Abstract
The snake venom C-type lectin alboaggregin A (or 50-kd alboaggregin) from Trimeresurus albolabris was previously shown to be a platelet glycoprotein (GP) Ib agonist. However, investigations of the signal transduction induced in platelets showed patterns of tyrosine phosphorylation that were different from those of other GPIb agonists and suggested the presence of an additional receptor. In this study, the binding of biotinylated alboaggregin A to platelet lysates, as well as affinity chromatography evaluations of platelet lysates on an alboaggregin A–coated column, indicated that this other receptor is GPVI. Additional experiments with reagents that inhibit either GPIb or GPVI specifically supported this finding. These experiments also showed that both GPIb and GPVI have a role in the combined signaling and that the overall direction this takes can be influenced by inhibitors of one or the other receptor pathway.
Introduction
Snake venoms contain many components that play a role in incapacitating or killing prey. Whereas venoms from some snake families contain mainly neurotoxins, others, such as those from the Viperidae and Crotalidae families, have principally hemorrhagic effects.1,2 Among the components that affect hemostasis are those that act on coagulation factors, platelets, and endothelial cells. These proteins are divided into several classes, with a major class being the proteases,3,4 which can activate coagulation factors or platelets or affect fibrinogen by cleavage of specific sequences or receptors. Another major class is the disintegrins,5,6 which function by binding to and blocking integrins, major adhesion receptors on platelets, and other cells. Many of these contain RGD (Arg-Gly-Asp) sequences, but other active sequences have also been found. Snake metalloproteases include hemorrhagins,7 which contain a zinc-binding sequence (HEXXH) that can destroy the basal membrane in vessel walls. Domains of disintegrins and metalloproteases are combined in the ADAMS class of proteins,5 6 which function by binding to and cleaving receptors. Several members of this class are known to affect collagen-platelet interactions by cleaving receptors or binding to collagen.
Another family of proteins that has been investigated extensively is the snake C-type lectins,8,9 which were given this name because of the folding structure they contain, thus resembling members of the C-type lectin family, which are calcium-dependent (hence, the “C”) and bind to sugars (hence, “lectin”). During the past decade, several snake C-type lectins that interact with coagulation factors10 as well as with platelet receptors have been described. Many, such as echicetin, jararaca glycoprotein (GP) Ib BP, tokaracetin, agkicetin, CHH-A, and CHH-B, were found to interact with platelet GPIb as inhibitors,9,11-14 whereas others, such as alboaggregin B, mamushigin, and flavocetins A and B,15,16 are activators. Still others, such as convulxin, were shown to interact with the collagen receptor GPVI17,18 or are thought to act by means of the integrin collagen receptor α2β1.19 It was also suggested that all these snake C-type lectins act by binding to a single target protein, although little is known about their actual binding sites. Three-dimensional structures have been determined for 2 snake C-type lectins: factor IX-binding protein from Trimeresurus flavoviridis20 and flavocetin A, a GPIb-binding protein.21
Alboaggregins A, B, and C are C-type lectins fromTrimeresurus albolabris, the white-lipped tree viper, that were shown to bind to platelet GPIb.14,15,22 Alboaggregin B has a simple heterodimeric structure23 and agglutinates platelets by binding to GPIb but does not cause major activation. Alboaggregin A is tetrameric and activates platelets strongly, presumably by means of a clustering mechanism. Both alboaggregins agglutinate fixed platelets by cross-linking receptors between platelets. The primary structure of alboaggregin A, consisting of a sequence of 4 subunits, has been determined.22 Studies of the signaling in platelets induced by alboaggregin A have suggested that it represents signaling by means of GPIb.14,22,24Because alboaggregin A gives much stronger signals than other GPIb ligands, it was proposed that it cross-links to a higher degree than the other agonists and could therefore be used to explore GPIb signaling pathways that activate platelets.24 However, because the pattern of tyrosine phosphorylation induced in platelets by alboaggregin A differs considerably from that caused by other ligands of GPIb, an alternative explanation for its strong agonist effects on platelets could be that it binds not only to GPIb but also to another platelet receptor. In this study, we demonstrated that this alternative explanation is correct: we found evidence that, along with binding to GPIb, alboaggregin A binds to platelet GPVI and that this is the major receptor involved in platelet activation by this snake C-type lectin.
Materials and methods
Materials
Bovine serum albumin (BSA), apyrase grade VIII, EDTA, protein A-Sepharose, N-hydroxysuccinimidyl–activated agarose, biotinamidocaproate N-hydroxysuccinimide ester (BcapNHS), avidin-alkaline phosphatase, 5-bromo-4-chloro-3-indoyl phosphate p-toluidine salt (BCIP), p-nitro blue tetrazolium chloride (NBT), alkaline phosphatase–conjugated and peroxidase-conjugated goat and antimouse antibodies (Abs), wheat germ agglutinin (WGA), thrombin, streptavidin R–phycoerythrin conjugate (streptavidin-PE), and Triton X-100 were from Sigma (St Louis, MO). Sepharose CL-2B and Sephadex G10 were from Amersham Pharmacia Biotech (Freiburg, Germany). The fast-protein liquid chromatography (FPLC) Q2 column was from BioRad Laboratories AG (Glattbrugg, Switzerland), and the Fractogel EMD TMAE-650 (S) column was from Merck (Darmstadt, Germany). Iloprost was a kind gift from Schering AG (Zürich, Switzerland), the SuperSignal chemiluminescence detection systems were from Pierce (Rockford, IL), and autoradiography (Fuji RX) films were from Fujifilm (Dielsdorf AG, Switzerland). Methylated type I calf-skin collagen was a kind gift from Dr J. Rauterberg. Annexin V–fluorescein isothiocyanate conjugated (FITC) (molar ratio of FITC to protein, 1.0) was from Bender MedSystems (Vienna, Austria).
The monoclonal antibody (mAb) anti-GPIbα (SZ2) was from Coulter-Immunotech Diagnostics (Hamburg, Germany), the Fab fragment of mAb IV.3 to FcγRII was from Medarex (Annandale, NJ), the mAb against GPIbβ (GI27) was a kind gift from Dr S. Santoso, and the mAb to the thrombin-binding site on GPIbα (VM16d) was a kind gift from Dr A.V. Mazurov. Antiphosphotyrosine mAb 4G10 and polyclonal anti-linker for activated T-cells (LAT) (G807) were from Upstate Biotechnology Inc (Lake Placid, NY). Polyclonal Ab against FcεRIγ was a kind gift from Professor J.-P. Kinet. Polyclonal chicken Abs against human CD62P and FITC-labeled chicken IgY Abs were from WAK Chemie Medical GmbH (Bad Soden, Germany). Rabbit polyclonal Abs against human GPIb and human GPIX25 and human GPVI and the anti-GPVI Fab and F(ab′)2 were prepared as described previously.26
Echicetin was purified from lyophilized Echis carinatus sochureki venom (Latoxan, Rosans, France)9,27 and convulxin was purified from lyophilized Crotalus durissus terrificus venom (Sigma, St Louis, MO)17 as described previously. Lyophilized trimeresurus (T) albolabris venom was from Latoxan. Polyvinylidene difluoride (PVDF) membranes (PolyScreen) were from DuPont NEN. Octanoyl-N-methylglucamide (ONMG) and nonanoyl-N-methylglucamide (NNMG) were from Oxyl Chemie (Bobingen, Germany).
Purification of alboaggregin A
Lyophilized crude T albolabris venom was dissolved in 0.05 M Tris buffer (pH 8.0), and undissolved material was removed by centrifugation. The venom was fractionated on a FPLC Fractogel TMAE-650 (S) ion-exchange column using a linear gradient of sodium chloride (NaCl) from 0 to 0.7 M. Fractions (10 μL) were assayed by adding them to 500 μL fixed platelets, with stirring at 1100 rpm at 37°C in an aggregometer (Lumitec, France). Platelet agglutination was monitored by assessing changes in light transmittance. Three of the eluted peaks agglutinated fixed platelets. The second peak (eluted with 0.3 M NaCl), which had the strongest response, was dialyzed against 0.05 M Tris buffer (pH 8.0) and purified by additional ion-exchange FPLC chromatography on a Q2 column using a linear gradient of NaCl from 0 to 0.4 M. The first peak (eluted with 0.15 M NaCl) agglutinated fixed platelets (tested as described above) and contained pure alboaggregin A, as controlled by gel electrophoresis. The protein was stored at 4°C until used.
Preparation of platelets for gel filtration
Venous blood from healthy adult volunteers who had not taken any medication that affects platelet function for at least 2 weeks before the study was obtained by venipuncture of the antecubital vein. Blood was anticoagulated with trisodium citrate (9 parts blood and 1 part trisodium citrate [0.108 M]). Platelet-rich plasma (PRP) was prepared by centrifugation at 250g for 10 minutes at room temperature. Platelets were gel filtered on Sephadex CL-2B equilibrated in buffer A (127 mM NaCl, 2.7 mM potassium chloride [KCl], 0.42 mM sodium phosphate, monobasic [NaH2PO4], 12 mM sodium bicarbonate, 1 mM magnesium chloride, 5.5 mM glucose, and 3.5% BSA [pH 7.35]) containing 0.1 U/mL apyrase.28
Platelet washing, aggregation, fixation, phosphorylation times, and immunoprecipitation
Human platelets were isolated from buffy coats less than 20 hours after blood collection (Central Laboratory, Swiss Red Cross Blood Transfusion Service). To each buffy coat, 30 mL of 100 mM citrate (pH 6.5) was added. PRP and the platelet pellet were isolated by successive centrifugation steps. Platelets were resuspended in buffer B (113 mM NaCl; 4.3 mM potassium phosphate, dibasic; 4.3 mM sodium phosphate, dibasic, 4.4 mM NaH2PO4, and 5.5 mM glucose [pH 6.5]) and centrifuged at 250g for 5 minutes. The platelet-rich supernatant was centrifuged at 1000g for 10 minutes, and platelets were again washed with buffer B. Washed platelets were resuspended in buffer C (20 mM HEPES, 140 mM NaCl, 4 mM KCl, and 5.5 mM glucose [pH 7.4]) and the platelet count was adjusted to 5 × 108 platelets/mL by dilution with buffer C. Samples were kept at room temperature until used for aggregation studies.
Platelet aggregation was monitored by assessing light transmission in an aggregometer (Lumitec), with continuous stirring at 1100 rpm at 37°C. Platelets were preincubated in buffer containing 2 mM calcium chloride (CaCl2) at 37°C for 2 minutes before the measurement was started by adding the samples for analysis. Platelets were fixed by incubation with 0.5% formaldehyde for 0.5 hour, followed by washing twice with phosphate-buffered saline (PBS; pH 7.4). For the tyrosine phosphorylation time-range experiments, aliquots (700 μL; 5 × 108 platelets/mL) of control, resting platelets, and activated platelets at appropriate time points, were solubilized in PBS containing 1% sodium dodecyl sulfate (SDS) with 1 mM phenylmethylsulfonyl fluoride (PMSF), 5 mM EDTA, 2 mMN-ethyl-maleimide (NEM), and 2 mM sodium orthovanadate.
For immunoprecipitation, aliquots (700 mL; 5 × 108platelets/mL) of control, resting, and activated platelets were solubilized in PBS containing 1.2% Triton X-100 with 1 mM PMSF, 5 mM EDTA, 2 mM NEM, and 2 mM sodium orthovanadate. After centrifugation, platelet lysates, which were precleared with protein A-Sepharose, were stirred for 2 hours with specific antibodies before 20 μL protein A-Sepharose was added. The lysates were then incubated for 6 to 8 hours.
Alboaggregin A-agarose and biotinylation of alboaggregin A
FPLC-purified alboaggregin A (500 μg) was dialyzed against PBS (pH 7.8). N-hydroxysuccinimidyl-activated agarose (500 μL) was washed 3 times in PBS (pH 7.8) and incubated with dialyzed alboaggregin A overnight at 4°C. Reactive groups were blocked with 200 mM glycine (2 hours at 4°C). For biotinylation, alboaggregin A dialyzed against PBS (pH 8) was incubated with BcapNHS (20 μg/mg alboaggregin A) for 1 hour at room temperature. Free BcapNHS was removed by Sephadex G-10 gel filtration.
Biotinylation of platelets, preparation of Triton X-100 platelet lysate, WGA affinity chromatography, and alboaggregin A affinity chromatography
Human platelets were isolated from 16 buffy coats as described above in the presence of 10 μM Iloprost and 5 mM EDTA. Washed platelets were diluted with PBS to 5 × 109/mL and incubated with 100 μg BcapNHS for 1 hour at room temperature. Free BcapNHS was separated by washing 3 times with PBS (pH 6.8). Biotinylated platelets were solubilized in PBS containing 1.2% Triton X-100 with 1 mM PMSF, 5 mM EDTA, and 2 mM NEM2. After centrifugation (40 000g for 1 hour at 4°C), the supernatant was applied to a column of WGA-Sepharose 4B equlibrated with buffer D (10 mM Tris, 130 mM NaCl, and 2.5 mM EDTA [pH 7.4]). The column was washed thoroughly with buffer D containing 0.2% ONMG. The bound material was eluted with 2.5% N-acetylglucosamine in buffer E (10 mM Tris, 30 mM NaCl, and 2.5 mM EDTA [pH 7.4]) containing 0.2% ONMG. Fractions containing membrane glycoproteins were pooled and loaded on an affinity chromatography column coated with alboaggregin A and equilibrated with buffer D. The column was washed thoroughly with buffer D containing 0.2% ONMG and then with buffer E containing 0.2% NNMG. The bound material was eluted with 0.08% SDS in buffer E. Fractions containing membrane glycoproteins that bound to alboaggregin A were analyzed by electrophoresis.
Flow cytometry
Samples were analyzed by using a fluorescence-activated cell-sorter flow cytometer (FACScan; Becton Dickinson, Heidelberg). Excitation was with an argon laser at 488 nm. The FACScan was used in a standard configuration with a 530-nm bandpass filter. Platelets were gated and data were obtained from fluorescence channels in a logarithmic mode. A total of 5000 events were analyzed for each data point.
Alboaggregin A–induced procoagulant activity and α-granule release
Annexin-V binding was measured. Gel-filtered platelets were diluted to 2.5 × 107 platelets/mL with buffer A containing 4 mM CaCl2. Platelets (100 μL) were incubated with alboaggregin A (0.1-2.5 μg/mL) for 25 minutes at room temperature, with gentle shaking, in a total volume of 125 μL in buffer A containing 4 mM CaCl2. Alboaggregin A–treated or control platelets were incubated with annexin V–FITC in the dark at room temperature, with gentle shaking. After 15 minutes, 0.5 mL buffer A containing 4 mM CaCl2 was added. After another 15 minutes, samples were analyzed by flow cytometry. Inhibitory antibodies were added directly to the platelets after gel filtration. After 5 minutes, platelets were incubated with alboaggregin A and treated as described above. Specific binding was calculated by subtracting unspecific binding determined in the presence of 20 mM EDTA.
CD62P expression was measured as a marker of α-granule release. Gel-filtered platelets were diluted to 2.5 × 107platelets/mL with PBS (pH 7.4). Platelets (100 μL) were incubated with alboaggregin A (0.1-5 μg/mL) for 3 minutes and fixed with formaldehyde. Platelets were then washed, resuspended in PBS (pH 7.4), and incubated with FITC-labeled anti-CD62P Ab (10 μg/mL). After 1 hour, platelets were again washed and analyzed by using flow cytometry. Specific binding was calculated by subtracting nonspecific binding determined with FITC-labeled chicken IgY.
Alboaggregin A binding to fixed platelets and its inhibition
Aliquots of formaldehyde-fixed platelets (100 μL; 2.5 × 107 platelets/mL in PBS [pH 7.4]) were preincubated for 5 minutes with increasing amounts of inhibitors and then incubated with biotinylated alboaggregin A (0.5 μg/mL) for 20 minutes. Subsequently, platelets were washed and incubated with streptavidin-PE for 30 minutes. The platelets were then washed, resuspended in PBS, and analyzed by flow cytometry. Unspecific streptavidin-PE binding to platelets was subtracted. Saturation binding of biotinylated alboaggregin was determined in a separate experiment (data not shown).
Results
Purification of alboaggregin A
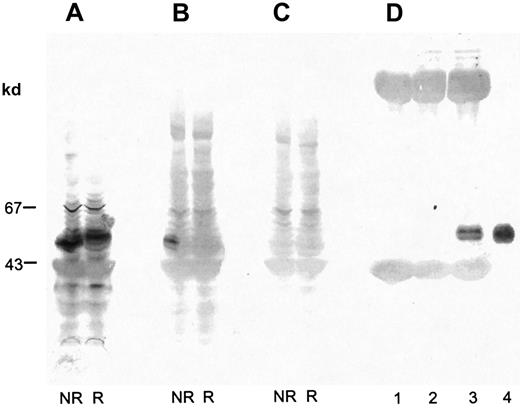
Alboaggregin A was purified from lyophilized crude T albolabris venom by ion-exchange FPLC using a Fractogel EMD TMAE-650 (S) column and a Q2 column. The final product showed a broad, 50-kd band under nonreducing conditions and 3 bands, at 14, 15, and 16 kd, under reducing conditions, when analyzed by SDS–polyacrylamide gel electrophoresis (PAGE) and silver staining (Figure1). The 14-kd band was noticeably stronger than those at 15 and 16 kd and contained both β subunits. The apparent molecular masses of subunits were comparable with those reported previously.9,22 After reduction and alkylation, the 4 subunits of alboaggregin A were separated by high-performance liquid chromatography (HPLC), and N-terminal sequences were obtained. The N-terminal amino acid sequences of alboaggregin A subunits were the same as those described previously.22
Determination of apparent molecular mass of alboaggregin A.
SDS-gel electrophoresis of FPLC-purified alboaggregin A under nonreducing (lane 1) and reducing (lane 2) conditions. The molecular mass standards were albumin (67 kd), soybean trypsin inhibitor (20 kd), and α lactalbumin (14 kd).
Determination of apparent molecular mass of alboaggregin A.
SDS-gel electrophoresis of FPLC-purified alboaggregin A under nonreducing (lane 1) and reducing (lane 2) conditions. The molecular mass standards were albumin (67 kd), soybean trypsin inhibitor (20 kd), and α lactalbumin (14 kd).
Effects of anti-GPIb Abs, echicetin, and convulxin on alboaggregin A binding to fixed platelets
Alboaggregin A binding to formaldehyde-fixed platelets was inhibited by anti-GPIbα mAb SZ2, polyclonal anti-GPIbα Ab, the C-type lectin echicetin (a GPIb-binding protein), and the C-type lectin convulxin (a GPVI-binding protein) (Figure2). Formaldehyde-fixed platelets were incubated with increasing amounts of Abs (20-100 μg/mL) or C-type lectins (1.6-140 μg/mL) for 5 minutes before the biotinylated alboaggregin A (0.5 μg/mL) was added. The inhibitory effect of convulxin, directed against GPVI, was much stronger than the effect of the inhibitors directed against GPIb. Saturation binding of biotinylated alboaggregin A and inhibition of biotinylated alboaggregin A binding by unlabeled alboaggregin A were determined in separate experiments (data not shown).
Effects of anti-GPIb Abs, echicetin, and convulxin on alboaggregin A binding to fixed platelets.
Formaldehyde-fixed platelets were preincubated with increasing amounts of mAb SZ2, anti-GPIb Ab, echicetin, or convulxin and incubated with 0.5 μg/mL biotinylated alboaggregin A and streptavidin-PE. ● Platelets in the presence of echicetin, ○ platelets in the presence of convulxin, ▪ platelets in the presence of mAb SZ2, and ▴ platelets in the presence of polyclonal rabbit anti-GPIb Abs. The fluorescence of 5000 platelets per measuring point was determined by flow cytometry. Data are the mean results from 3 experiments using platelets from different donors.
Effects of anti-GPIb Abs, echicetin, and convulxin on alboaggregin A binding to fixed platelets.
Formaldehyde-fixed platelets were preincubated with increasing amounts of mAb SZ2, anti-GPIb Ab, echicetin, or convulxin and incubated with 0.5 μg/mL biotinylated alboaggregin A and streptavidin-PE. ● Platelets in the presence of echicetin, ○ platelets in the presence of convulxin, ▪ platelets in the presence of mAb SZ2, and ▴ platelets in the presence of polyclonal rabbit anti-GPIb Abs. The fluorescence of 5000 platelets per measuring point was determined by flow cytometry. Data are the mean results from 3 experiments using platelets from different donors.
Alboaggregin A–induced agglutination and aggregation
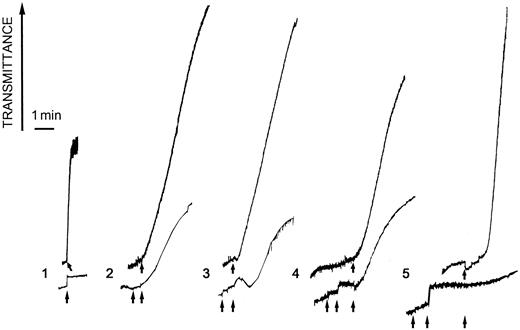
Alboaggregin A agglutinated formaldehyde-fixed platelets in the absence of any cofactor (Figure 3, lane 1). Maximum agglutination was reached at 1 μg/mL. Additional aggregation experiments were done with unfixed platelets. To evaluate the role of GPIb and GPVI in alboaggregin A–induced aggregation, echicetin was used to inhibit GPIb and Fab fragments of GPVI Ab were used to inhibit GPVI. Thrombin and collagen, respectively, were used as agonists to control the efficiency of inhibition by these agents. Echicetin (15 μg/mL), a GPIb-binding protein, partly blocked alboaggregin A–induced (0.5 μg/mL) and thrombin-induced (0.05 U/mL) aggregation of washed platelets (Figure 3, lane 2 and lane 3). Fab fragments of anti-GPVI Abs partly blocked alboaggregin A–induced (0.5 μg/mL) platelet aggregation and completely blocked collagen-induced (0.36 μg/mL) aggregation (Figure 3, lane 4 and lane 5).
Agglutination and aggregation response from platelets treated with alboaggregin A.
Washed formaldehyde-fixed platelets (700 μL) were stirred at 1100 rpm at 37°C and agglutination was induced by (1) 1 μg/mL alboaggregin A (buffer as control). Unfixed, washed platelets (700 μL) were stirred at 1100 rpm at 37°C in the presence (lower tracing) or absence (upper tracing) of 15 μg/mL echicetin (2 and 3). After 1 minute, 0.5 μg/mL alboaggregin A or 0.05 U/mL thrombin (control; 3) was added. In the same way, unfixed platelets were stirred in the presence (lower tracing) or absence (upper tracing) of Fab fragments of anti-GPVI Ab (4 and 5). After 1 minute, 0.5 μg/mL alboaggregin A or 0.36 μg/mL collagen (control; 5) was added.
Agglutination and aggregation response from platelets treated with alboaggregin A.
Washed formaldehyde-fixed platelets (700 μL) were stirred at 1100 rpm at 37°C and agglutination was induced by (1) 1 μg/mL alboaggregin A (buffer as control). Unfixed, washed platelets (700 μL) were stirred at 1100 rpm at 37°C in the presence (lower tracing) or absence (upper tracing) of 15 μg/mL echicetin (2 and 3). After 1 minute, 0.5 μg/mL alboaggregin A or 0.05 U/mL thrombin (control; 3) was added. In the same way, unfixed platelets were stirred in the presence (lower tracing) or absence (upper tracing) of Fab fragments of anti-GPVI Ab (4 and 5). After 1 minute, 0.5 μg/mL alboaggregin A or 0.36 μg/mL collagen (control; 5) was added.
Binding of biotinylated alboaggregin A to platelet lysate
Proteins in platelet lysate were separated by SDS-PAGE (7%-17% acrylamide gradient) and electroblotted on PVDF membranes. The membranes were incubated with biotinylated alboaggregin A and avidin-coupled alkaline phosphatase or with anti-GPVI Ab and antirabbit IgG–coupled alkaline phosphatase. Both were stained with BCIP and NBT. Under nonreduced conditions, biotinylated alboaggregin A bound to a band with the same molecular mass as GPVI (Figure4B). Unspecific binding of avidin phosphatase to platelet lysate could be excluded (Figure 4C). Figure4D, lane 3, clearly shows binding of alboaggregin A to GPVI immunoprecipitated with anti-GPVI antibodies and to GPVI purified on a convulxin-coated column (lane 4). Figure 4D, lanes 1 and 2, shows immunoprecipitations with the preimmune serum of the anti-GPVI Ab and an anti-GPV Ab as controls.
Binding of biotinylated alboaggregin A to platelet lysate.
Platelet lysate was separated by SDS-PAGE (7%-17% acrylamide gradient) and transferred to PVDF membranes. Proteins were incubated with (A) anti-GPVI Ab followed by antirabbit IgG–coupled alkaline phosphatase, (B) biotinylated alboaggregin A and avidin-coupled alkaline phosphatase, or (C) avidin-coupled alkaline phosphatase and detected with BCIP and NBT. (D) Proteins from platelet lysate immunoprecipitated with rabbit preimmune serum anti-GPVI (lane 1), rabbit anti-GPV Ab (lane 2), rabbit anti-GPVI Ab (lane 3), or isolated GPVI purified on a convulxin column (lane 4) was separated by SDS-PAGE (7%-17% acrylamide gradient) and transferred to PVDF membranes. The proteins were incubated with biotinylated alboaggregin A before detection with avidin-coupled alkaline phosphatase, BCIP, and NBT.
Binding of biotinylated alboaggregin A to platelet lysate.
Platelet lysate was separated by SDS-PAGE (7%-17% acrylamide gradient) and transferred to PVDF membranes. Proteins were incubated with (A) anti-GPVI Ab followed by antirabbit IgG–coupled alkaline phosphatase, (B) biotinylated alboaggregin A and avidin-coupled alkaline phosphatase, or (C) avidin-coupled alkaline phosphatase and detected with BCIP and NBT. (D) Proteins from platelet lysate immunoprecipitated with rabbit preimmune serum anti-GPVI (lane 1), rabbit anti-GPV Ab (lane 2), rabbit anti-GPVI Ab (lane 3), or isolated GPVI purified on a convulxin column (lane 4) was separated by SDS-PAGE (7%-17% acrylamide gradient) and transferred to PVDF membranes. The proteins were incubated with biotinylated alboaggregin A before detection with avidin-coupled alkaline phosphatase, BCIP, and NBT.
Binding of biotinylated platelets to alboaggregin A
The fractions enriched in biotinylated platelet glycoproteins from WGA column chromatography were loaded on an alboaggregin A–agarose affinity column. Fractions eluted with increasing amounts of SDS were analyzed by using SDS-PAGE (7%-17% acrylamide gradient) and transferred to PVDF membranes. The membranes were then incubated with avidin-coupled alkaline phosphatase or with anti-GPVI, anti-GPIb, anti-GPIbβ, or anti-GPIX Abs and peroxidase-conjugated antirabbit Abs. Membranes were stained with BCIP and NBT or bands detected with chemiluminescence. Fractions that eluted with 0.08% SDS contained GPIb, GPIbβ, GPIX, and GPVI (Figure 5).
Binding of biotinylated platelets to alboaggregin A.
Biotinylated platelet proteins eluted from an alboaggregin A affinity column with increasing amounts of SDS were separated by SDS-PAGE (7%-17% acrylamide gradient) and transferred to PVDF membranes. (A) Membranes were incubated with avidin-coupled alkaline phosphatase and detected with BCIP and NBT. (B) Membranes were incubated with anti-GPVI Ab, anti-GPIbβ (framed bands), and a peroxidase-conjugated second Ab and detected by using chemiluminescence. (C) Membranes were incubated with avidin-coupled alkaline phosphatase and stained with BCIP and NBT. (D) Membranes were incubated with anti-GPIb Ab, anti-GPIX (framed bands), and a peroxidase-conjugated second Ab and stained by using chemiluminescence. In each, lane 1 is the WGA-bound fraction of Triton X-100 phase of biotinylated platelet glycoproteins (starting material); lane 2, the flow-through fraction; lane 3, the first fraction eluted with 0.08% SDS; lane 4, the second fraction eluted with 0.08% SDS; lane 5, the fraction eluted with 0.2% SDS; and lane 6, the immunoprecipitation of starting material with (A) anti-GPVI Abs and (C) anti-GPIb Abs.
Binding of biotinylated platelets to alboaggregin A.
Biotinylated platelet proteins eluted from an alboaggregin A affinity column with increasing amounts of SDS were separated by SDS-PAGE (7%-17% acrylamide gradient) and transferred to PVDF membranes. (A) Membranes were incubated with avidin-coupled alkaline phosphatase and detected with BCIP and NBT. (B) Membranes were incubated with anti-GPVI Ab, anti-GPIbβ (framed bands), and a peroxidase-conjugated second Ab and detected by using chemiluminescence. (C) Membranes were incubated with avidin-coupled alkaline phosphatase and stained with BCIP and NBT. (D) Membranes were incubated with anti-GPIb Ab, anti-GPIX (framed bands), and a peroxidase-conjugated second Ab and stained by using chemiluminescence. In each, lane 1 is the WGA-bound fraction of Triton X-100 phase of biotinylated platelet glycoproteins (starting material); lane 2, the flow-through fraction; lane 3, the first fraction eluted with 0.08% SDS; lane 4, the second fraction eluted with 0.08% SDS; lane 5, the fraction eluted with 0.2% SDS; and lane 6, the immunoprecipitation of starting material with (A) anti-GPVI Abs and (C) anti-GPIb Abs.
Alboaggregin A–induced platelet procoagulant activity and α-granule release
The exposure of negatively charged phospholipids on gel-filtered platelets treated with alboaggregin A (0.1-2.5 μg/mL) and detected by annexin V-FITC is shown in Figure6. The expression of negatively charged aminophospholipids, mainly phosphatidylserine, depended on the concentration of alboaggregin A. Maximal expression of negatively charged aminophospholipids was reached at approximately 0.5 μg/mL alboaggregin A. This was also the threshold concentration at which aggregation of these platelets could be induced (Figure 3, lane 2). VM16d, a mAb to GPIbα, which inhibits thrombin binding but not von Willebrand factor (vWf) binding, and mAb SZ2, which prevents both thrombin and vWf binding to GPIbα, inhibited the procoagulant response of platelets to low but not to high doses of alboaggregin A (Figure 6). The antibodies were used at 20 μg/mL and were incubated with gel-filtered platelets for 10 minutes before treatment with alboaggregin A (0.1-2.5 μg/mL). Blockage of GPVI with Fab fragments of anti-GPVI Abs or blockage of GPIb with anti-GPIb Abs inhibited procoagulant responses to low doses of alboaggregin A (Figure 6A). The optimal antibody concentrations were determined separately.
Alboaggregin A–induced platelet procoagulant activity, α-granule release, and the effect of Abs against GPIb and GPVI.
(A) Gel-filtered platelets were preincubated with or without mAb SZ2 (20 μg/mL), mAb VM16d (20 μg/mL), or Fab fragments of anti-GPVI Abs. After treatment with alboaggregin A (0.05-2.5 mg/mL) and annexin V-FITC, the fluorescence of 5000 platelets per measuring point was determined by flow cytometry. (B) Gel-filtered platelets were preincubated with or without mAb SZ2 (20 μg/mL) or Fab fragments of anti-GPVI Abs. After treatment with alboaggregin A (0.1-2.5 mg/mL), fixation with formaldehyde, and addition of FITC-labeled anti-CD62P mAb, the fluorescence of 5000 platelets per measuring point was determined by flow cytometry. Data are the mean results from 3 experiments using platelets from different donors. ● 25 000 platelets/μL, ○ 25 000 platelets/μL in the presence of mAb SZ2, ■ 25 000 platelets/μL in the presence of mAb VM16d, and ▵ 25 000 platelets/μL in the presence of Fab fragments of anti-GPVI Abs.
Alboaggregin A–induced platelet procoagulant activity, α-granule release, and the effect of Abs against GPIb and GPVI.
(A) Gel-filtered platelets were preincubated with or without mAb SZ2 (20 μg/mL), mAb VM16d (20 μg/mL), or Fab fragments of anti-GPVI Abs. After treatment with alboaggregin A (0.05-2.5 mg/mL) and annexin V-FITC, the fluorescence of 5000 platelets per measuring point was determined by flow cytometry. (B) Gel-filtered platelets were preincubated with or without mAb SZ2 (20 μg/mL) or Fab fragments of anti-GPVI Abs. After treatment with alboaggregin A (0.1-2.5 mg/mL), fixation with formaldehyde, and addition of FITC-labeled anti-CD62P mAb, the fluorescence of 5000 platelets per measuring point was determined by flow cytometry. Data are the mean results from 3 experiments using platelets from different donors. ● 25 000 platelets/μL, ○ 25 000 platelets/μL in the presence of mAb SZ2, ■ 25 000 platelets/μL in the presence of mAb VM16d, and ▵ 25 000 platelets/μL in the presence of Fab fragments of anti-GPVI Abs.
Granule release from gel-filtered platelets treated with alboaggregin A (0.1-5 μg/mL) and detected with FITC-labeled anti-CD62P Ab is shown in Figure 6B. Inhibition of GPIb or GPVI clearly reduced granule release. The concentrations of antibodies used were those mentioned above.
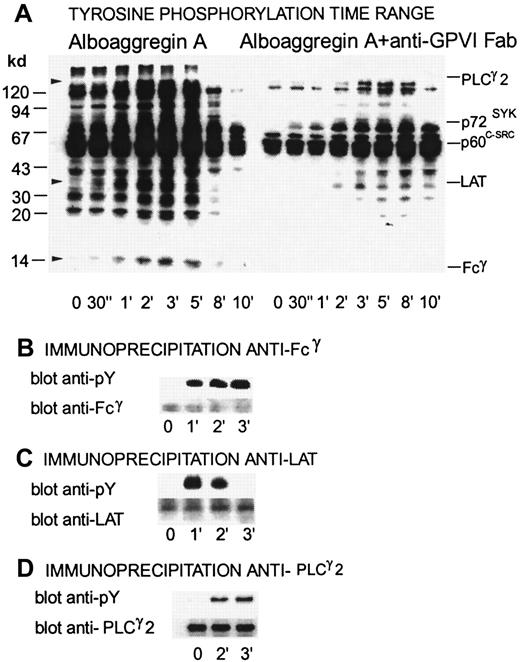
Alboaggregin A–induced tyrosine phosphorylation, inhibitory effects of Fab fragments of anti-GPVI Ab, and immunoprecipitation of Fcγ, LAT, and phospholipase C (PLC) γ2
Figure 7 shows a time range of tyrosine phosphorylation for platelets activated with 0.5 μg/mL alboaggregin A compared with platelets that were preincubated with Fab fragments of anti-GPVI Ab. Several proteins, including PLCγ2, Syk, Lyn, Fyn, and FcRγ, showed raised levels of tyrosine phosphorylation after activation with alboaggregin A. Fcγ, LAT, and PLCγ2 were clearly tyrosine phosphorylated after platelet activation with alboaggregin A at either 1 minute (Fcγ), 2 minutes (PLCγ2), or 30 seconds (LAT), whereas resting platelets had no or very low levels of tyrosine phosphorylation. Figure 7 shows that tyrosine phosphorylation of several proteins during activation with alboaggregin A was inhibited by Fab fragments of anti-GPVI Abs. Tyrosine phosphorylation of LAT was delayed for more than 2 minutes and tyrosine phosphorylation of Fcγ was inhibited completely under these conditions, indicating that Fcγ phosphorylation by alboaggregin A occurs mainly by means of activation of GPVI. In Figure 7B, 7C, and 7D, the upper bands show the level of tyrosine phosphorylation in immunoprecipitates from resting and alboaggregin A–activated platelets with anti-Fcγ, anti-LAT, and anti-PLCγ2 Abs, respectively. The lower bands show the same blots after stripping and reblotting with the corresponding antibodies to confirm that equal amounts of each component were immunoprecipitated from each platelet preparation.
Time dependence of tyrosine phosphorylation of proteins from platelets activated by alboaggregin A in the presence of Fab fragments of anti-GPVI Abs.
Washed platelets (700 μL) were stirred at 1100 rpm at 37°C with or without Fab fragments of GPVI Abs. After the addition of 0.5 μg/mL alboaggregin A, aliquots were removed at the times indicated and dissolved in SDS buffer containing inhibitors. (A) After separation by SDS-PAGE (7%-17% acrylamide gradient) and transfer to PVDF membranes, the proteins were incubated with the antiphosphotyrosine mAb 4G10 before detection by using a peroxidase-linked second Ab and chemiluminescence. (B-D, upper bands) Lysates of resting platelets and platelets activated with 0.5 μg/mL alboaggregin A. Aliquots were immunoprecipitated with antibodies against (B) Fcγ, (C) LAT, or (D) PLCγ2. After SDS-PAGE and Western blotting, the proteins were detected with 4G10 antiphosphotyrosine antibody. (B-D, lower bands) The membranes were then stripped and treated with anti-Fcγ, anti-LAT, or anti-PLCγ2 Abs.
Time dependence of tyrosine phosphorylation of proteins from platelets activated by alboaggregin A in the presence of Fab fragments of anti-GPVI Abs.
Washed platelets (700 μL) were stirred at 1100 rpm at 37°C with or without Fab fragments of GPVI Abs. After the addition of 0.5 μg/mL alboaggregin A, aliquots were removed at the times indicated and dissolved in SDS buffer containing inhibitors. (A) After separation by SDS-PAGE (7%-17% acrylamide gradient) and transfer to PVDF membranes, the proteins were incubated with the antiphosphotyrosine mAb 4G10 before detection by using a peroxidase-linked second Ab and chemiluminescence. (B-D, upper bands) Lysates of resting platelets and platelets activated with 0.5 μg/mL alboaggregin A. Aliquots were immunoprecipitated with antibodies against (B) Fcγ, (C) LAT, or (D) PLCγ2. After SDS-PAGE and Western blotting, the proteins were detected with 4G10 antiphosphotyrosine antibody. (B-D, lower bands) The membranes were then stripped and treated with anti-Fcγ, anti-LAT, or anti-PLCγ2 Abs.
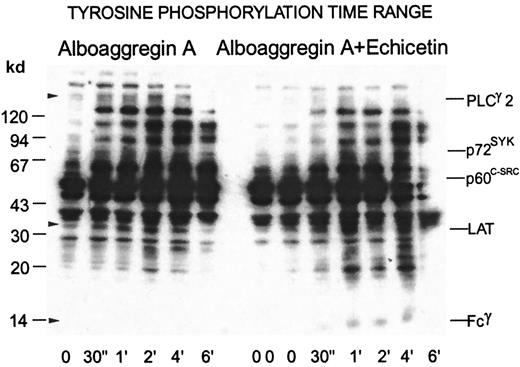
Tyrosine phosphorylation signal transduction by alboaggregin A and the inhibitory effect of echicetin
Figure 8 shows a time range of tyrosine phosphorylation for platelets activated with 0.3 μg/mL alboaggregin A compared with platelets that were preincubated with 15 μg/mL echicetin. In the presence of echicetin or SZ2 (data not shown), the overall pattern of tyrosine phosphorylation was somewhat reduced but mainly was slower; however, phosphorylation of certain proteins, notably Fcγ and LAT, increased.
Time dependence of tyrosine phosphorylation of proteins from platelets activated by alboaggregin A in the presence of echicetin.
Washed platelets (700 μL) were stirred at 1100 rpm at 37°C with or without echicetin (15 μg/mL). After the addition of 0.3 μg/mL alboaggregin A, aliquots were removed at the times indicated and dissolved in SDS buffer containing inhibitors. The time point 00 represents the control before the addition of echicetin and alboaggregin A, whereas the time point 0 was after echicetin was added. After separation by SDS-PAGE (7%-17% acrylamide gradient) and transfer to PVDF membranes, the proteins were incubated with the antiphosphotyrosine Ab 4G10 before detection by using a peroxidase-linked second Ab and chemiluminescence.
Time dependence of tyrosine phosphorylation of proteins from platelets activated by alboaggregin A in the presence of echicetin.
Washed platelets (700 μL) were stirred at 1100 rpm at 37°C with or without echicetin (15 μg/mL). After the addition of 0.3 μg/mL alboaggregin A, aliquots were removed at the times indicated and dissolved in SDS buffer containing inhibitors. The time point 00 represents the control before the addition of echicetin and alboaggregin A, whereas the time point 0 was after echicetin was added. After separation by SDS-PAGE (7%-17% acrylamide gradient) and transfer to PVDF membranes, the proteins were incubated with the antiphosphotyrosine Ab 4G10 before detection by using a peroxidase-linked second Ab and chemiluminescence.
Discussion
Because the signaling induced in platelets by the C-type lectin alboaggregin A did not correspond to that expected for a simple GPIb agonist, the specificity of alboaggregin A for platelet receptors was examined in detail by using several approaches. Alboaggregin A (50-kd alboaggregin) was purified from lyophilized T albolabris venom, essentially by using previously described methods,14,22 as a single, 50-kd nonreduced band and as 3 bands at 14, 15, and 16 kd in the reduced protein (Figure 1). The reduced band at 14 kd stained more strongly than the other 2 and contained both the β and β′ subunits. The N-terminal sequences of the 4 subunits separated by HPLC confirmed its classification. Alboaggregin A binding to fixed platelets was inhibited not only by GPIb-specific reagents, such as the mAb SZ2 and the snake C-type lectin echicetin, but also by the GPVI-specific reagent convulxin. This suggested that alboaggregin A has binding sites not only for GPIb but also for GPVI (Figure 2). The effects of GPIb and GPVI antagonists on aggregation were also tested, and both echicetin and SZ2 (data not shown), as well as Fab fragments of anti-GPVI antibodies, partly inhibited platelet aggregation (Figure 3). Alboaggregin A, like the GPIb-specific alboaggregin B15 but unlike the GPVI-specific convulxin,17 18 agglutinated fixed platelets.
Alboaggregin A was labeled with biotin and used in a ligand blotting experiment with platelet lysate separated by SDS-PAGE and blotted on PDVF membrane. The biotinylated alboaggregin A detected specifically a broad band at about 60 kd on the blot from the nonreduced lane but did not detect any band specifically on the blot from the reduced lane (Figure 4B). Polyclonal antibodies to GPVI detected bands at 60 kd and 65 kd, respectively, in lanes run in parallel. The biotinylated alboaggregin A also detected a 60-kd band in immunoprecipitates with an anti-GPVI antibody from platelet lysates and in bound material from a convulxin-agarose affinity column (Figure 4). Biotinylated alboaggregin A detected GPVI but failed to detect GPIb, probably because the conformation of GPVI was maintained by its disulfide bridges, thus allowing renaturing on the blot, whereas for GPIbα, the leucine-rich repeats (forming at least part of the binding site) could not renature. We therefore decided to check the binding sites under nondenaturing conditions.
Alboaggregin A was coupled to agarose and used for affinity chromatography assessments of nonionic detergent-solubilized platelet lysate. After extensive washing, the bound material was eluted with SDS. Both it and the last washes were analyzed by SDS-PAGE. Western blotting was also done with antibodies to GPIbα, GPIbβ, GPIX, and GPVI. The results showed that the bound and eluted material was strongly enriched in both GPIb-IX and GPVI. No other major proteins bound strongly to the affinity column, although a few were removed at the start of the elution and probably represented proteins associated with GPIb-IX and GPVI rather than with alboaggregin A directly. These results confirm that alboaggregin A binds to both these receptors.
Induction of platelet procoagulant activity in response to alboaggregin A was examined by using binding of annexin V to surface-exposed, negatively charged phospholipids or by measuring the generation of thrombin with a chromogenic assay.29 These studies clearly showed that alboaggregin A could induce this effect and was only slightly affected by antibodies against GPIb (Figure 6). Agonists specific for GPVI were previously shown to be efficient inducers of platelet procoagulant activity,30,31 whereas GPIb has been only marginally implicated in this function32 or in response to thrombin as the specific agonist.29 The tyrosine signal-transduction pathways of platelets treated with alboaggregin A were previously investigated by several groups14,24 and found to resemble closely those induced by collagen or convulxin.17 33 Thus, Fcγ, LAT, and PLCγ2 are strongly phosphorylated on tyrosine in response to alboaggregin A (Figures 7 and 8).
The effects of inhibitors of either GPIb or GPVI on tyrosine phosphorylation induced by alboaggregin A were therefore examined. Fab fragments of polyclonal anti-GPVI had a strong inhibitory effect on overall tyrosine phosphorylation, although some bands, particularly those at 60 and 64 kd, still showed a strong increase, whereas other bands (in the range of 30-40 kd and 100-120 kd) showed a weaker increase and were later than in the control platelets (Figure 7). Tyrosine phosphorylation of Fcγ was totally abolished, and that of the band corresponding to LAT was strongly reduced. Tyrosine phosphorylation of other bands (in the range of 20-30 kd and 70-100 kd) was completely inhibited. On the other hand, when GPIb was inhibited by using either echicetin (Figure 8) or the mAb SZ2 (data not shown), there was an overall reduction in the amount of tyrosine phosphorylation and a decrease in tyrosine phosphorylation rate, but the effect was not as strong as when GPVI was blocked. Obtaining a clear effect required a reduction in the amount of alboaggregin A used for platelet activation. However, some bands showed an increase in phosphorylation compared with the uninhibited alboaggregin A–activated control. With this amount of alboaggregin A as agonist, Fcγ was not detectable in a tyrosine phosphorylated state in the positive controls but was clearly phosphorylated in the GPIb-blocked platelets. The same was true for other GPVI signaling-pathway molecules, including LAT and p72Syk. Thus, it appears that when either GPIb or GPVI is blocked, the signaling pathway is tipped toward that of the other receptor.
These findings suggest that the structure of alboaggregin A,22 together with its binding properties, is an αβα′β′–type structure with binding sites for both GPIb and GPVI. Because either GPIb or GPVI antagonists can inhibit most binding of alboaggregin A to platelets, it is possible that binding to both GPIb and GPVI simultaneously on different platelets is necessary for platelet agglutination. On the other hand, the shift in receptor signaling caused by either type of antagonist suggests that although platelet-platelet interactions by means of alboaggregin A are mostly prevented, a clustering of one or the other receptor can still occur at the single-platelet level, either because each alboaggregin A molecule has 2 (sterically close) binding sites for each receptor or because when alboaggregin A binds to receptors on platelets, it can still self-aggregate to cluster receptors of a single type (or both reasons). A self-aggregating mechanism has already been suggested to explain the surprising strength of the GPVI agonist convulxin in activating platelets.17 Thus, the greater effectiveness of alboaggregin A in activating platelets compared with other GPIb agonists that have been tested may be explained by its use of GPVI as a coreceptor.
The question remains why the use of GPIb as a coreceptor is advantageous for the snake. Several explanations appear possible. One is that the larger number of GPIb molecules per platelet (at least 25 000 and probably 50 000) compared with GPVI molecules (2000-5000) permits a more efficient use of alboaggregin A in activating platelets and organizing links between platelets. Alternatively (or in addition), cross-linking GPIb and GPVI may amplify the signaling from each by means of a cross-talk mechanism. The results described here support both possibilities and indicate that it is not only GPVI that provides a signal. At lower concentrations of alboaggregin A, signaling by means of GPIb in addition to GPVI permits a strong activation of platelet tyrosine phosphorylation while shifting the mechanism away from the Fcγ route. This may mean that FcγRIIA, which has been implicated in GPIb function, may have a larger role here. Alboaggregin A will thus be an interesting tool for exploring cross-talk between 2 receptors that have critical roles in the early stages of platelet adhesion and activation in primary hemostasis.
Acknowledgments
We thank Dr J.-P. Kinet, Dr A.V. Mazurov, and Dr S. Santoso, respectively, for the FcεRIγ, VM16d, and GPIbβ antibodies and Dr J. Rauterberg for the methylated type I calf-skin collagen.
Work at the Theodor Kocher Institute was supported by grant 31-52396.97 from the Swiss National Science Foundation to K.J.C.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
K.J. Clemetson, Theodor Kocher Institute, University of Berne, Freiestrasse 1, CH-3012 Berne, Switzerland; e-mail: clemetson@tki.unibe.ch.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal