Abstract
The thrombopoietic status of patients with uremia remains unclear. This issue was addressed with particular reference to marrow megakaryocytopoiesis and endogenous thrombopoietin (TPO) levels. A study was conducted in 114 patients on hemodialysis, 43 patients on continuous ambulatory peritoneal dialysis, and 48 age-matched controls. Reticulated platelets, a marker of marrow megakaryocytopoiesis, were measured by flow cytometry. Serum TPO levels, platelet-associated IgG (PAIgG) levels, and hepatitis C virus (HCV) antibody titers were also measured by enzyme-linked immunosorbent assay. Circulating and reticulated platelet counts were significantly lower in the patients on dialysis than in the controls. Thrombocytopenia (less than 150 × 109/L) was most frequent in the HCV-positive hemodialysis patients, who had a higher incidences and higher PAIgG titers. The following results were obtained in the HCV-negative dialysis patients: (1) platelet counts chronologically decreased with years on hemodialysis; (2) platelet counts were associated with the reticulated platelet counts; (3) serum TPO levels were significantly elevated in the dialysis patients, responding to the decrease of reticulated platelets; (4) hematocrits had a positive correlation with serum TPO levels, and serum TPO levels were significantly higher in the patients on hemodialysis who did not require recombinant human erythropoietin therapy than in the other patients. In conclusion, thrombocytopenia is a frequent finding in patients on dialysis. The failure of megakaryocyte production could be the principal cause of the platelet reduction, and the peripheral destruction and sequestration of platelets may be concomitantly involved. Elevation of serum TPO may in part serve as an aid to erythropoiesis in dialysis patients.
Introduction
Thrombopoietin (TPO), the ligand for thec-mpl proto-oncogene, has recently been purified and cloned by 5 groups.1-5 It regulates the growth and maturation of megakaryocytes in bone marrow. When recombinant TPO is administered to normal mice and nonhuman primates, it significantly increases the number of megakaryocyte colony-forming cells in the bone marrow, resulting in an increase in the circulating platelet count.1,6 In addition to its thrombopoietic action, TPO has been shown to have a variety of biologic effects on erythroblasts, leukocytes, and osteoclasts,7-11 but the clinical implications of these biologic actions have never been studied in patients with uremia.
There have been no conclusive studies on the thrombopoietic status of chronically uremic patients.12-14 The kidney is thought to be one of the organs that constitutively produces TPO,2,3,15 suggesting that the insufficient thrombopoietic activity, if it exists, may be involved in the loss of TPO activity in patients on dialysis. Circulating TPO levels in thrombopoietic disorders, such as idiopathic thrombocytopenic purpura, aplastic anemia, and myelodysplastic syndrome, have been shown to vary.16-18 These clinical findings and the results of several animal studies19 20 have suggested that the circulating platelet count, megakaryocyte mass in the bone marrow, or both may be important regulators of serum TPO concentrations. Now that TPO is recognized as a major regulator of megakaryocyte production, the measurement of endogenous TPO concentrations may provide new clues that will allow better understanding of the thrombopoietic states of patients with chronic uremia. However, nothing is known about endogenous TPO levels or the role of TPO in the regulation of hematopoiesis in these patients.
Although bone marrow aspiration is a direct procedure for evaluating hematopoiesis in the bone marrow, it is not feasible to perform it in large populations, and measurement of reticulated platelets—that is, RNA-rich younger platelets released into circulation—may be a good alternative diagnostic aid for evaluating thrombocytopoiesis in the bone marrow.21,22 Kienast and Schmitz23studied human peripheral platelets by flow cytometric analysis using the dye thiazole orange (TO), which enters living cells and binds to RNA, to detect reticulated platelets. They suggested that the RNA-rich reticulated platelet count may be a useful indicator of thrombocytopoiesis in the bone marrow.
We addressed the following issues in dialysis patients, with particular reference to TPO and reticulated platelets: thrombopoietic status, possible causes of the altered thrombopoiesis if present, and clinical significance of endogenous TPO in the erythropoietic status. In addition, we specifically examined the implications of hepatitis C virus (HCV) infection, frequently observed in patients on dialysis,24 25 in the thrombopoiesis of the patients studied.
Patients, materials, and methods
Patients
The study included 114 patients undergoing hemodialysis (HD), 43 patients on continuous ambulatory peritoneal dialysis (CAPD), and 48 age-matched normal controls. Forty (35.1%) of the patients on HD were HCV infected, but none of them had ever manifested clinical symptoms or signs of hepatitis or of transaminase abnormalities. Examination by abdominal ultrasonography or computed tomography confirmed that splenomegaly was absent in all patients. None of the patients had received interferon alpha. None of the CAPD patients or controls was infected with HCV. The causes of the renal failure were chronic glomerulonephritis (87 patients), gestosis (6 patients), polycystic kidney disease (5 patients), nephrosclerosis (4 patients), chronic pyelonephritis (4 patients), gout (2 patients), hypoplasia (2 patients), Alport syndrome (2 patients), and hydronephrosis (2 patients). Patients on HD for collagen diseases and diabetes mellitus were excluded. The study was approved by the institutional review board of the hospital and was carried out according to the principles of the Declaration of Helsinki. Informed consent was obtained from all patients included in the study.
Samples
Blood specimens were obtained before the start of treatment at the first HD session of the week. Whole blood for the blood counts and flow cytometric analysis was collected by venipuncture into tubes containing trisodium EDTA. Serum samples were prepared simultaneously and stored at −80°C until the serum levels of TPO and erythropoietin (EPO) were measured. Cells were counted with an automatic electronic counter. Serum chemistry values, intact parathyroid hormone levels (i-PTH), and β2 microglobulin levels were determined by conventional analytical methods. A third-generation enzyme-linked immunosorbent assay (ELISA) was used to determine anti-HCV antibodies.
TPO assay
The serum TPO concentration was determined with a commercially available ELISA kit (Quantikine Human TPO Immunoassay; R&D Systems, Minneapolis, MN). Briefly, 200 μL rhTPO standard, serum sample, or blank was added in duplicate to the wells of a microtiter plate precoated with an anti-TPO monoclonal antibody, and the plate was incubated for 3 hours at 4°C. After washing, 200 μL horseradish-peroxidase-conjugated anti-TPO antibody was added, and the plate was incubated for 1 hour at 4°C. The color was developed by using tetramethylbenzidine as the substrate, and the reaction was stopped by adding 50 μL acid solution to each well. Absorbance was recorded at 450 nm, and the TPO concentration of the sample was calculated from the corresponding standard curve. The manufacturer's instructions state that the assay recognizes recombinant and natural TPO and has a detection limit of less than 15 pg/mL.
EPO assay
Serum EPO concentrations were determined with a commercially available RIA kit (Recombigen; Japan DPC, Tokyo, Japan). In brief, 200 μL recombinant human-α EPO standard, serum sample, or blank was added in duplicate to the tubes and mixed with 250 μL anti-EPO monoclonal antibody. The tubes were incubated for 2 hours at 37°C, gently mixed with 100 μL sodium iodide I 125-labeled rh-α EPO, and again incubated for 3 hours at 37°C. After 1 mL anti-rabbit goat serum was added to each tube for B/F separation, the tube was centrifuged at 2000 rpm for 15 minutes at 4°C. The isotopic count for each tube was measured with a γ counter (ARC-950; Aloka, Tokyo, Japan) after the supernatant was aspirated. The EPO concentration of the sample was calculated from the standard B/B0curve.
Measurement of reticulated platelets by thiazole orange staining and flow cytometric analysis
TO was purchased from Becton Dickinson (Retic-COUNT; San Jose, CA). The TO staining procedure was performed basically according to the manufacturer's protocol. A double-labeling technique was performed on whole blood samples to analyze platelets alone by flow cytometry. In brief, 5 μL well-mixed whole blood was stained with 5 μL phycoerythrin (PE)-tagged monoclonal antibody directed against glycoprotein Ib (CD 42b; Becton Dickinson) in 50 μL Tyrode's buffer (140 mM NaCl, 2.7 mM KCl, 5.5 mM dextrose, 0.42 mM Na2HPO4, 12 mM NaHCO3, 10 mM disodium EDTA with 2 mg/mL bovine serum albumin) for 30 minutes at room temperature. After cells were washed 3 times, they were incubated with 1 mL Retic-COUNT (Becton Dickinson) reagent for 30 minutes in the dark at room temperature. At the same time, a control sample was prepared for each patient sample by adding 1 mL phosphate-buffered saline with 0.1% sodium azide instead of TO. The samples were immediately subjected to flow cytometry (FACScan; Becton Dickinson) for analysis. In the analysis, platelets were selected by setting a first gate that separated the platelets from the erythrocytes, leukocytes, and debris in a forward-scatter (FSC) versus side-scatter (SSC) gate in logarithmic amplifiers. To identify more accurately the platelet population, we used a second gate setting for logarithmic amplification of FSC versus FL2 fluorescence (585 nm). Ten thousand cells of the CD42b PE-tagged platelet population within this platelet gate were evaluated by TO staining. To exclude cell autofluorescence and instrument background, an unstained control sample was prepared simultaneously for each patient sample. A threshold line was determined on each unstained control sample in such a way that in the logarithmic amplification of FSC versus FL1 fluorescence (580 nm), 99% of unstained platelets was detected under this line. The subset of highly FL1 fluorescent platelets over the threshold line was considered to represent TO-stained reticulated platelets. Data acquisition and analysis were performed with CellQuest software (Becton Dickinson). The percentage of positive cells in the platelets (% reticulated platelets) and the reticulated platelet counts (% reticulated platelets/100 × peripheral platelet counts) were calculated.26,27 The reticulated platelet counts have been confirmed to be a more reliable indicator of current marrow thrombopoietic status than the percentage reticulated platelets26-28; thus, we principally used the former parameter in the evaluation. All experiments were conducted in triplicate. The average intra-assay coefficient of variation was 8.40% (3 HD samples, n = 6 for each).
Measurement of platelet-associated immunoglobulin
PAIgG was determined by a competitive micro-ELISA method, as described by Tsubakihara et al.29 In brief, platelets were separated from whole blood that had been collected in EDTA, and they were washed 4 times with phosphate-buffered saline containing 1.1% bovine serum albumin. ELISA was performed in 96-well microplates coated with purified human IgG. A horseradish-peroxidase-conjugated anti-human IgG antibody was incubated simultaneously with the samples and visualized with o-phenylenediamine. Competitive binding of peroxidase-conjugated anti-human IgG antibody between platelets and wells coated with human IgG was analyzed. The amount of peroxidase bound to the wells was determined using a standard curve and expressed as nanograms IgG per 107 platelets. Absorbance was measured on an ELISA plate reader with the filter at 480 nm. The actual measurement of PAIgG was performed by SRL (Tokyo, Japan).
Statistics
Data are expressed as mean ± SD unless otherwise stated. The differences between mean values were evaluated by using the 2-tailed Student t test for unpaired data, andP < .05 was considered statistically significant.
Results
Platelet counts
Platelet counts and the incidence of thrombocytopenia in the HD, CAPD, and age-matched healthy subjects were examined first. The mean of the 5 most recent platelet counts was used to evaluate the dialysis patients. Results are shown in Table1. The duration of dialysis was longest in the HCV-positive HD patients. Thrombocytopenia was defined as a platelet count less than 150 × 109/L. Platelet counts of the HCV-positive HD patients were the lowest of all of the subjects studied. Platelet counts of the HCV-negative HD and CAPD patients were significantly lower than those of the controls. Platelet counts of the HCV-negative patients were significantly lower than of the patients with CAPD. The incidence of thrombocytopenia was highest in the HCV-positive HD patients (55%; 22 of 40 patients). Next, the influence of prescribed drugs on the platelet counts was studied in regard to allopurinol, angiotensin-converting enzyme inhibitors, histamine (H-2) receptor agonists, and recombinant human erythropoietin (rhEPO). Platelet counts did not significantly differ between any of the groups, whether the group had or had not taken each of the drugs.
Platelet counts of dialysis patients and healthy subjects
| Group . | No. subjects . | Age (y) . | Dialysis duration (y) . | Platelet counts (×109/L) . | Incidence of thrombocytopenia (%) . |
|---|---|---|---|---|---|
| Controls | 48 | 49.6 ± 7.46 | — | 242.9 ± 38.4 | — |
| HD patients | 114 | 50.9 ± 14.8 | 13.9 ± 6.46 | 174.9† ± 50.6 | 31.6 (36 of 114) |
| HCV-positive | 40 | 49.8 ± 12.8 | 18.6* ± 13.9 | 145.3‡ ± 38.3 | 55.0 (22 of 40) |
| HCV-negative | 74 | 51.7 ± 15.5 | 11.56* ± 5.96 | 195.6† ± 51.4 | 18.9 (14 of 74) |
| CAPD patients | 43 | 48.5 ± 10.5 | 6.78 ± 4.76 | 210.61-153 ± 47.2 | 9.30 (4 of 43) |
| Group . | No. subjects . | Age (y) . | Dialysis duration (y) . | Platelet counts (×109/L) . | Incidence of thrombocytopenia (%) . |
|---|---|---|---|---|---|
| Controls | 48 | 49.6 ± 7.46 | — | 242.9 ± 38.4 | — |
| HD patients | 114 | 50.9 ± 14.8 | 13.9 ± 6.46 | 174.9† ± 50.6 | 31.6 (36 of 114) |
| HCV-positive | 40 | 49.8 ± 12.8 | 18.6* ± 13.9 | 145.3‡ ± 38.3 | 55.0 (22 of 40) |
| HCV-negative | 74 | 51.7 ± 15.5 | 11.56* ± 5.96 | 195.6† ± 51.4 | 18.9 (14 of 74) |
| CAPD patients | 43 | 48.5 ± 10.5 | 6.78 ± 4.76 | 210.61-153 ± 47.2 | 9.30 (4 of 43) |
P < .01 vs HCV-negative patients, CAPD patients, or both.
P < .01 vs control and CAPD patients.
P < .01 vs control, HCV-negative patients, and CAPD patients.
P < .05 vs control.
Data are shown as the mean ± SD.
PAIgG values in HD patients with thrombocytopenia
To test the possibility that autoimmune mechanisms induce peripheral destruction or sequestration of platelets, the PAIgG values were studied in all patients with thrombocytopenia on HD (n = 36). Data are shown in Figure 1. The reference range of the PAIgG measurements was 9.0 to 25 ng/107platelets (data were from SRL). The proportion of HCV-positive HD patients who were PAIgG-positive was 81.8% (18 of 22 patients) as opposed to only 42.9% (6 of 14 patients) of the HCV-negative patients. The PAIgG titers in the HCV-positive patients were 105.2 ± 94.2 ng/107 platelets and nearly 4 times higher than those in the HCV-negative patients (28.2 ± 18.8 ng/107platelets). All 6 HCV-negative patients with positive PAIgG levels were women who had experienced blood transfusions or pregnancy.
PAIgG values of HD patients with thrombocytopenia.
PAIgG was measured by competitive micro-ELISA. The reference range was 9.0 to 25 ng/107 platelets. The proportion of HCV-negative HD patients who were PAIgG-positive (more than 25 ng/107platelets) was 42.9%, whereas 81.8% of the HCV-positive HD patients were PAIgG-positive. The mean titer of PAIgG was 105.2 ± 94.2 ng/107 platelets in the HCV-positive HD patients (n = 22) and 28.2 ± 18.1 ng/107 platelets in the HCV-negative HD patients (n = 14), and the difference was significant (P = .005).
PAIgG values of HD patients with thrombocytopenia.
PAIgG was measured by competitive micro-ELISA. The reference range was 9.0 to 25 ng/107 platelets. The proportion of HCV-negative HD patients who were PAIgG-positive (more than 25 ng/107platelets) was 42.9%, whereas 81.8% of the HCV-positive HD patients were PAIgG-positive. The mean titer of PAIgG was 105.2 ± 94.2 ng/107 platelets in the HCV-positive HD patients (n = 22) and 28.2 ± 18.1 ng/107 platelets in the HCV-negative HD patients (n = 14), and the difference was significant (P = .005).
Changes in platelet count in the HCV-negative HD patients during the years of HD
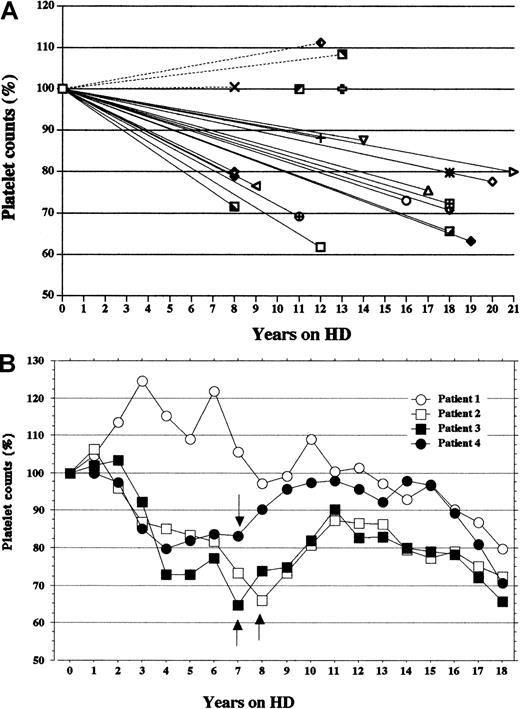
To better understand the simple impact of HD treatment on the platelet counts, we reviewed the counts in the 22 HCV-negative HD patients who had received HD treatment for 8 to 21 years (mean age, 52.9 ± 12.5 years; 11 women, 11 men; mean HD duration, 13.8 ± 4.29 years). All individual changes in platelet counts expressed as percentages of the initial counts, from the start to the endpoint of HD treatment, are shown in Figure2A. Three patients showed mild increases (0.5%, 8.4%, and 11.2%), and 2 patients showed no change, but the other 17 (77.3%) patients showed clear decreases in platelet counts. The mean reduction in platelet counts during the period in these 17 patients was 25.2% ± 7.51% (range, 11.8% to 38.2%), and the rate of reduction was 1.91% ± 0.83% per HD year (range, 0.89% to 3.55% per HD year). The trends in the platelet counts in the 4 patients (2 women, 2 men) of the 17 patients whose HD treatments started simultaneously 18 years earlier are shown in Figure 2B. The platelet counts for each year are the means of 24 counts each year. Overall, the platelet counts gradually decreased, showing considerable fluctuation over 18 HD years. At approximately the middle of the clinical course in 3 patients (patients 2, 3, and 4), rhEPO replacement therapy was begun. Afterward, in spite of the continuation of rhEPO therapy, platelet counts began to decrease again and fell to less than 75% of the initial values. Platelet counts for patient 1, who had never received rhEPO replacement therapy during that period, showed a decrease that became steady after 10 years of hemodialysis.
Chronologic trends in platelet count in the HCV-negative HD patients after the start of HD therapy.
Values plotted for each year are the means of 24 measurements (twice a month) during that year. The platelet counts were reviewed for 8 to 21 years in the 22 HCV-negative HD patients. All data are expressed as percentages of the initial platelet counts. (A) Individual changes in platelet counts between the beginning of HD treatment and the present. Platelet counts decreased throughout the years on HD in 77.3% (17 of 22) of patients. (B) Annual trends in platelet counts over 18 years on HD in the 4 HCV-negative HD patients with the same number of years on HD treatment. Patients 1 and 3 are women. At approximately the middle of the HD years in patients 2, 3, and 4, rhEPO replacement therapy was begun. Arrows indicate the start of rhEPO therapy.
Chronologic trends in platelet count in the HCV-negative HD patients after the start of HD therapy.
Values plotted for each year are the means of 24 measurements (twice a month) during that year. The platelet counts were reviewed for 8 to 21 years in the 22 HCV-negative HD patients. All data are expressed as percentages of the initial platelet counts. (A) Individual changes in platelet counts between the beginning of HD treatment and the present. Platelet counts decreased throughout the years on HD in 77.3% (17 of 22) of patients. (B) Annual trends in platelet counts over 18 years on HD in the 4 HCV-negative HD patients with the same number of years on HD treatment. Patients 1 and 3 are women. At approximately the middle of the HD years in patients 2, 3, and 4, rhEPO replacement therapy was begun. Arrows indicate the start of rhEPO therapy.
Platelet counts, reticulated platelet counts, and serum TPO levels in HCV-negative dialysis patients without rhEPO treatment
As described above, it is likely that HCV infection affects thrombopoietic status in patients on dialysis. Therefore, we studied only the HCV-negative dialysis patients who had not received rhEPO at least for 3 months to evaluate the pure influence of chronic uremia on megakaryocytopoiesis in the bone marrow. Platelet and reticulated platelet counts and serum TPO levels were studied in 45 HCV-negative HD patients, 30 CAPD patients, and 33 age-matched controls. The data are summarized in Table 2. Platelet counts were significantly lower in the HD and CAPD patients than in the controls. Platelet counts were significantly lower in the HD patients than in the CAPD patients, but reticulated platelet counts were not different. Serum TPO levels of dialysis patients were significantly elevated than in age-matched controls (Table 2). Significant TPO elevation was more prominent in the CAPD patients, whose duration of dialysis was shorter than in HD patients.
Reticulated platelets in HCV-negative dialysis patients without rhEPO replacement therapy
| Group . | No. subjects . | Age (y) . | Dialysis duration (y) . | Platelet counts (×109/L) . | Absolute counts of reticulated platelets (×109/L) . | Serum TPO levels (pg/mL) . |
|---|---|---|---|---|---|---|
| Control | 33 | 46.1 ± 10.9 | — | 242.9 ± 38.4 | 20.4 ± 13.1 | 90.9 ± 45.8 |
| HD patients | 45 | 51.6 ± 14.9 | 11.5* ± 6.31 | 191.5*,† ± 36.7 | 8.66† ± 5.36 | 111.7† ± 37.3 |
| CAPD patients | 30 | 45.9 ± 11.7 | 7.20 ± 5.31 | 222.3† ± 40.0 | 10.1† ± 4.78 | 183.5†,‡ ± 52.8 |
| Group . | No. subjects . | Age (y) . | Dialysis duration (y) . | Platelet counts (×109/L) . | Absolute counts of reticulated platelets (×109/L) . | Serum TPO levels (pg/mL) . |
|---|---|---|---|---|---|---|
| Control | 33 | 46.1 ± 10.9 | — | 242.9 ± 38.4 | 20.4 ± 13.1 | 90.9 ± 45.8 |
| HD patients | 45 | 51.6 ± 14.9 | 11.5* ± 6.31 | 191.5*,† ± 36.7 | 8.66† ± 5.36 | 111.7† ± 37.3 |
| CAPD patients | 30 | 45.9 ± 11.7 | 7.20 ± 5.31 | 222.3† ± 40.0 | 10.1† ± 4.78 | 183.5†,‡ ± 52.8 |
P < .01 vs CAPD;
P < .01 vs control;
P < .01 vs HD patients.
Data are shown as the mean ± SD.
Reticulated platelets were analyzed by TO staining and flow cytometry.
All experiments were conducted in triplicate.
Correlations between platelet counts, reticulated platelet counts, dialysis duration, and serum TPO levels
Reticulated platelet counts in each modality of dialysis patients were significantly positively correlated with peripheral platelet counts (Table 3). These findings suggested that the platelet counts were regulated mainly on a bone marrow basis in the dialysis patients. Moreover, reticulated platelet counts decreased with years on dialysis treatment. Neither platelet nor reticulated platelet counts had a significant relation with any of the drugs prescribed or with serum creatinine, urea nitrogen, iPTH, or β2 microglobulin levels.
Correlations between platelet counts, reticulated platelet counts, dialysis duration, and serum TPO levels of HCV-negative dialysis patients without rhEPO replacement therapy
| . | Correlations . | Correlation coefficients . | P . |
|---|---|---|---|
| HD (n = 45) | Plt vs Ret | 0.436 | .0024 |
| Plt vs TPO | −0.237 | .1180 | |
| TPO vs Ret | −0.396 | .0067 | |
| Ret vs dialysis duration | −0.338 | .0226 | |
| TPO vs dialysis duration | 0.359 | .0148 | |
| CAPD (n = 30) | Plt vs Ret | 0.491 | .0117 |
| Plt vs TPO | −0.214 | .3076 | |
| TPO vs Ret | −0.496 | .0107 | |
| Ret vs dialysis duration | −0.445 | .0247 | |
| TPO vs dialysis duration | 0.449 | .0234 |
| . | Correlations . | Correlation coefficients . | P . |
|---|---|---|---|
| HD (n = 45) | Plt vs Ret | 0.436 | .0024 |
| Plt vs TPO | −0.237 | .1180 | |
| TPO vs Ret | −0.396 | .0067 | |
| Ret vs dialysis duration | −0.338 | .0226 | |
| TPO vs dialysis duration | 0.359 | .0148 | |
| CAPD (n = 30) | Plt vs Ret | 0.491 | .0117 |
| Plt vs TPO | −0.214 | .3076 | |
| TPO vs Ret | −0.496 | .0107 | |
| Ret vs dialysis duration | −0.445 | .0247 | |
| TPO vs dialysis duration | 0.449 | .0234 |
Plt, platelet counts; Ret, reticulated platelet counts; TPO, serum thrombopoietin levels.
Serum TPO levels of each modality of dialysis patient showed a significant inverse correlation with the reticulated platelet counts, but not with the circulating platelet counts. In addition, serum TPO levels significantly increased with years on dialysis in each dialysis modality (Table 3). The low TPO (TPO less than 100 pg/mL) HD group had a significantly higher reticulated platelet count than the high TPO HD group (11.0 ± 4.70 vs 7.27 ± 5.32 × 109/L;P < .05). The low TPO (TPO less than 180 pg/mL) CAPD group had a significantly higher reticulated platelet count than the high TPO CAPD group (11.0 ± 4.79 vs 8.04 ± 4.74 × 109/L; P < .05). No specific differences between TPO levels and platelet counts were observed. These findings indicated that endogenous TPO levels were regulated predominantly by megakaryocyte mass in the marrow of both groups of dialysis patients, but not by peripheral platelet counts. Serum TPO levels were not significantly correlated with serum creatinine, urea nitrogen, i-PTH, or β2 microglobulin levels or with weekly doses (IU/kg) of rhEPO.
Effect of elevated TPO on erythropoiesis in dialysis patients
We addressed the clinical question whether endogenous TPO has any erythropoietic activity in dialysis patients. The significant inverse or positive correlations were obtained between hematocrits, reticulated platelet counts, and serum TPO levels in the HCV-negative HD (n = 40) and CAPD patients (n = 25) who were not treated with rhEPO (Table4). Furthermore, the groups with high TPO level had significantly greater hematocrits than the groups with low TPO levels, regardless of dialysis modalities (36.5% ± 3.07% vs 34.3% ± 3.76% in the HD group, P < .05; 35.5% ± 3.33% vs 33.0% ± 2.04% in the CAPD group,P < .05). In addition, the serum TPO and endogenous EPO levels were simultaneously measured in HD patients regularly treated with rhEPO (n = 33) and in those who did not require rhEPO therapy for at least 1 year (n = 33). Hematocrits were significantly higher in the HD patients who did not have rhEPO replacement therapy than in the group who did, though their endogenous EPO levels were almost the same. Serum TPO concentrations were significantly higher in patients without rhEPO replacement therapy than in those who received rhEPO replacement therapy (Table 5). There were no significant differences in age, HD duration, platelet counts, serum creatinine, urea nitrogen, transferrin saturation, or ferritin levels between the 2 groups.
Correlations of hematocrits with platelet counts, reticulated platelet counts, and serum TPO levels of HCV-negative dialysis patients without rhEPO replacement therapy
| . | Correlations . | Correlation coefficients . | P . |
|---|---|---|---|
| HD (n = 45) | Hct vs Plt | −0.057 | .7136 |
| Hct vs Ret | −0.325 | .0289 | |
| Hct vs TPO | 0.449 | .0017 | |
| CAPD (n = 30) | Hct vs Plt | −0.201 | .3399 |
| Hct vs Ret | −0.690 | .0001 | |
| Hct vs TPO | 0.573 | .0023 |
| . | Correlations . | Correlation coefficients . | P . |
|---|---|---|---|
| HD (n = 45) | Hct vs Plt | −0.057 | .7136 |
| Hct vs Ret | −0.325 | .0289 | |
| Hct vs TPO | 0.449 | .0017 | |
| CAPD (n = 30) | Hct vs Plt | −0.201 | .3399 |
| Hct vs Ret | −0.690 | .0001 | |
| Hct vs TPO | 0.573 | .0023 |
Hct, hematocrits; Plt, platelet counts; Ret, reticulated platelet counts; TPO, serum thrombopoietin levels.
Comparisons between serum levels of hematopoietic growth factors of HCV-negative HD patients with and without rhEPO replacement therapy
| Subjects . | Age (y) . | HD duration (y) . | Platelet counts (×109/L) . | Hct (%) . | TPO (pg/mL) . | EPO (mU/mL) . |
|---|---|---|---|---|---|---|
| HD patients with rhEPO (n = 33) | 55.9 ± 12.1 | 12.2 ± 5.90 | 189.4 ± 58.0 | 30.2 ± 2.96 | 95.1 ± 35.5 | 17.7 ± 7.65 |
| HD patients without rhEPO (n = 33) | 56.2 ± 9.40 | 14.6 ± 6.29 | 189.2 ± 49.2 | 34.95-150 ± 3.29 | 120.35-151 ± 48.2 | 17.8 ± 12.3 |
| Subjects . | Age (y) . | HD duration (y) . | Platelet counts (×109/L) . | Hct (%) . | TPO (pg/mL) . | EPO (mU/mL) . |
|---|---|---|---|---|---|---|
| HD patients with rhEPO (n = 33) | 55.9 ± 12.1 | 12.2 ± 5.90 | 189.4 ± 58.0 | 30.2 ± 2.96 | 95.1 ± 35.5 | 17.7 ± 7.65 |
| HD patients without rhEPO (n = 33) | 56.2 ± 9.40 | 14.6 ± 6.29 | 189.2 ± 49.2 | 34.95-150 ± 3.29 | 120.35-151 ± 48.2 | 17.8 ± 12.3 |
P < .01 vs HD patients with rhEPO;
P < .02 vs HD patients with rhEPO.
Data are shown as the mean ± SD.
Hct, hematocrits; TPO, thrombopoietin; EPO, erythropoietin.
All experiments were conducted in duplicate.
Discussion
Our study demonstrated that reductions of platelet counts were more frequent in patients on dialysis, particularly in the HCV-positive HD patients, and that the failure of megakaryocyte production in the marrow is an underlying cause of this platelet reduction in both HD and CAPD patients. In HCV-negative HD patients, megakaryocytopoiesis was shown to decrease with years on HD, possibly contributing to their annual decreases in platelet counts. Peripheral destruction–sequestration of platelets, on the basis of an autoimmune mechanism, may be a concomitant cause of the reduction in platelet counts, especially in the HCV-positive HD patients. Serum TPO concentrations were elevated in the dialysis patients, possibly in response to the reduced megakaryocyte mass in the marrow, which confirmed that TPO deficiency is never the primary cause of the failure of megakaryocytopoiesis in the marrow. This provoked elevation of TPO may be in part responsible for the variation in erythropoietic status among individual HD patients.
The incidence of thrombocytopenia was highest in the HCV-positive HD patients. The cause of thrombocytopenia in these patients was mostly a combination of failure of marrow thrombocytopoiesis and peripheral platelet destruction–sequestration. No studies correlate platelet lifespan or the association between PAIgG titers and platelet turnover. However, recent studies30 clarify the mechanisms of PAIgG generation and its association with peripheral platelet destruction in autoimmune disorders such as idiopathic thrombocytopenia purpura and systemic lupus erythematosus. Nonuremic patients with HCV have been shown31-33 to have high incidences of several autoimmune antibodies, including PAIgG and anti-phospholipid antibodies, and those antibodies may account for their thrombocytopenia. The formation of PAIgG may be partly attributable to HCV infection of the patients' platelets, bone marrow cells, or both.31,34 Although immune responses are thought to be reduced in HD patients,35,36 autoimmune antibodies are frequently produced.36-38 Our study showed that PAIgG is produced even in HCV-negative HD patients with thrombocytopenia, but production is more marked in those with HCV. The frequency and degree of thrombocytopenia in HD patients have been matters of controversy.12-14 The proportion of HCV-positive patients included in previous studies may have been related to the differing results in the past.
Reduced megakaryocyte production in the marrow, which was confirmed with the evaluation of the number of reticulated platelets in this study, is likely to be a common condition underlying thrombocytopenia in all dialysis patients. Our results agreed with the findings of Tàssies et al,39 who measured reticulated platelets in dialysis patients by a similar method.40 The reticulated platelet count is almost a useful indicator of marrow megakaryocytopoiesis, but it is not perfect. The appearance of reticulated platelets may be the manifestation of the end product of megakaryocytopoiesis, and the number of reticulated platelets may not reflect ineffective platelet production, in which megakaryocyte breakdown and platelet consumption may occur in the marrow cavity. We may bear this flaw of the measure in mind until direct findings of quantitative megakaryocyte and platelet turnover studies are established.
A major organ that constitutively produces TPO is considered to be liver, but it is also produced in the proximal tubules of the kidney.2,3,15 We thus hypothesized that diminished TPO production in the kidney may be in part associated with thrombocytopenia in dialysis patients. However, our study clearly showed that the deficiency of endogenous TPO was a negligible cause of thrombocytopenia or hypo-megakaryocytopoiesis in the HD and CAPD patients. What, then, induces the loss of reticulated platelets in dialysis patients? The platelet counts of the HCV-negative HD patients were related to the reticulated platelet counts that decreased with years on dialysis treatment (Table 3). Our retrospective analysis of the platelet counts in the 22 HCV-negative HD patients and the individual chronologic trends in the platelet counts of 4 patients with the same number of years on HD treatment confirmed that the platelet counts decreased with the number of years on HD even after rhEPO, which may induce thrombopoietic activity,41,42 was administered (Figure 2A-B). Both findings suggested that undialyzable marrow-suppressive toxins that gradually accumulate43despite HD treatment may be involved in the failure of platelet production.
Serum TPO levels were elevated in the HD and CAPD patients relative to the control subjects. This indicates that TPO secretion from an unknown source(s) may be altered in response to the decrease in platelet or megakaryocyte mass, or both, or that TPO destruction and removal may be reduced in those patients. The mechanisms regulating serum TPO levels remain unclear, but the TPO mRNA levels in TPO-producing organs, such as the liver and kidney, have been shown not to change appreciably in response to either thrombocytopenia or thrombocytosis.44,45 Accumulation of TPO, which exists in serum in glycosylated form with a molecular size of approximately 70 kd, may occur in dialysis patients. However, the number or binding affinity of the c-mpl protein (a receptor for TPO) on circulating platelets and megakaryocytes in marrow are generally thought to be the major route for the destruction and removal of TPO in serum,19,20,46 as in macrophage-colony stimulating factor.47 Our study showed that serum TPO levels were inversely correlated with reticulated platelet counts in the HCV-negative HD and CAPD patients, but not with their circulating platelet counts (Table 3). These findings suggest that the elevation of serum TPO levels in dialysis patients is induced predominantly by the failure of megakaryocyte production in the marrow. Stromal cells in marrow, which may sense the decrease in megakaryocytes, release TPO and may locally affect the turnover of megakaryocytes in marrow,48-50 and they could be candidates for a potential source of TPO that contributes to the elevation of its serum levels in the HD patients. We could not find a clear answer to why TPO levels were significantly higher in the CAPD patients, whose reticulated platelet counts were not less than those of the HD patients. Adsorption of TPO on dialyzer membranes should be considered, or the longer dialysis duration of the HD patients could be associated with this difference.
Our data imply that the higher levels of TPO in the dialysis patients may be favorable for erythropoiesis. The positive correlation between hematocrit and TPO, and the comparative study of patients with and without rhEPO replacement therapy, suggested that TPO may have erythropoietic activity or enhance endogenous EPO activity in vivo. TPO and EPO share sequences that are highly involved in erythropoietic activity; thus, TPO has been expected to function as an erythropoietic factor in vitro.1-5 In this regard, Era et al8 demonstrated that TPO enhanced the erythropoietic activity of rhEPO in an experiment using a yolk sac cell line. Similar results were also obtained in human CD34+ bone marrow cells and cord blood cells.9 Our own results may support that TPO serves as an erythropoietic factor in some patients. However, more solid evidence, such as a clinical effect of rhTPO administration on erythropoiesis in normal or uremic subjects, will be needed to state the erythropoietic effect of TPO.
In conclusion, our study demonstrated the following: (1) the reduction of platelet counts is a frequent finding in dialysis patients, especially HCV-positive HD patients; (2) the reduced megakaryocyte production in bone marrow possibly underlies a decrease in platelet counts, and the peripheral destruction–sequestration of platelets on an autoimmune basis may be concomitantly involved; (3) the TPO deficiency that would be expected because of loss of kidney mass is negligible as a cause of the thrombocytopenia, or the serum TPO levels are elevated in dialysis patients regardless of dialysis modality, possibly as a response to the reduction of megakaryocyte mass in marrow; and (4) the elevated TPO levels may in part serve as an aid to erythropoiesis in dialysis patients escaping rhEPO therapy.
Acknowledgment
We thank the research staff of Kirin Pharmaceutical for their constructive and helpful discussions.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Minoru Ando, Division of Nephrology, Tokyo Metropolitan Geriatric Medical Center, 35-2, Sakae-Cho, Itabashi-Ku, Tokyo, 173-0015, Japan; e-mail:aannddoo@f3.dion.ne.jp.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal