μL of the cell suspension was added to the wells and allowed to attach for 2 hours at 37°C. The wells were washed 3 times to remove unattached cells and fixed with 2% P-formaldehyde, after which adhered cells were counted. The amount of attached cells was expressed as a percentage value relative to the numbers of cells attached to normal fibrin.48
Statistics
Experiments were performed with duplicate wells and expressed as percent of control. For statistical analysis the ANOVA was used followed by a modified t test according to Bonferroni.
Results
Coagulation parameters of normal plasma and plasmaNieuwegein
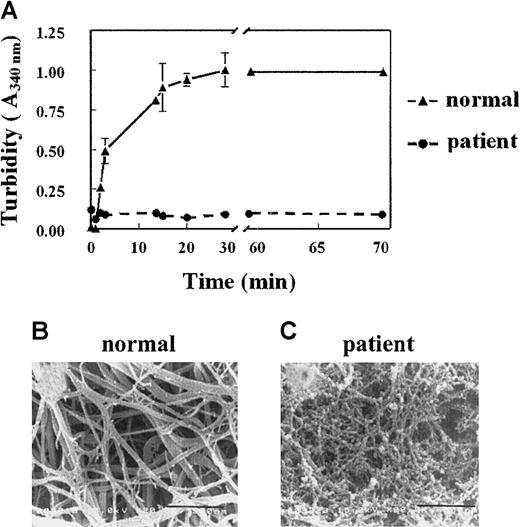
A 35-year old man showed a prolonged activated partial thrombin time, which was determined routinely prior to a biopsy procedure. The clotting time of this plasma, as determined by the Clauss assay, was prolonged (clotting times for normal plasma and patient plasma were 4.5 and 15 seconds, respectively). This delayed clotting occurred despite a normal plasma concentration of fibrinogen (1.7 mg/mL), suggesting an abnormality in the patient's fibrinogen molecule. This fibrinogen is further referred to as fibrinogenNieuwegein. When plasma was clotted by the addition of thrombin (1 U/mL), the turbidity at 340 nm increased progressively with normal plasma but not with plasmaNieuwegein (Figure 1A). Differences in turbidity were also observed when normal plasma and the patient's plasma were clotted with Reptilase or when purified fibrinogens were used (data not shown).
Fibrin structure of fibrinNieuwegein.
(A) Plasma was clotted by the addition of 1 U/mL thrombin. Turbidity at 340 nm was measured in time. Triangles represent control plasma, and circles, plasmaNieuwegein. Data are expressed as mean ± SEM of duplicate experiments with duplicate wells. (B,C) Scanning electron microscopic analysis of the formed fibrin structure from normal plasma (B) and plasmaNieuwegein (C). Bars represent 900 nM.
Fibrin structure of fibrinNieuwegein.
(A) Plasma was clotted by the addition of 1 U/mL thrombin. Turbidity at 340 nm was measured in time. Triangles represent control plasma, and circles, plasmaNieuwegein. Data are expressed as mean ± SEM of duplicate experiments with duplicate wells. (B,C) Scanning electron microscopic analysis of the formed fibrin structure from normal plasma (B) and plasmaNieuwegein (C). Bars represent 900 nM.
Determination of the fibrin structure by scanning electron microscopy
To visualize the fibrin networks, normal plasma and plasmaNieuwegein were clotted by the addition of 1 U/mL thrombin and the formed network was analyzed by scanning electron microscopy. Consistent with the turbidity data, the fibrin network formed from fibrinogenNieuwegein(fibrinNieuwegein) was tighter than the network formed from normal fibrinogen and consisted of thin fibrin fibers instead of the thick fibrin bundles of normal fibrin (Figure 1B,C).
SDS-PAGE and immunoblot analysis of fibrin monomers and plasma
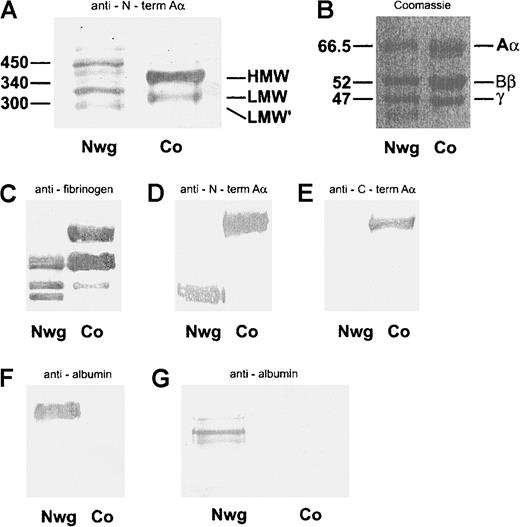
The different molecular forms of fibrinogen in plasmaNieuwegein and control plasma were analyzed under nonreducing conditions (Figure 2A). Although control plasma contained the pattern of fibrinogen molecules consistent with high-molecular-weight (HMW) fibrinogen (340 kd), low-molecular-weight (LMW) fibrinogen (300 kd), and LMW fibrinogen (270 kd) (Figure 2A, lane 2),49 50 a large heterogeneity of fibrinogen molecules with aberrant molecular masses was found in plasmaNieuwegein (Figure 2A, lane 1).
Analysis of plasma fibrin monomers.
(A) Analysis of plasma run on a nonreduced 5% SDS-PAGE gel and immunoblotted with mAb Y18/HRP, directed against the N-terminus of the Aα-chain (A). (B-F) Coomassie staining of fibrin monomers run on a reduced 10% SDS-PAGE gel (B). Immunoblots of plasma, run on a reduced 10% SDS-PAGE gel, with rabbit-antihuman fibrinogen/HRP (C), mAb Y18/HRP directed against the NH2-terminus of the Aα-chain (D), G8/HRP directed against the COOH-terminus of the Aα-chain (E). Immunoblot of fibrin monomers with rabbit-antialbumin/HRP (F). Analysis of plasma run on a nonreduced 5% SDS-PAGE gel and immunoblotted with rabbit-antihuman albumin/HRP (G). Nwg indicates fibrin(ogen)Nieuwegein, and Co, control fibrin(ogen).
Analysis of plasma fibrin monomers.
(A) Analysis of plasma run on a nonreduced 5% SDS-PAGE gel and immunoblotted with mAb Y18/HRP, directed against the N-terminus of the Aα-chain (A). (B-F) Coomassie staining of fibrin monomers run on a reduced 10% SDS-PAGE gel (B). Immunoblots of plasma, run on a reduced 10% SDS-PAGE gel, with rabbit-antihuman fibrinogen/HRP (C), mAb Y18/HRP directed against the NH2-terminus of the Aα-chain (D), G8/HRP directed against the COOH-terminus of the Aα-chain (E). Immunoblot of fibrin monomers with rabbit-antialbumin/HRP (F). Analysis of plasma run on a nonreduced 5% SDS-PAGE gel and immunoblotted with rabbit-antihuman albumin/HRP (G). Nwg indicates fibrin(ogen)Nieuwegein, and Co, control fibrin(ogen).
To further evaluate the nature of the altered structure of the fibrinogenNieuwegein molecule, the individual fibrinogen chains were characterized. To this end, fibrin monomers were derived from plasmaNieuwegein by Reptilase treatment, and subsequently analyzed by SDS-PAGE under reducing conditions. Whereas in control plasma 3 bands corresponding to the Aα-, Bβ- and γ-chain were found (Figure 2B, lane 2), an additional band was observed for fibrinNieuwegein (Figure 2B, lane 1). Immunoblotting of plasma run on a 5% SDS-PAGE with rabbit-antihuman fibrinogen showed a signal for the Aα-, Bβ-, and γ-chain in the normal fibrinogen, and for the Bβ- and γ-chain and an additional band with an apparent mass of 46 kd in fibrinogenNieuwegein (Figure 2C). Y18, which specifically recognizes the amino-terminal part of the Aα-chain, reacted with the Aα-chain in normal fibrinogen and with the 46-kd band of fibrinogenNieuwegein, suggesting that this 46-kd band corresponds to a shortened Aα-chain (Figure2D). This was confirmed by the use of G8, which recognizes the carboxyl-terminal part of the Aα-chain; whereas this G8 reacts with the Aα-chain in normal fibrinogen, no signal was observed with fibrinogenNieuwegein (Figure 2E). These results indicate that the carboxyl-terminal part of the Aα-chain is lacking in fibrinogenNieuwegein. The 66-kd band at the Aα-chain position of fibrinogenNieuwegein was at the height of albumin and found to cross-react with rabbit-antihuman albumin, demonstrating that this band predominantly represents albumin (Figure2F, lane 1). Indeed, immunoblotting of plasmaNieuwegeinwith rabbit-antihuman albumin revealed 3 different bands (Figure 2G), whereas no bands were observed for normal plasma.
Amplification and DNA sequence analysis of exon V of the fibrinogen Aα-chain gene
Sequence analysis of an amplified DNA fragment spanning the base pairs encoding amino acids 391 to 625 of the Aα-chain of fibrinogenNieuwegein revealed that in the triplet coding for Aα 453 (proline) a cytosine residue is inserted, altering the sequence from CCT to CCC. This insertion alters the reading frame, changing the GAT sequence, the codon for Aα 454 (asparagine), to a termination codon (TGA). The normal sequence for the Aα-chain was not detected in the amplified DNA, indicating that the patient is homozygous for this mutation. This insertion results in a truncated Aα-chain lacking the amino acids 454 to 625. As shown for fibrinogenMarburg34 and fibrinogenMilanoIII,51 this premature chain termination will give rise to an Aα-chain with an apparent mass of 46 kd on a SDS-PAGE gel according to Laemmli and also explains the presence of fibrinogen-albumin complexes, due to disulfide formation between the unpaired cysteine at position Aα 442 and a free cysteine present in albumin.
Influence of dysfibrinogenNieuwegein on in vitroangiogenesis
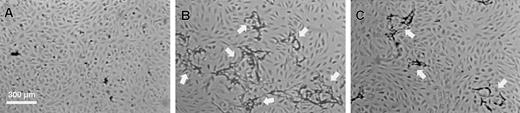
To investigate the effect of the structural defect of fibrinogenNieuwegein on tube formation, fibrin matrices prepared from this fibrinogen were used in an established in vitro angiogenesis model.47 When hMVEC seeded on top of a control fibrin matrix were stimulated with basic fibroblast growth factor and tumor necrosis factor-α (bFGF/TNFα), they invaded the underlying fibrin matrix and formed capillary-like tubular structures (Figure 3B). This process was accompanied by local degradation as was evident from the accumulation of fibrin degradation products in the conditioned medium. Cross-sectional analysis confirmed the presence of tubular structures with a lumen covered with endothelial cells. In agreement with our previous findings,47 no tubular structures were formed in the absence of bFGF/TNF-α (Figure 3A). When fibrinNieuwegein was used as a matrix, a marked reduction in fibrin degradation products in the conditioned media (666 ± 162 ng/mL as compared to 7289 ± 217 ng/mL, n = 3) and a reduction (75% ± 6%, n = 5) in tubular structure formation occurred (Figure 3C). Because the outgrowth of vessels depends on the local degradation of the underlying fibrin matrix by the urokinase-plasminogen activator (u-PA)/plasmin system, the concentrations of u-PA and plasminogen activator inhibitor (PAI)-1 antigen47 were determined in the conditioned media. No differences in accumulation of these proteins in conditioned media from cells cultured on top of a control and a fibrinNieuwegeinmatrix were found (data not shown).
Formation of capillary-like tubular structures by hMVEC in a fibrinNieuwegein matrix.
The hMVEC were seeded in confluency on the fibrin matrices. Under control conditions they remained as a monolayer on the fibrin (A). Stimulated with 5 ng/mL bFGF and 1 ng/mL TNF-α they formed invasive capillary-like tubular structures in normal fibrin matrices (B). Capillary-like tubular structure formation by bFGF/TNF-α–stimulated cells was decreased in fibrinNieuwegein matrices (C). After 4 days pictures were taken. Arrows indicate capillary-like tubular structures. Bar represents 300 μM.
Formation of capillary-like tubular structures by hMVEC in a fibrinNieuwegein matrix.
The hMVEC were seeded in confluency on the fibrin matrices. Under control conditions they remained as a monolayer on the fibrin (A). Stimulated with 5 ng/mL bFGF and 1 ng/mL TNF-α they formed invasive capillary-like tubular structures in normal fibrin matrices (B). Capillary-like tubular structure formation by bFGF/TNF-α–stimulated cells was decreased in fibrinNieuwegein matrices (C). After 4 days pictures were taken. Arrows indicate capillary-like tubular structures. Bar represents 300 μM.
Localization of αv-integrins on the surface of endothelial cells present in the capillary-like tubular structures in a fibrin matrix
Deletion of the carboxyl-terminal part of the Aα-chain in fibrinogenNieuwegein eliminates the RGD sequence at position Aα 572-574, which constitutes an adhesion site for endothelial αvβ3-integrin. To investigate whether this may be the cause of the diminished tube formation, we first confirmed the presence of αv-integrins on endothelial cells. Immunohistochemical analysis of hMVEC cultured on top of a normal fibrin matrix showed the presence of αv-integrins on the surface of hMVEC present in capillary-like tubular structures in the matrix, as well as in cells present on top of the fibrin matrix (Figure4).
Immunostaining for αv-integrins on the cell surface of hMVEC stimulated with bFGF/TNF-α.
After an incubation period of 5 days with bFGF/TNF-α, the fibrin matrices were sectioned and endothelial cells were stained for αv-integrins. Arrows point to endothelial cells present in the monolayer on top of the matrix and to cells in the capillary-like tubular structure in the fibrin.
Immunostaining for αv-integrins on the cell surface of hMVEC stimulated with bFGF/TNF-α.
After an incubation period of 5 days with bFGF/TNF-α, the fibrin matrices were sectioned and endothelial cells were stained for αv-integrins. Arrows point to endothelial cells present in the monolayer on top of the matrix and to cells in the capillary-like tubular structure in the fibrin.
Role of RGD sequences in fibrin and endothelial αvβ3 during cell adhesion to and tube formation in a fibrin matrix
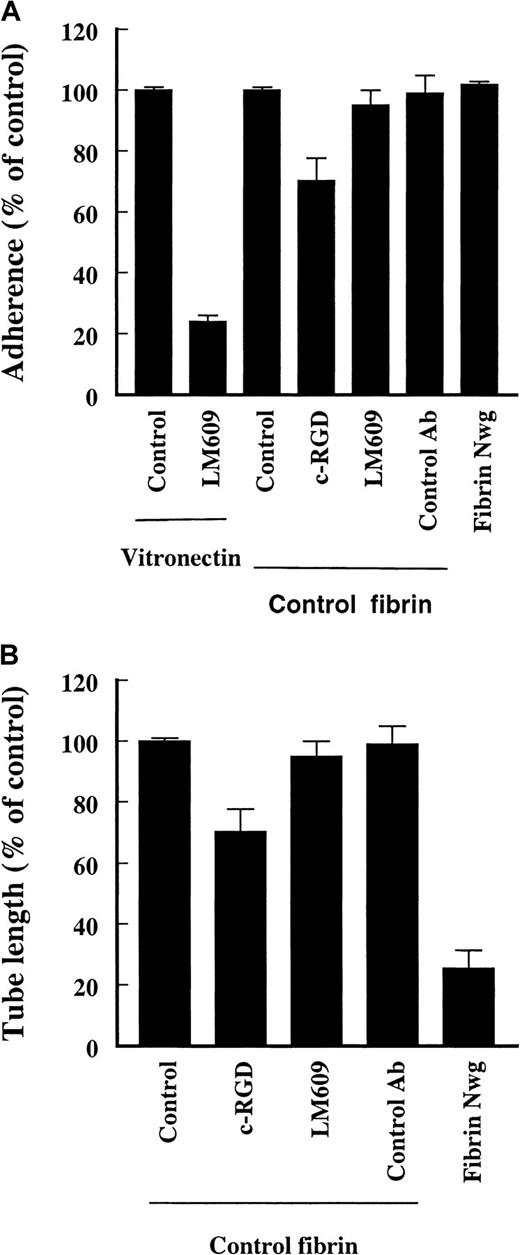
A possible interaction between the RGD sequences in fibrin and the endothelial αvβ3 receptor during cell adhesion and tube formation was evaluated using cyclic-RGD peptides (c-RGD, 10 μg/mL) and an inhibiting mAb against αvβ3 (LM609, 10 μg/mL). This antibody completely blocks the adhesion of hMVEC to a vitronectin matrix, which indicates that endothelial cells have a functional αvβ3-complex (Figure5A, compare to Figure 4). The attachment of endothelial cells to an underlying fibrin matrix was slightly reduced by 20% ± 9% (n = 2) by the presence of c-RGD; addition of 25 to 50 μg/mL c-RGD reduced the adhesion of cells in a dose-dependent fashion. The adhesion of cells was unaffected by the presence of LM609 (95% ± 4% of control values, n = 3) (Figure 5A). Adhesion of endothelial cells to fibrinNieuwegein was not impaired either.
Effect of c-RGD (10 μg/mL) and anti-αvβ3-antibody (LM609, 10 μg/mL) on the adherence to and invasion in fibrin matrices by hMVEC.
(A) Cells were allowed to adhere to vitronectin in the presence or absence of LM609 (10 μg/mL) or to fibrin in the presence c-RGD (10 μg/mL), anti-FITC mAb (10 μg/mL) (control Ab), and LM609 (10 μg/mL). Cells were also allowed to adhere to a matrix prepared from fibrinogenNieuwegein. After 2 hours the adhered cells were counted and expressed as percent of control (no addition). Results from 6 cultures in 3 independent experiments are given and expressed as mean ± SD. (B) The hMVEC were seeded on top of the fibrin matrices prepared from normal and fibrinogenNieuwegein and were stimulated to invade by 5 ng/mL bFGF and 1 ng/mL TNF-α alone or in the presence of c-RGD (10 μg/mL), anti-FITC (10 μg/mL), or LM609 (10 μg/mL). After 6 days the formed tube length was measured and expressed as percent of control (bFGF/TNF-α without addition of antibodies). Data are the mean ± SD of 6 cultures in 3 independent experiments. (*representsP < 0.05)
Effect of c-RGD (10 μg/mL) and anti-αvβ3-antibody (LM609, 10 μg/mL) on the adherence to and invasion in fibrin matrices by hMVEC.
(A) Cells were allowed to adhere to vitronectin in the presence or absence of LM609 (10 μg/mL) or to fibrin in the presence c-RGD (10 μg/mL), anti-FITC mAb (10 μg/mL) (control Ab), and LM609 (10 μg/mL). Cells were also allowed to adhere to a matrix prepared from fibrinogenNieuwegein. After 2 hours the adhered cells were counted and expressed as percent of control (no addition). Results from 6 cultures in 3 independent experiments are given and expressed as mean ± SD. (B) The hMVEC were seeded on top of the fibrin matrices prepared from normal and fibrinogenNieuwegein and were stimulated to invade by 5 ng/mL bFGF and 1 ng/mL TNF-α alone or in the presence of c-RGD (10 μg/mL), anti-FITC (10 μg/mL), or LM609 (10 μg/mL). After 6 days the formed tube length was measured and expressed as percent of control (bFGF/TNF-α without addition of antibodies). Data are the mean ± SD of 6 cultures in 3 independent experiments. (*representsP < 0.05)
During the process of capillary-like tubular structure formation in the underlying fibrin matrix the presence of 10 μg/mL c-RGD hardly reduced the extent of tube formation. Addition of higher concentrations of c-RGD (50-100 μg/mL) reduced the outgrowth to 80%. The addition of LM609 did not alter the extent of outgrowth (80% ± 9% and 95% ± 3% of control values, n = 3, respectively) (Figure 5B). These data indicate that αvβ3-integrins on endothelial cells are not essential for attachment to and invasion in a fibrin matrix.
Influence of the fibrin structure on in vitroangiogenesis
The effect of the abnormal polymerization of fibrinogenNieuwegein on capillary-like structure formation was further analyzed in 2 ways. First, because the fibrinogen molecules in plasmaNieuwegein can form complexes with albumin, either one or 2 per molecule, albumin was added to the polymerizing fibrinogen in a 1:1 or 2:1 molar ratio to investigate the effect of albumin on fibrin structure and the formation of capillary-like tubular structures. The extent of tube formation was only slightly affected by the highest concentration of albumin tested (data not shown). This suggests that the presence of albumin per se in a fibrin matrix does not significantly influence fibrin formation and as such tube formation. These results, however, do not exclude the possibility that covalent binding of albumin to fibrinogenNieuwegein affects fibrin polymerization and as such tube formation.
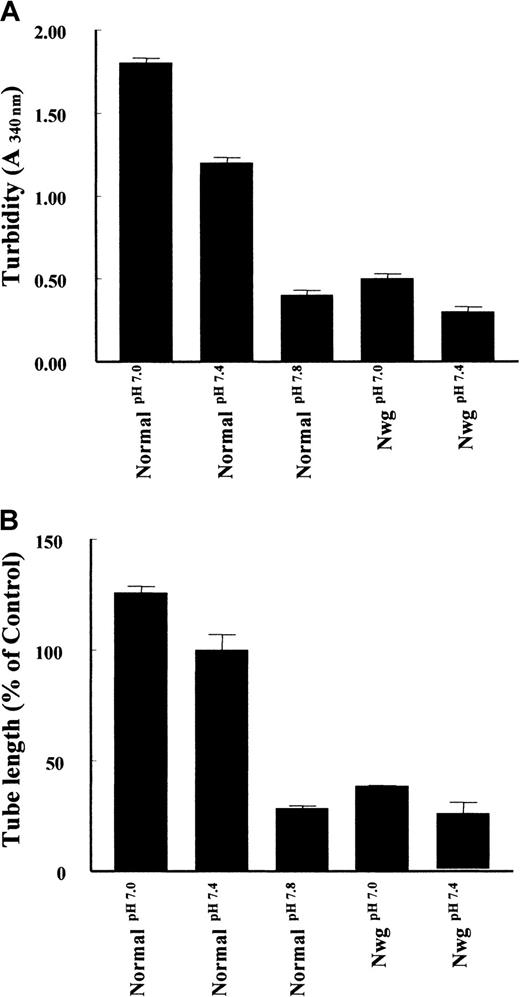
Second, as shown in Figure 6A, the pH of the polymerization buffer influences the structure of the fibrin matrices.12 An increase of pH during the polymerization of normal fibrin from pH 7.0 to 7.8 results in a decrease of turbidity. Analysis of tube formation reveals a parallel decrease in the tube length formed. Similarly, an increase in turbidity of fibrin matrices prepared from fibrinogenNieuwegein was observed when the pH of the buffer was decreased to pH 7.0 (Figure 6A), with a parallel increase (53% ± 19%) in tube formation (Figure 6B). These findings suggest that the configuration of the fibrin network is an important determinant of the outgrowth of tubes in a fibrin matrix.
Effect of fibrin structure on capillary tube formation.
Fibrin matrices from normal fibrinogen and fibrinogenNieuwegein were polymerized at pH 7.0, 7.4, and 7.8. (A) Turbidity at 340 nm was measured after 2 hours of polymerization. (B) The hMVEC were seeded on top of these fibrin matrices and stimulated with 5 ng/mL bFGF and 1 ng/mL TNF-α to form invasive capillary-like tubular structures. Capillary-like tubular structure formation was quantified after 4 days and expressed as percent of control (normal fibrin at pH 7.4). Data are mean ± SD of 6 cultures in 3 independent experiments.
Effect of fibrin structure on capillary tube formation.
Fibrin matrices from normal fibrinogen and fibrinogenNieuwegein were polymerized at pH 7.0, 7.4, and 7.8. (A) Turbidity at 340 nm was measured after 2 hours of polymerization. (B) The hMVEC were seeded on top of these fibrin matrices and stimulated with 5 ng/mL bFGF and 1 ng/mL TNF-α to form invasive capillary-like tubular structures. Capillary-like tubular structure formation was quantified after 4 days and expressed as percent of control (normal fibrin at pH 7.4). Data are mean ± SD of 6 cultures in 3 independent experiments.
Effect of cross-linking of fibrin on the extent and maintenance of tube formation
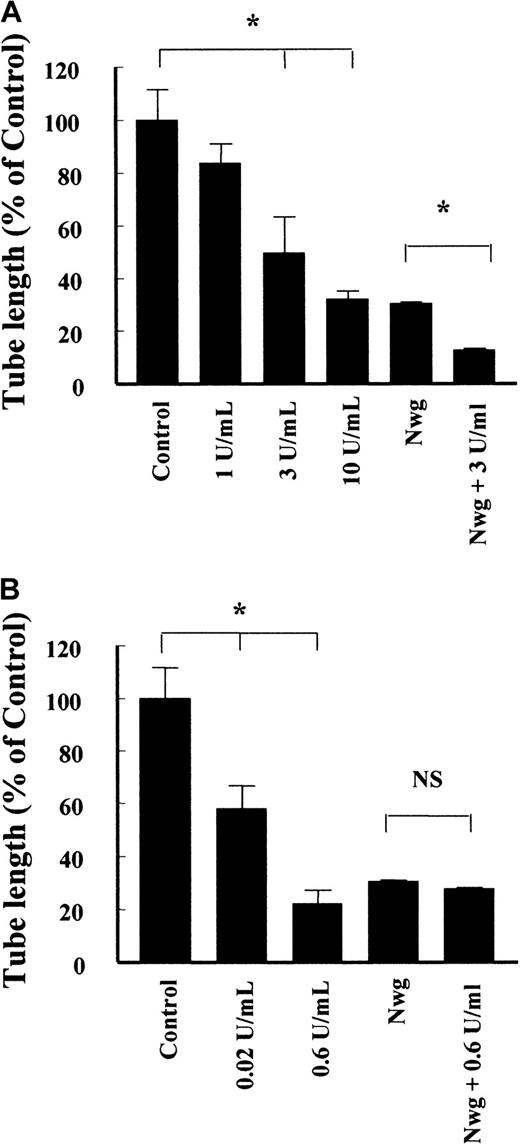
Not only the bundle structure is an important factor in the architecture of the fibrin matrix, but also the extent of cross-linking. Factor XIIIa, which is present in the circulation, covalently links 2 γ-chains in adjacent fibrin molecules, while TG, which is released during wounding of a vessel, covalently links 2 Aα-chains. The presence of factor XIIIa during the polymerization of normal fibrin caused a concentration-dependent reduction of tube formation (Figure 7A), resulting in a 68% ± 3% (n = 4) decrease at 10 U/mL factor XIIIa. Cross-linking of the fibrinNieuwegein with 3 U/mL factor XIIIa resulted in a proportional decrease (42% ± 1%, n = 5) of the length of tubes formed.
Effect of factor XIIIa and tissue TG on the formation of capillary-like tubular structures in fibrin.
Fibrin matrices prepared from normal fibrinogen and fibrinogenNieuwegein were cross-linked by the addition of different concentrations of factor XIIIa (A) and tissue TG (B). The hMVEC were seeded on top of the fibrin and stimulated with 5 ng/mL bFGF and 1 ng/mL TNF-α to form invasive capillary-like tubular structures. The total length of capillary-like tubular structures was quantified after 4 days and expressed as percent of control (normal fibrin). Data are mean ± SD of 8 cultures in 4 independent experiments (* represents P < 0.05). NS indicates not significant.
Effect of factor XIIIa and tissue TG on the formation of capillary-like tubular structures in fibrin.
Fibrin matrices prepared from normal fibrinogen and fibrinogenNieuwegein were cross-linked by the addition of different concentrations of factor XIIIa (A) and tissue TG (B). The hMVEC were seeded on top of the fibrin and stimulated with 5 ng/mL bFGF and 1 ng/mL TNF-α to form invasive capillary-like tubular structures. The total length of capillary-like tubular structures was quantified after 4 days and expressed as percent of control (normal fibrin). Data are mean ± SD of 8 cultures in 4 independent experiments (* represents P < 0.05). NS indicates not significant.
Addition of TG during the polymerization of normal fibrin reduced the length of the formed tubes by 42% ± 9% (n = 5) and 78% ± 5% (n = 5) for 0.02 U/mL and 0.6 U/mL TG, respectively (Figure 7B). Addition of 0.6 U/mL TG to polymerizing fibrinNieuwegein did not affect the extent of tubes formed (Figure 7B). This is consistent with the absence of a cross-linking site for TG in fibrinNieuwegein due to the deletion of the carboxyl-terminal part of the Aα-chain.
Interestingly, cross-linking of the normal fibrin matrix with TG retards the initiation of capillary-like tubular structure formation and as such the increase in formed tube length after 4 days. During a prolonged period of 7 days, the tubular structures in the non–cross-linked fibrin matrices were regressed to invaginations in the fibrin matrix. The structures formed in cross-linked matrices, however, appeared to be stable during the prolonged stimulation (data not shown). These data suggest that cross-linking of the fibrin matrix by TG not only affects the initiation and extent of tubular structure formation but also the maintenance of the structures formed.
Discussion
In this paper we describe the effect on capillary-like tubular structure formation of a new congenital dysfibrinogenemia, fibrinogenNieuwegein, which is characterized by the deletion of amino acids Aα 454-610 of the Aα-chain. This deletion is the result of insertion of a single nucleotide in codon Aα 453, which results in the appearance of a stop codon at Aα 454. The structural defect of fibrinogenNieuwegein results in the presence of a covalently linked albumin to the free SH-group in the unpaired cysteine at Aα 442, in the deletion of the RGD-sequence at Aα 572-574, and in the disappearance of the cross-linking site for TG. Analysis of the effect of this structural defect on fibrin formation and on the formation of tube-like structures showed that the more dense fibrin network formed from fibrinogenNieuwegeindelayed ingrowth of human endothelial cells. This delayed tube formation was further retarded by factor XIIIa but not by TG.
FibrinogenNieuwegein has characteristics that are very similar to the previously described dysfibrinogen Marburg34 and Milano III.51 In both congenital dysfibrinogenemias clotting time is prolonged and the fibrin network formed is tight. The deletion of carboxyl-terminal amino acids 454-610 in fibrinogenNieuwegein and that of amino acids 461-610 in fibrinogenMarburg result in the presence of an unpaired cysteine at position Aα 442, which normally forms an intrachain disulfide bridge with cysteine at position Aα 472.52 The free SH-group of the unpaired cysteine covalently links to a free SH-group in albumin, which results to the formation of fibrinogen-albumin complexes. Interestingly, in other congenital dysfibrinogenemias, such as fibrinogenDusart53and fibrinogenChapel Hill,54fibrinogen-albumin complexes are also formed. In these dysfibrinogenemias, the substitution of arginine Aα 554 by cysteine results in the presence of a free SH-group that covalently links to a free SH-group in albumin. These latter 2 dysfibrinogens are also associated with a delayed clotting time and the formation of dens fibrin networks. This suggests that in fibrinogenNieuwegeinand fibrinogenMarburg the delayed clotting times and the abnormal network formation are caused by the covalently linked albumin rather than by the absence of the carboxyl-terminal part of Aα-chain. The Aα-chain of fibrinogen plays an important role in the lateral association of the fibrin monomers, and the presence of cross-linked albumin molecules leads to steric hindrance of the association and consequently to the formation of a rigid network.14,15 Such a steric hindrance leading to abnormal lateral association is also seen with fibrinogenCaracasII, in which the conversion of a serine into an asparagine leads to an additional carbohydrate group linked to the carboxyl-terminus of the Aα-chain.55
Because of the deletion of the carboxyl-terminal part of Aα-chain in fibrinogenNieuwegein, the RGD-sequence at position Aα 572-574 is missing. This RGD-sequence is reported to interact with endothelial αvβ3-integrin and during fibrin-endothelial cell interaction in vitro30-32suggesting it also plays an important role during the process of angiogenesis in vivo. hMVEC express αvβ3(this paper and reference 56) and this integrin is essential for the attachment of these cells to a vitronectin matrix.48 In contrast to the expectation that the absence of the RGD-sequence in fibrinogenNieuwegein may result in a retarded tube formation,28 our results show that an interaction of αvβ3-integrin with the RGD at position Aα 572-574 is not crucial for endothelial cell attachment to and tube formation in a pure fibrin matrix. This does not exclude, however, that other receptors,24,25 including other integrins or cooperative interaction of αvβ3-integrin with other integrins,57 or other RGD-sequences in fibrinogen are apparently more important for fibrin-endothelial cell interactions in our in vitro angiogenesis model. In vivo the nature of the temporary matrices is more complex than fibrin alone, and during interaction of endothelial cells with these matrices the role for αvβ3 is indispensable.31 32Our data, however, suggest that a role for αvβ3 during endothelial cell-fibrin interaction may be overemphasized.
The results of this paper and previous work11-13 show that the architecture of the formed fibrin matrix plays a critical role during the formation of capillary-like tubular structures. Turbid and malleable fibrin matrices prepared from intact fibrinogen have an extensive ingrowth of tubular structures, while transparent and rigid matrices retard this process. In line with this, tube formation in the transparent fibrinNieuwegein matrix is delayed. By lowering the pH during polymerization the structure of the fibrinNieuwegein network could be partially normalized, which led to an increased tube formation. The formation of capillary-like tubular structures is dependent on pericellular fibrin degradation by invading endothelial cells,12 and the fact that the dense fibrin network is less degraded than the normal fibrin matrix explains the slower ingrowth of endothelial cells herein.12 Not only the fibrinolytic sensitivity of the matrices influences the outgrowth, but also the differences in pore size between the normal and fibrinNieuwegein network could account for the differences in formation of tubular structures in the underlying matrix. Other congenital dysfibrinogenemias with a structural defect that leads to abnormal fibrin polymerization may similarly be expected to show retarded ingrowth.
Cross-linking of fibrin by factor XIIIa and TG further stabilizes the network and thus further decreases the plasmin-mediated degradation of the fibrin matrix.58 As a consequence endothelial cells more slowly degrade the matrix during migration and tube formation, which results in a retarded ingrowth of tubes. In vivo it has been shown that an increased stability of the temporary matrix promotes proper wound healing by ensuring that the matrix persists until tissue regeneration is completed.59 In fibrinNieuwegein cross-linking by factor XIIIa resulted in a diminished tube formation, similar to the decrease observed in normal fibrin. However, cross-linking by TG, which is more relevant for wound repair, did not occur as a result of the deletion of the TG cross-linking site in the carboxyl-terminal part of the Aα-chain. Due to the nature of the fibrinNieuwegein network, the temporary matrix formed at an injury site has a stability comparable to a TG cross-linked matrix in a normal individual. Indeed, we observed a similar capillary-like formation in TG-treated fibrin matrices formed from normal and the patient's fibrinogen. This may explain why in the anamnesis of the patient until now no abnormal wound healing has been reported.
In summary, this report describes a new congenital dysfibrinogenemia characterized by a deletion of the carboxyl-terminal part of the Aα-chain containing an important RGD sequence and a TG cross-linking site. This fibrin delays tube formation, not because of the absence of the αvβ3 adhesion sequence, but due to the altered fibrin structure. As a result of the deletion in fibrinNieuwegein further stablization by TG does not occur. Taken together, the characterization of fibrinogenNieuwegein provides further insight in the structural features required for angiogenesis in a fibrin environment.
Gaubius Laboratory, TNO-PG, Leiden, The Netherlands; Vascular Biology, Thrombosis Research Institute, London, United Kingdom; St Antonius Ziekenhuis, Nieuwegein, The Netherlands; Pharming, Leiden, The Netherlands; Institute for Cardiovascular Research, Vrije Universiteit, Amsterdam, The Netherlands.
Submitted March 27, 2000; accepted October 23, 2000.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Victor W. M. van Hinsbergh, Gaubius Laboratory, TNO-PG, Zernikedreef 9, 2333 CK Leiden, The Netherlands; e-mail: vwm.vanhinsbergh@pg.tno.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal