Abstract
Chemokines appear to have an important role in the seeding of lymphoid progenitors in the thymus, the regulation of the coordinated movements of the maturing T cells within this organ, and the egress of the resulting naive T cells to secondary lymphoid organs. CCR9, the specific receptor for the β-chemokine TECK/CCL25, is selectively expressed in thymus, lymph node, and spleen. Using a specific anti-CCR9 polyclonal antibody, K629, and a semiquantitative reverse transcriptase–polymerase chain reaction procedure, a detailed study of CCR9 expression in the thymus and secondary lymphoid organs was performed. The results show that CD4+CD8+ double-positive thymocytes have the highest CCR9 expression in thymus. Single-positive CD8+ thymocytes continue to express this receptor after abandoning the thymus as mature naive T cells, as suggested by the existence of a CD8+CD69lowCD62LhighCCR9+ cell subset. Consistent with this, CD8+lymphocytes from lymph nodes, spleen, and Peyer patches express a functional CCR9, as its expression correlates with migration in response to CCL25. Conversely, CD4+ thymocytes lose CCR9 before abandoning the thymus, and CD4+ T cells from secondary lymphoid organs also lack CCR9 expression. Analysis of CCR9 expression in thymocytes from mice of different ages showed that CCR9 levels are affected by age, as this receptor is more abundant, and its response to CCL25 is more potent in newborn animals. Collectively, these results suggest that CCR9 has a role in thymocyte development throughout murine life, with clear differences between the CD4+ and CD8+ lineages.
Introduction
T-cell development in the thymus starts after bone marrow lymphoid multipotent precursors reach the fetal thymic primordium, where thymocyte maturation is an organized differentiation process that gives rise to a functional T-cell compartment.1 During maturation, thymocytes migrate sequentially through thymic regions, starting in the subcapsular region where immature pre-T cells are found. Then they proceed to the cortex and the medulla, where mature CD4+ and CD8+ T cells reside.2,3 Pertussis toxin–sensitive mechanisms control the trafficking of lymphoid precursors to the thymus4 as well as the thymocyte movement and distribution within and outside the thymus,5-7 which is consistent with the involvement of chemokine-dependent processes. Chemokines are a family of small cationic proteins that exert a highly specific control of the migration of leukocytes by inducing their directional movement along chemokine gradients and by activating adhesion molecules.8-10 Based on conserved structural motifs, 4 chemokine subfamilies have been defined (CC, CXC, C, and CX3C), with most proteins belonging to the CC and CXC subfamilies. Here we use the recently proposed systematic nomenclature for chemokines.10 Chemokines interact with their target cells through specific receptors. The binding specificity and signaling ability of 18 chemokine receptors have been described so far, all of them G protein–coupled 7-transmembrane receptors that are inhibited by pertussis toxin.11
A number of thymus-expressed chemokines are reported to participate in the control of T-cell development. Trafficking of bone marrow hematopoietic progenitors may be controlled by CXCL12/SDF-1 (stromal-cell–derived factor–1) (the CXCR4 ligand), a reported chemoattractant for human CD34+ hematopoietic progenitor cells.12 Depending on their maturation stage, human and murine thymocytes are chemoattracted by different chemokines; most immature cells are responsive to CXCL12, whereas more mature T cells respond to CCL4/MIP-1β (macrophage inflammatory protein–1β) (a CCR1/CCR5 ligand) and to CCL19/MIP-3β and CCL21/SLC (secondary lymphoid tissue chemoattractant) (CCR7 ligands).13-15CCL25/TECK is an interesting chemokine that was first identified in the thymus, which chemoattracts thymocytes in a pertussis toxin–sensitive manner.16 We have recently described human and murine CCR9 as the specific receptor for the β-chemokine CCL25.17Besides its strict ligand specificity (CCL25 is the only CCR9 ligand known), this receptor is also interesting because of its selective expression in thymus, lymph node, and spleen.17 We have now further characterized mouse CCR9 expression using flow cytometry and semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR) approaches which show that CCR9 expression is modulated throughout thymocyte differentiation and is selectively maintained in CD8 T cells from secondary lymphoid organs. We also present data suggesting that CCR9 activity may be necessary for T-cell development throughout murine life.
Materials and methods
Isolation of primary cell populations
Thymus, spleen, lymph node, and Peyer patches were obtained from 4- to 6-week-old BALB/c mice, except where indicated otherwise; the organs were gently disrupted and filtered through nylon mesh to remove aggregates. To remove adherent cells, thymocytes were incubated in plastic flasks in RPMI 1640 (Gibco-BRL Life Technologies, Paisley, England) with 10% fetal calf serum (FCS) for 30 minutes at 37°C. In the case of RAG-2–deficient mice, thymocytes were prepared at different times after the intraperitoneal (i.p.) administration of 75 μg anti-CD3ε mAb 2C11 (Pharmingen, San Diego, CA) in 300 μL phosphate-buffered saline (PBS). To separate CD69+ from CD69− thymocytes, antimouse CD69-FITC (fluorescein isothiocyanate) (Pharmingen), paramagnetic anti-FITC microbeads (Miltenyi Biotech, Auburn, CA), and magnetic-activated cell sorter (MACS) separation columns (Miltenyi Biotech) were used. The samples obtained contained approximately 88% CD69+ and 98% CD69− cells, respectively. Splenocytes were resuspended in 2 mL 0.83% (wt/vol) NH4Cl to lyse erythrocytes. Peripheral blood mononuclear cells (PBMCs) were obtained by centrifuging heparinized blood on Nycoprep 1.077 cushions (Nycomed, Oslo, Norway); PBMCs were then collected from the interface and washed twice with PBS to remove platelets.
For semiquantitative RT-PCR analysis, thymus, spleen, and lymph node lymphocyte cells were purified into different subsets.
Thymocyte subsets.
Double-positive (DP) thymocytes were sorted as CD4+CD8+ cells from a cell suspension after double-staining with FITC-conjugated anti-CD4 and phycoerythrin (PE)-conjugated anti-CD8. Total CD4+, CD69+CD4+, and CD69−CD4+ single-positive (SP) thymocytes were sorted after depletion of CD8+ thymocytes from a thymic cell suspension by complement-mediated cytotoxicity using a cytotoxic anti-CD8 monoclonal antibody (mAb). CD8+, CD69+CD8+, and CD69−CD8+ SP thymocytes were sorted after CD4+ thymocyte depletion from a thymic cell suspension by complement-mediated cytotoxicity using a cytotoxic anti-CD4 mAb. Pre-T cells were sorted as CD4−CD8−CD25+ cells after depletion of CD4+ and CD8+ thymocytes by complement-mediated cytotoxicity using anti-CD4 and anti-CD8 cytotoxic mAb.
Spleen and mesenteric lymph node lymphocytes.
B cells, CD4+ T cells, and CD8+ T cells were sorted from spleen or mesenteric lymph node cell suspensions after triple-immunofluorescent staining with FITC-conjugated anti-B220, PE-conjugated anti-CD8, and tricolor-conjugated anti-CD4. Cells were sorted on a fluorescence-activated cell sorter (FACS) (FACSort flow cytometer; Becton Dickinson, Mountain View, CA). The sorted cell populations were more than 98% pure on re-analysis (data not shown).
Cell lines and reagents
The murine interleukin-3 (IL-3)–dependent Ba/F3 cell line (Dr D Milligan, Arris Pharmaceuticals, San Francisco, CA) was cultured in RPMI 1640 with 10% FCS, 10% conditioned medium from the IL-3–producing cell line WEHI.3B, and supplements. Plasmid constructs were prepared using the expression vector pCIneo (Promega Company, Palo Alto, CA); human CXCR4, CCR6, and CCR9; and murine CCR6, CCR8, and CCR9. The resulting plasmids were used to transiently or stably transfect Ba/F3 cells by electroporation. Mouse CCL25/TECK was used (R&D Systems, Minneapolis, MN).
Generation of rabbit antimouse CCR9 polyclonal antibodies
A peptide comprising murine CCR9 amino acids 3-22 was synthesized and coupled to KLH. Outbred New Zealand rabbits were injected intradermically in multiple sites with 300 μg peptide-KLH conjugate in complete Freund adjuvant. At weeks 4 and 7, intramuscular boosts were given using 150 μg of the same material in incomplete Freund adjuvant. Rabbit serum was collected 7-10 days after the last boost, antibody titers were determined by enzyme-linked immunosorbent assay (ELISA), and serum K629 was chosen for this study. Anti-CCR9 polyclonal antibodies (pAbs) were purified on a CCR9 peptide (3-22) affinity column, then biotinylated using EZ-link-Sulfo-NHS-LC-Biotin (Pierce, Rockford, IL).
Flow cytometry studies
The following mAbs were used in this study (brand names in parentheses): antimouse B220-FITC (clone RA3.6B2), CD4-TC and CD4-PE (L3T4), CD8-FITC and CD8-TC (Ly2), CD69-FITC and CD69-PE (H1.2F3), and CD62L-PE (Mel-14) (Pharmingen); goat F(ab′)2 antirabbit immunoglobulin G (IgG) (H+L)-RPE, antimouse B220-TC (RA3.6B2), and streptavidin-RPE (Southern Biotechnology, Birmingham, AL); CD4-Red613 (H129.19, Gibco); and antirabbit Ig-FITC (Amersham International, Little Chalfont, England). For staining, 5 × 105-106 cells were washed and resuspended in PBSst (PBS, 2% bovine serum albumin [BSA], and 0.05% sodium azide) with 20 μg/mL mouse IgG and then incubated for 20 minutes at 4°C. Primary antibodies were added, with blocking mouse IgG, followed by incubation at 4°C for 40 minutes. Following 2 washes in PBSst, subsequent antibodies were added, always with mouse IgG, followed by incubation for 30 minutes at 4°C. Four-color stainings were performed with the following combinations of mAbs and pAbs: affinity-purified rabbit antimouse CCR9 pAb, followed by antirabbit Ig-FITC, antimouse CD4-Red-613, CD8-TC, and CD69-PE or CD62L-PE. Three-color stainings were performed with purified rabbit antimouse CCR9 pAb, which was followed by antirabbit Ig-FITC, antimouse CD4-Red-613, and CD8-TC. Alternatively, 3-color experiments were done with biotinylated rabbit antimouse CCR9 followed by antimouse CD4-TC (or B220-TC), CD8-FITC, and streptavidin-RPE. Control stainings with pre-immune rabbit serum, an irrelevant affinity-purified rabbit serum raised against a mouse protein, or PBSst plus second antibody were performed routinely. All controls used gave a similar staining signal. Samples were analyzed in an EPICS XL flow cytometer (Coulter Electronics, Miami, FL), and events corresponding to approximately 2.5 × 105 cells were collected for each sample.
Chemotaxis assays
Cell migration was assayed in Transwell inserts (Costar, Cambridge, MA) with a 5-μm-pore diameter. Cells were resuspended in RPMI with 1% BSA and 25 mM HEPES (4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid) (107cells per mL), and 100-μL aliquots were loaded into the upper inserts. Chemokine aliquots of 600 μL, prepared in the same medium, were placed in the lower wells. Two or more replicate wells were used for each point. After a 2-hour incubation, inserts were removed, and migrated cells were counted in an EPICS XL flow cytometer. To determine the migration of the lymphocyte subpopulations, the number of cells in each subset of the migrated cell population was determined by appropriate staining assays.
Semiquantitative RT-PCR studies
Total RNA from mouse tissues or FACS-sorted cell subsets was extracted using Tri-reagent (Sigma Chemical, St Louis, MO). A total of 5 μg RNA from tissue or the total amount obtained from 5 × 104-106 cells was reverse-transcribed with random hexamers and the Superscript enzyme (Gibco). Semiquantitative real-time fluorescence PCR was performed in a LightCycler instrument (Roche, Mannheim, Germany) using SYBR green as fluorogenic dye. Briefly, 3 serial dilutions of each complementary DNA (cDNA) were subjected to 35-45 PCR cycles for 1 second at 93°C, 25 seconds at 68°C, and 2 seconds at 80°C in a 15-μL mixture containing 1 × LightCycler-DNA Master SYBR Green I (Roche), 4.5 mM MgCl2, and 0.4 μM each of forward and reverse gene-specific primers. To reduce nonspecific amplification, theTaq-containing component (LightCycler-DNA Master SYBR Green I) was pre-incubated with an anti-Taq antibody (Clontech Laboratories, Palo Alto, CA) as recommended by the manufacturer. SYBR green fluorogenic emission was acquired at 80°C to further minimize the formation of low-temperature–melting nonspecific DNA products. Oligonucleotides used for the cDNAs were: mouse CCR9: 5′-CACCATGATGCCCACAGAAC-3′ and 5′-GATGAGAAGCACACAGCTGTAG-3′; β-actin: 5′-AGGCTCTTTTCCAGCCTTCCT-3′ and 5′-CTAGAAGCATTTGCGGTGGACGATGGAGGGGCC-3′.
Northern blot analysis
Total RNA from mouse thymus was extracted with Tri-reagent (Sigma) and fractionated by electrophoresis on a denaturing formaldehyde-agarose gel. RNA samples were transferred to a Hybond-N (Amersham) membrane and ultraviolet cross-linked. The membrane was prehybridized and hybridized in Rapid Hyb buffer (Amersham) as recommended by the supplier.
Results
The pAb K629 binds selectively to CCR9-expressing transfectant cells
Rabbit pAb K629 raised against a peptide corresponding to CCR9 amino acids 3-22 was used to study CCR9 expression in different lymphoid subpopulations. To confirm specificity, pAb K629 was used in staining assays with Ba/F3 transfectants expressing different human and mouse chemokine receptors. Only Ba/F3 cells expressing mouse CCR9 bound K629 (Figure 1); there was no cross-reactivity with other Ba/F3-expressed chemokine receptors tested including hCCR9, which is 86% identical to CCR9.17 The immunizing peptide competed for CCR9 recognition by K629 (data not shown).
Rabbit pAb antiserum K629 specifically recognizes mouse CCR9.
Murine Ba/F3 cells transfected with different human or mouse chemokine receptors were stained with rabbit antimouse CCR9 pAb K629 and antirabbit Ig-FITC, and the results were analyzed by flow cytometry. Ba/F3 transfectants expressing the following chemokine receptors were used: mouse CCR9 (shaded histogram), human CCR6 (–-), mouse CCR6 (...), mouse CCR8 (– —), human CXCR4 (––), human CCR9 (–-), and the void vector pCIneo (…..).
Rabbit pAb antiserum K629 specifically recognizes mouse CCR9.
Murine Ba/F3 cells transfected with different human or mouse chemokine receptors were stained with rabbit antimouse CCR9 pAb K629 and antirabbit Ig-FITC, and the results were analyzed by flow cytometry. Ba/F3 transfectants expressing the following chemokine receptors were used: mouse CCR9 (shaded histogram), human CCR6 (–-), mouse CCR6 (...), mouse CCR8 (– —), human CXCR4 (––), human CCR9 (–-), and the void vector pCIneo (…..).
CCR9 is expressed mainly in CD4+CD8+ DP and CD4−CD8+ SP thymocytes
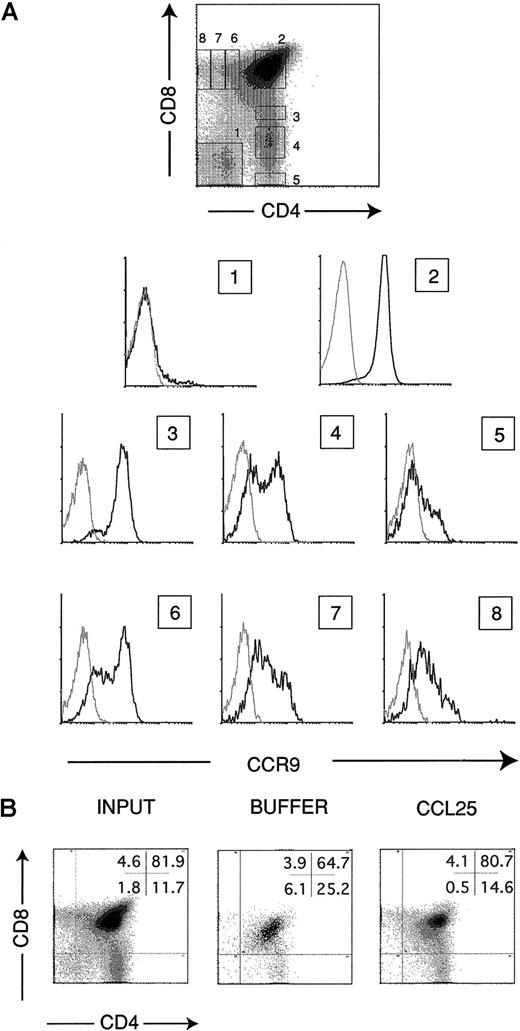
Based on messenger RNA (mRNA) analysis by Northern blotting, we previously reported thymus as the organ with maximum CCR9 mRNA expression, and greatly reduced expression of this chemokine receptor was noted in lymph nodes and spleen.17 Here, a more detailed study of CCR9 expression in thymus was performed using pAb K629. Thymocytes were stained in 3-color experiments to analyze CCR9 expression in subpopulations expressing different levels of CD4 and CD8 markers. The different thymocyte subpopulations studied expressed CCR9, although clear differences could be detected among them (Figure2A). Whereas only 4% to 6% of immature CD4−CD8− DN cells expressed the receptor, almost the entire CD4+CD8+ DP thymocyte subpopulation expressed CCR9. Among SP thymocyte subpopulations, both CD4+CD8− and CD4−CD8+SP subsets showed 2 cell subpopulations that differed in their CCR9 expression levels. Differences were clearly observed between CD4+ and CD8+ cell subsets, however, when gated cell subpopulations within the CD4 or CD8 lineages were analyzed for CCR9 expression. The results showed that CCR9 expression was progressively lost in maturing CD4+ cells, whereas more mature CD8+ thymocytes showed higher CCR9 levels (Figure2A).
Characterization of CCR9 expression on different thymocyte subsets.
(A) Thymocytes were stained in 3-color assays with the affinity-purified rabbit antimouse CCR9 pAb K629, which was followed by anti-CD4, anti-CD8, and antirabbit Ig-FITC (bold line). Numbered histograms show the CCR9 expression of the corresponding gated cell subpopulations indicated in the CD4/CD8 plot. Control stainings with an irrelevant rabbit antimouse serum are also shown (dotted line). (B) Flow cytometry analysis of thymocytes that have migrated in response to 300 nM CCL25 or control buffer. Thymocytes in the lower chamber of Transwell inserts were counted and stained with anti-CD4 and anti-CD8 pAbs. The percentage of cells in each subset is shown (upper right); analysis of input thymocytes is also shown. Representative results are shown of more than 10 independent experiments performed.
Characterization of CCR9 expression on different thymocyte subsets.
(A) Thymocytes were stained in 3-color assays with the affinity-purified rabbit antimouse CCR9 pAb K629, which was followed by anti-CD4, anti-CD8, and antirabbit Ig-FITC (bold line). Numbered histograms show the CCR9 expression of the corresponding gated cell subpopulations indicated in the CD4/CD8 plot. Control stainings with an irrelevant rabbit antimouse serum are also shown (dotted line). (B) Flow cytometry analysis of thymocytes that have migrated in response to 300 nM CCL25 or control buffer. Thymocytes in the lower chamber of Transwell inserts were counted and stained with anti-CD4 and anti-CD8 pAbs. The percentage of cells in each subset is shown (upper right); analysis of input thymocytes is also shown. Representative results are shown of more than 10 independent experiments performed.
The flow cytometry results were confirmed by a semiquantitative RT-PCR procedure using cDNA obtained from FACS-sorted thymocyte subsets and CCR9-specific primers (Table 1). Migration assays using CCL25 as a chemoattractant were performed to study whether the CCR9 protein detected in thymocytes was functional. In agreement with the CCR9 expression levels detected in each subpopulation, CD4+CD8+ DP thymocytes were the subset showing the greatest CCL25-mediated chemoattraction (Figure 2B). This migration was inhibited when the chemokine was present in both upper and lower compartments of the migration device, confirming that this response was mostly chemotactic (data not shown).
Semiquantitative reverse transcriptase-polymerase chain reaction analysis of mCCR9 expression of FACS–sorted lymphoid cell subpopulations
| . | mCCR9/β-actin, arbitrary units . |
|---|---|
| Thymus | |
| CD4−CD8−CD25+ | 5 |
| CD4+CD8+ | 100 |
| CD4+CD8− | 30 |
| CD4−CD8+ | 40 |
| CD4+CD8−CD69+ | 20 |
| CD4+CD8−CD69− | 5 |
| CD4−CD8+CD69+ | 30 |
| CD4−CD8+CD69− | 20 |
| Lymph nodes | |
| CD8+ | 14 |
| CD4+ | 1 |
| Spleen | |
| CD8+ | 8 |
| CD4+ | 1 |
| . | mCCR9/β-actin, arbitrary units . |
|---|---|
| Thymus | |
| CD4−CD8−CD25+ | 5 |
| CD4+CD8+ | 100 |
| CD4+CD8− | 30 |
| CD4−CD8+ | 40 |
| CD4+CD8−CD69+ | 20 |
| CD4+CD8−CD69− | 5 |
| CD4−CD8+CD69+ | 30 |
| CD4−CD8+CD69− | 20 |
| Lymph nodes | |
| CD8+ | 14 |
| CD4+ | 1 |
| Spleen | |
| CD8+ | 8 |
| CD4+ | 1 |
Anti-CD3ε treatment of RAG-2−/−mice up-regulates CCR9 protein expression in thymocytes
RAG-2–null (RAG-2−/−) animals do not express pre-TCR, and therefore their thymocyte maturation is blocked at the CD44−CD25+CD4−CD8−DN stage. CD3ε stimulation mimics pre-TCR signaling and is known to promote the DN to DP transition in RAG-2–deficient thymocytes. A recent report18 on the analysis of CCR9 mRNA in RAG-2–deficient mice showed that it was up-regulated in thymocytes when the animals were treated with a single i.p. injection of the anti-CD3ε mAb 2C11. We wanted to study whether the levels of functional CCR9 protein were also increased following that mRNA up-regulation. For this, the anti-CD3ε mAb 2C11 was injected toRAG-2–deficient mice, and total thymocyte samples were prepared at different times after the treatment and stained with the pAb K629. The results obtained were consistent with the results reported by Norment et al18 for CCR9 mRNA; 24 hours after the anti-CD3ε treatment of RAG-2–deficient mice, an increase in the CCR9 protein expression was already detected in the thymocytes (Figure 3). Analysis 48 and 96 hours after the treatment showed that the amount of CCR9 protein present in thymocyte membranes increased along that time interval. Chemotaxis assays showed that the CCL25-induced migration of thymocytes from RAG-2−/−–treated animals was also progressively stronger, as expected from their increasing CCR9 protein levels (data not shown). Control stainings showed that the anti-CD3ε treatment caused RAG-2−/− thymocytes to progress from a CD44−CD25+ to a CD44−CD25− phenotype, as expected (data not shown).
Anti-CD3ε treatment ofRAG-2−/− thymocytes up-regulates CCR9 expression.
Total thymocytes were prepared from RAG-2–deficient animals 24, 48, and 96 hours after a single ip administration of 75 μg anti-CD3ε mAb 2C11 in PBS and stained with pAb K629 to analyze CCR9 expression (bold-line histograms). Control stainings of thymocytes from untreated RAG-2–deficient animals are also shown (dotted-line histograms). Results shown are representative of 3 independent experiments performed.
Anti-CD3ε treatment ofRAG-2−/− thymocytes up-regulates CCR9 expression.
Total thymocytes were prepared from RAG-2–deficient animals 24, 48, and 96 hours after a single ip administration of 75 μg anti-CD3ε mAb 2C11 in PBS and stained with pAb K629 to analyze CCR9 expression (bold-line histograms). Control stainings of thymocytes from untreated RAG-2–deficient animals are also shown (dotted-line histograms). Results shown are representative of 3 independent experiments performed.
CCR9 is expressed mainly in more mature CD8+, but not CD4+, SP thymocytes
Four-color staining experiments were performed with SP thymocytes to analyze CCR9 expression in cells in distinct maturation stages, as indicated by CD4, CD8, CD69, and CD62L expression. Among CD4+ and CD8+ thymocytes, CD69−, CD69low, and CD69high subpopulations could be distinguished. CCR9 expression was associated mainly with CD69+ cells (Figure 4A). In agreement with these data, CCL25-induced migration was higher for the total CD69+ thymocyte subpopulation than for the CD69− thymocyte subset (Figure 4B). Some differences were detected between the CD4+ and the CD8+ SP cell subsets; indeed, CCR9 expression in CD4+ SP cells was shown mainly by CD4+CD69high thymocytes, whereas in CD8+ SP cells, CCR9 expression could be detected in both the CD8+CD69high and the CD8+CD69low cell subpopulations. We confirmed these results by semiquantitative PCR performed with FACS-sorted CD69+ and CD69− subsets of both CD4+ and CD8+ SP thymocyte subpopulations (Table 1). SP thymocytes modulate their CD62L levels from CD62Llow to CD62Lhigh before leaving the thymus as naive T cells. We studied CCR9 expression on thymocytes during that transition, and as Figure 4C shows, differences between the CD4+ and CD8+ thymocyte subsets were again detected. CCR9 expression was associated mainly to CD4+CD62Llow cells, whereas for CD8+ thymocytes, both CCR9+CD62Llowand CCR9+CD62Lhigh cells were present in the preparations.
Characterization of CCR9 expression on mature SP thymocytes.
Thymocytes were stained in 4-color experiments with anti-CD4 and anti-CD8 to gate in the SP thymocyte subpopulations. The gates used for CD4+ and CD8+ cells were those with numbers 5 and 8 in Figure 2, respectively. Gated cells were then analyzed for expression of CCR9 and CD69 (A) or CD62L (C). (B) CD69−and CD69+ thymocyte subsets were purified as described (“Materials and methods”), and migration in response to 300 nM CCL25 was assayed in Transwell inserts. A migration index was established as the ratio between cell number migrated in response to CCL25/cell number migrated to buffer. Results shown are representative of 4 independent experiments performed.
Characterization of CCR9 expression on mature SP thymocytes.
Thymocytes were stained in 4-color experiments with anti-CD4 and anti-CD8 to gate in the SP thymocyte subpopulations. The gates used for CD4+ and CD8+ cells were those with numbers 5 and 8 in Figure 2, respectively. Gated cells were then analyzed for expression of CCR9 and CD69 (A) or CD62L (C). (B) CD69−and CD69+ thymocyte subsets were purified as described (“Materials and methods”), and migration in response to 300 nM CCL25 was assayed in Transwell inserts. A migration index was established as the ratio between cell number migrated in response to CCL25/cell number migrated to buffer. Results shown are representative of 4 independent experiments performed.
CD8+ lymphocytes are the major CCR9-expressing cells in secondary lymphoid organs
A semiquantitative RT-PCR procedure was employed to confirm CCR9 mRNA expression in different lymphoid tissues. The results obtained concurred with those obtained in Northern blot analysis17and underscored that this β-chemokine receptor is expressed preferentially in thymus (Table 2). Low CCR9 expression was also detected in lymph nodes, Peyer patches, and spleen. A more detailed analysis of CCR9 protein expression on cell surface in lymphocyte subpopulations from these secondary lymphoid organs was carried out with pAb K629. The staining assays demonstrated that all CD8+ lymphocytes showed moderate CCR9 expression (Figure 5A). Conversely, CCR9+ lymphocytes less than 1% of total CD4+cells in all secondary lymphoid organs studied, which is in agreement with the semiquantitative RT-PCR results (Figure 5A, Table 1). In addition, approximately 1.5% to 3% of B220+ cells was consistently found to be CCR9+ lymphocytes. Migration assays performed with lymph node and spleen cells showed that CD8+ cells were chemoattracted by 300 nM CCL25 (Figure5B); in contrast, we were unable to detect clear migration by B220+ cells. Consistent with their lack of CCR9 expression, CD4+ T cells were unable to migrate.
Semiquantitative reverse transcriptase-polymerase chain reaction analysis of mCCR9 expression in different organs and tissues
| . | mCCR9/β-actin, arbitrary units . |
|---|---|
| Thymus | 100.00 |
| Lymph nodes | 3.50 |
| Spleen | 0.50 |
| Peyer patch | 1.40 |
| Small intestine* | 0.40 |
| PBMC | 1.00 |
| Liver | 0.05 |
| . | mCCR9/β-actin, arbitrary units . |
|---|---|
| Thymus | 100.00 |
| Lymph nodes | 3.50 |
| Spleen | 0.50 |
| Peyer patch | 1.40 |
| Small intestine* | 0.40 |
| PBMC | 1.00 |
| Liver | 0.05 |
Indicates without Peyer patches.
Characterization of CCR9 functional expression in secondary lymphoid organs.
(A) Cells from the sources indicated were stained with anti-CD8, anti-B220, or anti-CD4 and the biotinylated rabbit antimouse CCR9 pAb K629, and gated CD8+, B220+ and CD4+ cells were analyzed for CCR9 expression (bold line). Control stainings with PBSst are shown (dotted line). (B) Flow cytometry analysis of lymphocytes migrated in response to 300 nM CCL25 or to control buffer. Lymphocytes in the lower chambers of Transwell inserts were counted and stained with anti-CD4 or anti-B220 and anti-CD8 pAbs. The percentage of cells in each quadrant is shown (upper right). Representative results are shown from 3 independent experiments performed.
Characterization of CCR9 functional expression in secondary lymphoid organs.
(A) Cells from the sources indicated were stained with anti-CD8, anti-B220, or anti-CD4 and the biotinylated rabbit antimouse CCR9 pAb K629, and gated CD8+, B220+ and CD4+ cells were analyzed for CCR9 expression (bold line). Control stainings with PBSst are shown (dotted line). (B) Flow cytometry analysis of lymphocytes migrated in response to 300 nM CCL25 or to control buffer. Lymphocytes in the lower chambers of Transwell inserts were counted and stained with anti-CD4 or anti-B220 and anti-CD8 pAbs. The percentage of cells in each quadrant is shown (upper right). Representative results are shown from 3 independent experiments performed.
CCR9 protein expression in thymus decreases in mature and old mice
As the thymus involutes with age, we studied whether CCR9 was expressed in this lymphoid organ throughout murine life. Flow cytometry studies were thus performed with pAb K629 to analyze possible differences in the amount of CCR9 protein in thymocytes. Low CCR9 levels are expressed in thymocytes from day-15 fetuses, increase in thymocytes from day-17 fetuses, and reach maximum expression in newborn mice (Figure 6A). CCR9 expression decreased thereafter; this is illustrated by thymocytes from 1-month-old and 1-year-old animals, which showed lower, nearly similar CCR9 levels (Figure 6A). Semiquantitative RT-PCR analysis of CCR9 mRNA was performed in thymocytes from mice of different ages. Concurring with the results obtained with the pAb K629, CCR9 mRNA was detected in mouse thymocytes from day-15.5 fetuses, reached a maximum around birth, and then decreased with increasing animal age (Table3). Indeed, 45-day-old animals showed 2- to 3-fold lower CCR9 mRNA levels than did newborn mice. We also studied the response of these thymocytes to CCL25 in migration assays. Thymocytes from newborn animals showed the greatest chemotactic response (Figure 6B). Interestingly, in spite of showing similar CCR9 levels, CCL25-mediated chemoattraction was clearly stronger in 1-month-old animals than in 1-year-old mice. To study CCL25 expression throughout murine life, Northern blots were prepared with total thymus RNA from mice of various ages (day-16.5 fetuses to 8-month-old adult animals) and were analyzed with a CCL25-specific probe. The results showed that CCL25 mRNA levels were similar in all animals analyzed, regardless of age (data not shown).
Age-related effects on CCR9 expression and function in thymocytes.
(A) Total thymocytes from mice of the indicated age were stained with the biotinylated rabbit antimouse CCR9 pAb K629 followed by streptavidin-RPE. A control staining with PBSst is shown. (B) The migration response to 300 nM CCL25 by total thymocytes from mice of the indicated age was assayed in Transwell inserts, and a migration index was established as in Figure 3 (▪). The chemotactic component of this migration was estimated by placing CCL25 in the upper and lower chambers of the Transwell (■). Results are representative of 3 independent experiments performed.
Age-related effects on CCR9 expression and function in thymocytes.
(A) Total thymocytes from mice of the indicated age were stained with the biotinylated rabbit antimouse CCR9 pAb K629 followed by streptavidin-RPE. A control staining with PBSst is shown. (B) The migration response to 300 nM CCL25 by total thymocytes from mice of the indicated age was assayed in Transwell inserts, and a migration index was established as in Figure 3 (▪). The chemotactic component of this migration was estimated by placing CCL25 in the upper and lower chambers of the Transwell (■). Results are representative of 3 independent experiments performed.
Semiquantitative reverse transcriptase-polymerase chain reaction analysis of mCCR9 expression on thymocytes from animals of the indicated ages
| . | mCCR9/β-actin, arbitrary units . |
|---|---|
| Days postcoitum | |
| 15.5 | 11 |
| 16.5 | 18 |
| 18.5 | 62 |
| Newborns | 118 |
| Days postpartum | |
| 2 | 177 |
| 14 | 133 |
| 45 | 57 |
| . | mCCR9/β-actin, arbitrary units . |
|---|---|
| Days postcoitum | |
| 15.5 | 11 |
| 16.5 | 18 |
| 18.5 | 62 |
| Newborns | 118 |
| Days postpartum | |
| 2 | 177 |
| 14 | 133 |
| 45 | 57 |
Discussion
The physical architecture of the thymus provides an adequate environment for T-cell development by means of a complex and organized process involving sequential chemokine-controlled movement of maturing thymocytes through this lymphoid organ.1-3,5-7,13-15 We recently described human and murine CCR9 as the specific receptor for the β-chemokine CCL25, thus identifying what appear to be important elements in the maturation process.17 We have now further characterized murine CCR9 expression using flow cytometry and semiquantitative RT-PCR approaches.
In thymocytes, CCR9 is expressed at all stages of T-cell maturation, with maximum expression in CD4+CD8+ DP cells (Figure 2). Because most DN thymocytes do not express CCR9, it is tempting to speculate that the CCL25/CCR9 axis is not essential for the arrival of bone marrow lymphoid progenitors to the thymus. In consonance with this, thymus recolonization by T-cell precursors is reported to occur in the presence of a neutralizing antibody to CCL25.4 Altogether, these data suggest that other chemo-attractants are also involved in this process. Thymocytes fromRAG-2–deficient mice treated with anti-CD3ε progress from a CD44−CD25+ to a CD44−CD25− stage, as it happens in wild-type mice following pre-TCR signaling. Our results showing that this transition is associated with the up-regulation of the CCR9 protein in these thymocytes are in agreement with previous reports that showed the up-regulation of CCR9 mRNA.18
Positive selection in the thymus is a multistage process that involves transition through a CD4+CD8+CD69+intermediate phase and subsequent maturation phases that lead to the SP thymocyte stage.19 SP thymocytes then decrease their CD69 levels and increase expression of the homing receptor CD62L (L-selectin); these more mature cells are the major source of thymic emigrants.20 CCR9 expression in SP thymocytes was mainly associated to cells undergoing positive selection, as defined by their levels of the CD69 activation marker. This was also functionally corroborated, as the CD69+ thymocyte subset showed the highest CCL25-induced chemoattraction (Figure 4). A recent report showed that initiation and subsequent events in the positive selection of thymocytes are critically dependent on their sustained interaction with thymic epithelium, although interaction with MHC molecules is necessary only during the initiation stage.21 CCR9 is expressed by thymocytes undergoing positive selection, and its ligand, CCL25/TECK, is expressed by thymic epithelial cells.22 It is therefore tempting to suggest that in thymus, the CCL25/CCR9 axis delivers signals that play a role in the positive selection process of thymocytes.
Concurring with the results obtained with pAb K629, semiquantitative RT-PCR experiments showed that concomitantly with the CD69+to CD69− SP transition, there was a down-regulation of the CCR9 RNA message (Table 1). This down-regulation was particularly marked in the case of CD4+ SP cells, a result in consonance with the reported lack of response by mature CD4+ SP thymocytes to CCL25.14 Staining of pAb K629 detected the progressive disappearance of CCR9 from the membrane of thymocytes committed to the CD4+ lineage, which is in parallel to the progressive reduction in CD69 levels as they became mature CD4+ cells. In contrast, most mature CD8+ SP thymocytes still expressed CCR9, showing that the CCR9 down-regulation associated to maturation of this thymocyte subset is less dramatic than that detected in the CD4+ compartment. Results from analysis of CD62L levels in SP thymocytes also support the idea of CCR9 down-regulation within the SP thymocyte compartment, especially marked in the CD4 lineage, as maturation of these cells proceeds. A clear difference between CD4+ and CD8+ SP cells was again evident, as CCR9 expression was associated to CD4+CD62Llow cells. In the case of CD8+ thymocytes, apart from the CCR9+CD62Llow cell subset, another CCR9+CD62Lhigh cell subset was also present in the preparations. Altogether, these results add substantial details about the precise thymocyte subsets expressing this receptor in the context of mouse T-cell maturation in the thymus and confirm at the protein level what we and others have reported based on CCR9 mRNA analysis.17,18 22
We found that in secondary lymphoid organs, CCR9 is expressed mainly by CD8+ lymphocytes. These CD8+ T cells migrate in response to CCL25 (Figure 5). It therefore appears that CD8+ thymocytes still express CCR9 when they abandon the thymus, and CCR9 is also functionally expressed in CD8+ T cells from secondary lymphoid organs; this suggests a possible role for CCR9 in that migration process. As substantial CCL25 levels have not been found in organs other than thymus and small intestine,16,22 this putative role for CCR9 may be mediated by a distinct ligand produced in these lymphoid organs. CCR7 is a chemokine receptor with a very important role in T-cell migration to lymphoid organs. Studies in CCR7 null mice nevertheless showed that although greatly impaired, some T-cell migration to lymphoid organs still existed in these animals,23 which suggests that other chemokine receptors may contribute to this process. From our data it can be speculated that CCR9 could be one of these chemokine receptors.
The situation is markedly different for CD4+ cells, as the more mature CD4+ SP thymocytes express very low levels of CCR9, which is then practically undetectable in lymph node, Peyer patches, and spleen CD4+ T cells. In addition to CD8+ lymphocytes, a small subset of B220+ cells was consistently found to express CCR9. Nevertheless, these B lymphocytes were not clearly chemo-attracted by 300 nM CCL25 in migration assays (Figure 5). Similar results have been reported for a CCR9-expressing B-cell subpopulation from human peripheral blood lymphocytes.24 It is presently not possible to suggest a clear role for CCR9 in peripheral B cells. Consistent with the CCL25 expression reported in small intestine, we found CCR9 expression in a small lymphocyte subpopulation from Peyer patches (Figure 5, Table 2) and in intra-epithelial lymphocytes (IELs) (data not shown), although the CCR9 expression detected in mice is clearly lower than that reported for human IELs.24
Analysis of bone marrow showed that CCR9 was expressed by B220low lymphocytes from this primary lymphoid organ (data not shown). Interestingly, a recent report on the chemotactic responses of B cells as they progress through maturation in bone marrow showed that the cells respond to CCL25/TECK during an early B-lineage stage.25 Our finding of CCR9 expression in bone marrow B cells is thus in consonance with those results and suggests that as for T cells in the thymus, CCR9 plays a role in B-lymphocyte maturation in the bone marrow.
Advancing age is accompanied by a series of alterations in the immune system including thymic involution.19 Examination of CCR9 protein and mRNA levels showed that they were higher in newborns than in older animals or fetuses. This concurs with thymocyte population dynamics as described in the mouse, during which nearly all mouse thymocytes are CD4−CD8− DN cells until gestational day 15; some days later, most thymocytes are CD4+CD8+ DP cells,19 that is, cells which show the highest CCR9 expression. We found decreased CCR9 expression in thymocytes from older animals, also reflected as a diminished response to CCL25. Recent findings based on analysis of TCR rearrangement excision circles, a proposed marker for recent thymic emigrants, indicate that human thymus supports T-cell development throughout life, although the de novo T-cell generation rate was slower than that in younger persons.26 It can thus be speculated that the decreased CCR9 expression we find in thymocytes from older mice is an adjustment by the aging thymus to decrease the T-cell generation rate. We also provide evidence suggesting the existence of an additional mechanism to control CCR9 function in aged animals. Certainly, the migratory response to CCL25 is greatly reduced in thymocytes from 1-year-old mice, although their CCR9 protein levels are similar to those of thymocytes from 1-month-old mice. Analysis of CCL25 mRNA levels in mouse thymus showed that this β-chemokine is expressed from day-16.5 postcoitum embryos to 8-month-old animals, suggesting that the CCR9/CCL25 function is required for thymocyte maturation in fetuses, newborns, and adult animals (data not shown).
In summary, we report here a detailed characterization of mouse CCR9 expression. Our data suggest that this β-chemokine receptor could have several functions. First, CCR9 might have a role in thymocyte development that is necessary throughout the mouse life. Second, its preferential expression on secondary lymphoid organ CD8+ T cells suggests another role, which might include a contribution to the CD8+ cell trafficking to these organs. Finally, the reported CCL25 expression in small intestine16 22 and the CCR9 expression we detect in IELs and Peyer patch lymphocytes implicates CCR9 in the small intestine immune response.
Acknowledgments
We would like to thank Dr J. Gutiérrez for critical reading and comments on the manuscript and L. Gómez and M. Lozano for excellent technical assistance. We also thank M. C. Moreno-Ortı́z and I. López-Vidriero for help with flow cytometry analysis, Dr J. P. Albar and F. Roncal for providing the peptide used in immunization, and C. Mark for editorial assistance. The Departamento de Inmunologı́a y Oncologı́a was founded and is supported by the Spanish Research Council (CSIC) and by Pharmacia Corporation.
Supported in part by grant 08.1/0018/1998 (C.A.) from the Comunidad Autónoma de Madrid, Spain.
Submitted June 21, 2000; accepted October 18, 2000.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Gabriel Márquez, Departamento de Inmunologı́a y Oncologı́a, Centro Nacional de Biotecnologı́a/CSIC, Universidad Autónoma de Madrid, Cantoblanco, E-28049 Madrid, Spain; e-mail: gmarquez@cnb.uam.es.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal