Abstract
Cytochrome b5 reductase (b5R) deficiency manifests itself in 2 distinct ways. In methemoglobinemia type I, the patients only suffer from cyanosis, whereas in type II, the patients suffer in addition from severe mental retardation and neurologic impairment. Biochemical data indicate that this may be due to a difference in mutations, causing enzyme instability in type I and complete enzyme deficiency or enzyme inactivation in type II. We have investigated 7 families with methemoglobulinemia type I and found 7 novel mutations in the b5R gene. Six of these mutations predicted amino acid substitutions at sites not involved in reduced nicotinamide adenine dinucleotide (NADH) or flavin adenine dinucleotide (FAD) binding, as deduced from a 3-dimensional model of human b5R. This model was constructed from comparison with the known 3-dimensional structure of pig b5R. The seventh mutation was a splice site mutation leading to skipping of exon 5 in messenger RNA, present in heterozygous form in a patient together with a missense mutation on the other allele. Eight other amino acid substitutions, previously described to cause methemoglobinemia type I, were also situated in nonessential regions of the enzyme. In contrast, 2 other substitutions, known to cause the type II form of the disease, were found to directly affect the consensus FAD-binding site or indirectly influence NADH binding. Thus, these data support the idea that enzyme inactivation is a cause of the type II disease, whereas enzyme instability may lead to the type I form.
Introduction
Nicotinamide adenine dinucleotide reduced (NADH) cytochrome b5 reductase (b5R; EC 1.6.2.2) is a 300–amino acid, membrane-bound enzyme localized in the endoplasmic reticulum of all tissue cells. This enzyme transfers electrons from NADH to cytochrome b5 via its flavin adenine dinucleotide (FAD) prosthetic group.1 Reduced cytochromeb5 then donates its electrons to various acceptors involved in elongation and desaturation of fatty acids, cholesterol biosynthesis, and cytochrome P-450–mediated drug metabolism.2-5 In erythrocytes, a 25–amino acid shorter, soluble form of b5R exists, which is functional in methemoglobin reduction.6,7 Both forms of b5R are derived from a single gene,8 but recent studies have indicated that an erythroid-specific transcript generates the soluble form of b5R in humans.9
The primary structure of b5R is highly conserved among mammalian species.10 The 31-kd hydrophilic form of the human enzyme shows 92% sequence identity with the solubilized form of pig liver b5R, for which a 3-dimensional model of the crystal structure (1ndh) at 2.4 Å resolution is available.11 A preliminary account of the molecular structure of the human enzyme at 2.5 Å has also been published.12 The crystallographic analysis has revealed that 1ndh consists of 2 domains that are separated by a large interdomain cleft. The N-terminal domain, which harbors the FAD, is structurally related to the FAD-binding domain of ferredoxin-NADP+ reductase (FNR).13The C-terminal domain of 1ndh is involved in NADH binding, and its structure is somewhat different from the corresponding FNR domain. Despite the presence of a Rossmann fold14 and the structural homology with several other flavoenzymes,15-25the exact mode of NADH binding in 1ndh is unknown. Nevertheless, it has been suggested that the large interdomain cleft permits the nicotinamide moiety of NADH to access the re side of the isoalloxazine ring of the flavin without a conformational change of the C-terminal region.11 This would be different from the situation in FNR, where a displacement of a tyrosine residue is required for direct hydride transfer from NADPH to FAD.21 26
Deficiency of b5R may lead to 2 different clinical phenotypes. In type I methemoglobulinemia, cyanosis is the only clinical symptom.27,28 In type II methemoglobulinemia, cyanosis is accompanied by severe mental retardation and neurologic impairment.29,30 In type I, the b5R enzyme deficiency is profound in the erythrocytes (< 10% of normal activity) and moderate in other blood and tissue cells (20%-60% of normal activity).31-34 In type II, the b5R deficiency is generalized to all tissues.
The human b5R gene DIA1 is localized at chromosome 22q13 and contains 9 exons.8 Until now, 7 different mutations in this gene have been described to lead to type I b5R deficiency,33-39 and 11 different mutations to the type II disease.31,32,34 40-44 In general, type I mutations were found in “marginal portions” of the enzyme, giving rise to enzymatically active, but unstable proteins. Type II mutations cause exon skipping in messenger RNA (mRNA) and premature protein synthesis termination, or are localized in consensus FAD- or NADH-binding sites of b5R, leading to loss of protein expression or to expression of enzymatically inactive proteins. Due to the long half-life of erythrocytes and the lack of protein synthesis in these cells, type I b5R deficiency is mainly found in the red blood cells, whereas type II b5R deficiency is apparent in all tissue cells, with much more detrimental effects.
We now describe 7 new mutations in the b5R gene leading to type I methemoglobulinemia. These and previously published mutations were tested in a 3-dimensional structural model of human b5R for their effects on structure and function of b5R.
Patients, materials, and methods
Patients were classified as having methemoglobinemia when cyanosis was apparent, methemoglobin (metHb) in the blood was above 5% and/or metHb reductase in the erythrocytes was below 0.5 IU/g Hb. The patients were regarded to suffer from methemoglobinemia type I if neurologic abnormalities had not become manifest at the age of 6 to 9 months. EDTA or citrated blood samples were washed 3 times with 0.9% NaCl solution. MetHb reductase activity in the red cells was measured with the method of Hegesh and coworkers45 in a Beckman DU-65 spectrophotometer (Beckman Coulter, Fullerton, CA). The metHb levels were measured by the method of Evelyn and Malloy46with a Hewlett Packard 8451A diode-array spectrophotometer or with an OSM3 Hemoximeter from Radiometer (Copenhagen, Denmark). A Puregene DNA isolation kit (Gentra Systems, Minneapolis, MN) was used to isolate DNA from leukocytes. RNA was isolated from leukocytes by the method of Chirgwin and colleagues,47 followed by synthesis of complementary DNA (cDNA) as described by Bolscher and associates.48
DNA amplification and sequencing
Polymerase chain reaction (PCR) was performed in sealed glass capillaries containing 15 μL of reaction mixture consisting of 2 μL 10 × bovine serum albumin (BSA) solution (2.5 μg/μL BSA, Sigma Chemical, St Louis, MO), 1.5 μL Thermophilic DNA polymerase (Promega, Madison, WI) in 10 × buffer (100 mM Tris-HCl, pH 9.0, 500 mM KCl, 1% vol/vol Triton X-100), 0.75 μL MgCl2 25 mM, 0.6 μL dNTP solution (dATP, dCTP, dGTP, dTTP, 5 mM each; Gibco BRL, Gaithersburg, MD), 0.2 μL anti-Taq DNA polymerase (7 μM; 1.1 μg/μL in 50 mM KCl, 10 mM Tris-HCl, 50 vol % glycerol, pH 7.0; Clontech, Palo Alto, CA), 0.2 μL Taq DNA polymerase (5 U/μL; Promega), 0.5 μL flagged sense primer (10 μM) (Table 1), 1 μL antisense primer (10 μM) (Table 1), 5 μL genomic DNA solution (0.025 μg/μL in demineralized water) and filled up to 15 μL with demineralized water.
Sequences of the primers used for amplification of theb5R gene
| Exon and primer/fragment code . | Direction (sense or antisense) . | Primers . | Amplimer length (bp) . |
|---|---|---|---|
| FLAG | — | 5′ TGT AAA ACG ACG GCC AGT 3′ | — |
| Exon 1 | S | 5′ FLAG + CAG AAG CAG GCG GAG CGA CC 3′ | 252 |
| E1 | AS | 5′ GTC CCA GTC CCT CGC GAC GC 3′ | |
| Exon 2 | S | 5′ FLAG + TAG TGT GGA CAA GAA GCC GGT GT 3′ | 310 |
| E2 | AS | 5′ TAC TTC AGA GCA GAC CAT GC 3′ | |
| Exon 3 | AS | 5′ FLAG + ACG TCC CAG CTT CCT GCC TC 3′ | 352 |
| E3 | S | 5′ CGT TCA GCA GCT TTG GGC C 3′ | |
| Exon 4 | S | 5′ FLAG + GCA CCA GCA GGA GTG AAG GCA 3′ | 363 |
| E4 | AS | 5′ CAC CCA CAC CCC CTC CAC AG 3′ | |
| Exon 5 | S | 5′ FLAG + TGC AGA AGA GGC ACT TGT CC 3′ | 268 |
| E5 | AS | 5′ GTG TAA CCA AGG GAT TCC GA 3′ | |
| Exon 6 | S | 5′ FLAG + CCG GGC CTC ACC CCT TCT C 3′ | 473 |
| E6/7 | AS | 5′ CAG GGT AAG CTG AGT TTC CC 3′ | |
| Exon 7 | AS | 5′ FLAG + CAG GGT AAG CTG AGT TTC CC 3′ | 473 |
| E7/6 | S | 5′ CCG GGC CTC ACC CCT TCT C 3′ | |
| Exon 8 | S | 5′ FLAG + TGA AAG CTC CGC AAG CGT GT 3′ | 327 |
| E8 | AS | 5′ TCC AAA GCT CAA CGC CAC AA 3′ | |
| Exon 9 | S | 5′ FLAG + GCA TTT AAG AAT TCA CCG CC 3′ | 354 |
| E9 | AS | 5′ GGA GGT GAC TGG GTG AGC GT 3′ |
| Exon and primer/fragment code . | Direction (sense or antisense) . | Primers . | Amplimer length (bp) . |
|---|---|---|---|
| FLAG | — | 5′ TGT AAA ACG ACG GCC AGT 3′ | — |
| Exon 1 | S | 5′ FLAG + CAG AAG CAG GCG GAG CGA CC 3′ | 252 |
| E1 | AS | 5′ GTC CCA GTC CCT CGC GAC GC 3′ | |
| Exon 2 | S | 5′ FLAG + TAG TGT GGA CAA GAA GCC GGT GT 3′ | 310 |
| E2 | AS | 5′ TAC TTC AGA GCA GAC CAT GC 3′ | |
| Exon 3 | AS | 5′ FLAG + ACG TCC CAG CTT CCT GCC TC 3′ | 352 |
| E3 | S | 5′ CGT TCA GCA GCT TTG GGC C 3′ | |
| Exon 4 | S | 5′ FLAG + GCA CCA GCA GGA GTG AAG GCA 3′ | 363 |
| E4 | AS | 5′ CAC CCA CAC CCC CTC CAC AG 3′ | |
| Exon 5 | S | 5′ FLAG + TGC AGA AGA GGC ACT TGT CC 3′ | 268 |
| E5 | AS | 5′ GTG TAA CCA AGG GAT TCC GA 3′ | |
| Exon 6 | S | 5′ FLAG + CCG GGC CTC ACC CCT TCT C 3′ | 473 |
| E6/7 | AS | 5′ CAG GGT AAG CTG AGT TTC CC 3′ | |
| Exon 7 | AS | 5′ FLAG + CAG GGT AAG CTG AGT TTC CC 3′ | 473 |
| E7/6 | S | 5′ CCG GGC CTC ACC CCT TCT C 3′ | |
| Exon 8 | S | 5′ FLAG + TGA AAG CTC CGC AAG CGT GT 3′ | 327 |
| E8 | AS | 5′ TCC AAA GCT CAA CGC CAC AA 3′ | |
| Exon 9 | S | 5′ FLAG + GCA TTT AAG AAT TCA CCG CC 3′ | 354 |
| E9 | AS | 5′ GGA GGT GAC TGG GTG AGC GT 3′ |
Exon 1 of the b5R gene is very GC-rich and was therefore amplified with the following adaptations: 0.45 μL MgCl225 mM, 0.6 μL 7-deaza-dNTP solution 75% (3.75 mM 7deaza-dGTP, 1.25 mM dGTP, 5 mM dATP, 5 mM dCTP, 5 mM dTTP; Boehringer Mannheim, Mannheim, Germany) and addition of 3 μL GC-melt (5 M GC-melt, Clontech).
The PCR equipment consisted of an Air Thermo Cycler (ATC) (type: 1605, Idaho Technology, Idaho Falls, ID).
The cycle conditions were denaturation 5 seconds at 96°C, annealing 20 seconds at 65°C, and elongation 20 seconds at 72°C, for 40 cycles. Fragments 5 and 6,7 had annealing temperatures of 62°C and 68°C, respectively. Fragment 1 had an annealing/elongation time of 80 seconds at 72°C and was amplified for 60 cycles.
The oligonucleotide flag on the forward PCR primers was complementary to the −21m13 forward fluorescent primer. Direct sequencing of the amplified products was performed with BigDye Primer Cycle Sequencing Ready Reaction kit −21m13 FWD from ABI Prism, (Applied Biosystems, Warrington, United Kingdom). For each of the bases deoxy-A, -C, -G or -T, 4 μL of “ready reaction-mix A or C or G or T,” 1 μL of the 1:10 water-diluted PCR product, and 25 μL Biozym Biowax was put in a 96-well plate. The cycle sequence reactions were performed on PCR equipment from Hybaid (Middlesex, United Kingdom) type; OmniGene (Biozym, Landgraaf, The Netherlands) under the following conditions: denaturation 1 second at 95°C, annealing/elongation 15 seconds at 60°C, for 40 cycles. Subsequently, products of the same PCR sample were pooled and desalted with the Sephadex G-50/multiscreen system (Millipore, Molsheim, France) according to the Dye Terminator Removal protocol (http://www.millipore.com/analytical/technote/multiscreen/tn053/). The dried samples were dissolved in 3 μL sequence loading buffer (Amersham Pharmacia Biotech, Uppsala, Sweden). The samples were denatured for 2 minutes at 95°C, put on ice and loaded on an ABI Prism 377 sequencer (Applied Biosystems, Foster City, CA), run module settings: Seq Run 36E-1200, dRhoda matrix, DP 5%BD–21m13FWD. The sequencer was casted with a 5% acrylamide longranger-gel prepared from a 50% stock solution (FMC BioProducts, Vallensbeak Strand, Denmark).
cDNA amplification and sequencing
The cDNA was amplified as was genomic DNA, with about 2 ng cDNA in 5 μL demineralized water. The primers used to amplify exons 4, 5, and 6 were sense 5′ GTGGGCCAGCACATCTACCT and antisense 5′ GATCATGCCCACGGACTTCA. The cycle conditions were denaturation 5 seconds at 96°C, annealing 20 seconds at 60°C, and elongation 30 seconds at 72°C, for 35 cycles at slope 9. Direct sequencing of the amplified products was performed with the BigDye Terminator cycle sequencing Ready Reaction kit (Applied Biosystems, no. 4303153). Four microliters of this reaction mixture, 4 μL demineralized water, 2 μL antisense primer (10 μM), 1 μL of the antisense product, and 25 μL Biowax (Biozym) were put in a 96-well polycarbonate plate. The cycle conditions were 2 seconds at 95°C and 2 minutes at 70°C for 35 cycles in an Omnigene Thermocycler (Hybaid) with simultaneous tube control and calibration factor 200. The sequencing samples were purified and loaded on an ABI Prism 377 sequencer (Applied Biosystems) as described for genomic DNA.
Restriction fragment analysis
Restriction fragment analysis was performed with the following enzymes.
NciI (BcnI) cuts after C′ in CC′GGG, indicative for the mutation G→A in exon 2 (R49Q; single-letter amino acid codes); the PCR product with the normal sequence will be digested.
BsiEI (Bsh1285I) cuts after G′ in CG′GCCG, indicative for the mutation C→T in exon 3 (P64L); the PCR product (made with a mismatch primer) with the normal sequence will be digested.
ApaI (Bsp1201) cuts after C[prime in GGGCC′C, indicative for the mutation C→T in exon 5 (L144P); the PCR product with the normal sequence will be digested.
BstUI (Bsh1236I) cuts after G′ in CG′CG, indicative for the mutation G→A in exon 6 (A178T); the PCR product with the normal sequence will be digested. With the same enzyme the T→G mutation in exon 8 (L238R) was tested; the PCR product with the mutated sequence will be digested.
Pag1 (BspH1) cuts after T′ in T′CATGA, indicative for the mutation G→A in exon 9 (V252M); the PCR product with the mutated sequence will be digested.
The assays were performed with 1 μL restriction enzyme, 1 μL restriction enzyme buffer, 4 μL demineralized water, and 5 μL of the PCR product. Digestion took place overnight at 37°C. The products were loaded on a 1% agarose gel with ethidium bromide and were run for 30 minutes at 100 mA.
Structure homology modeling
The globular fold of human b5R was predicted with MODELLER49 using the CHARMM forcefield.50 The 3-dimensional model of the crystal structure of pig liver b5R at 2.4 Å resolution (Brookhaven Protein Data Bank file: 1ndh; reference 11) served as the template file. The stereochemical quality of the homology model was verified by PROCHECK51 and the protein folding was assessed with PROFILE52 and PROSAII,53which evaluate the compatibility of each residue to its environment independently. After this initial verification, incompatible regions of the model were optimized with the simulated annealing procedure (molecular dynamics) of the XPLOR package,54 thereby fixing the other parts of the protein. A simulated annealing calculation was performed for 1000 steps at 900 K, with each step taking 0.5 femtosecond. Before and after molecular dynamics the model was energy minimized with the conjugate gradient algorithm of XPLOR, the minimization converged after 2000 cycles (gradient: 0.1 kcal/mol). Again, the model was checked and verified. NADH docking was performed with the program O,55 based on the mode of NADP(H) binding in the related structure of ferredoxin-NAD(P)+reductase.26 NADH forcefield constants for energy minimization were derived from CHARMM. Finally, the model was verified after several rounds of energy minimization.
Results
Family studies
In family V, both parents showed half-normal metHb reductase activity, correlating with the G757A (exon 9) mutation in one of their alleles (Figure 1A, Table2). This mutation leads to the replacement of Val to Met at position 252. The 2 affected children were homozygotes for the G757A mutation and showed almost no metHb reductase activity. Their brother lacked the mutation and was unaffected.
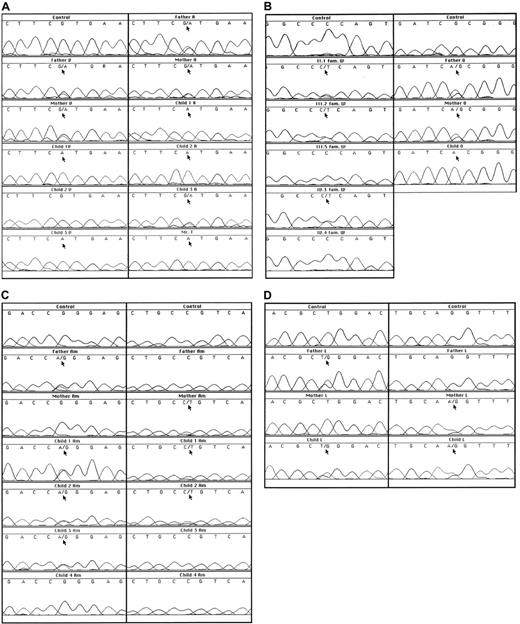
Sequence analyses of genomic DNA.
(A) Results of a fragment around G757 in families V, A, and T. (B) Results of a fragment around C434 in family W and a fragment around G535 in family O. (C) Results of a fragment around G149 and a fragment around C194 in family Am. (D) Results of a fragment around T716 and a fragment around the intron 4/exon 5 boundary in family L.
Sequence analyses of genomic DNA.
(A) Results of a fragment around G757 in families V, A, and T. (B) Results of a fragment around C434 in family W and a fragment around G535 in family O. (C) Results of a fragment around G149 and a fragment around C194 in family Am. (D) Results of a fragment around T716 and a fragment around the intron 4/exon 5 boundary in family L.
Summary of results
| Individual . | MetHb (%) . | MetHb reductase (IU/g Hb) . | Mutation . | Amino acid change . |
|---|---|---|---|---|
| Healthy controls (n = 0) | < 1.0 | 1.7-3.5 | ||
| Family V | ||||
| Father | not tested | 1.5 | G757A heterozygote | V252M |
| Mother | not tested | 1.3 | G757A heterozygote | V252M |
| Child 1 | not tested | 0.1 | G757A homozygote | V252M |
| Child 2 | not tested | 3.0 | G757G normal | |
| Child 3 | not tested | 0 | G757A homozygote | V252M |
| Family A | ||||
| Father | 0.4 | 0.7 | G757A heterozygote | V252M |
| Mother | 0.4 | 1.0 | G757A heterozygote | V252M |
| Child 1 | 10.7 | 0.1 | G757A homozygote | V252M |
| Child 2 | 10.0 | 0.1 | G757A homozygote | V252M |
| Child 3 | 0.5 | 1.5 | G757A heterozygote | V252M |
| Family T | ||||
| Mr T | 0.3 | 0.2 | G757A homozygote | V252M |
| Family W | ||||
| II.1 | 0.4 | 1.2 | C434T heterozygote | P144L |
| II.3 | not tested | 0 | not tested | |
| II.4 | not tested | 1.2 | not tested | |
| III.2 | 0.7 | 0.7 | C434T heterozygote | P144L |
| III.3 | not tested | 0.5 | not tested | |
| III.5 | 0.4 | 3.0 | C434C normal | |
| IV.1 | not tested | 1.7 | not tested | |
| IV.2 | not tested | 1.9 | not tested | |
| IV.3 | 0.3 | 1.1 | C434T heterozygote | P144L |
| IV.4 | 0.4 | 2.0 | C434C normal | |
| IV.5 | not tested | 1.0 | not tested | |
| Family Am | ||||
| Father | 0.7 | 2.3 | G149A heterozygote | R49Q |
| Mother | 0.1 | 1.6 | C194T heterozygote | P64L |
| Child 1 | 6.1 | 0.4 | G149A heterozygote | R49Q |
| C194T heterozygote | P64L | |||
| Child 2 | 2.0 | 0.8 | G149A heterozygote | R49Q |
| C194T heterozygote | P64L | |||
| Child 3 | 0.7 | 1.3 | G149A heterozygote | R49Q |
| Child 4 | not tested | 2.6 | normal | |
| Family L | ||||
| Father | 2.8 | 1.1 | T716G heterozygote | L238R |
| Mother | 1.7 | 1.1 | intron 4 − 1g → a heterozygote | Splice defect |
| Child | 9.1 | 0.2 | T716G heterozygote | L238R |
| Intron 4 − 1g → a heterozygote | Splice defect | |||
| Family O | ||||
| Father | 1.0 | 1.5 | G535A heterozygote | A178T |
| Mother | 2.0 | 1.1 | G535A heterozygote | A178T |
| Child | 31 | 0.1 | G535A homozygote | A178T |
| Individual . | MetHb (%) . | MetHb reductase (IU/g Hb) . | Mutation . | Amino acid change . |
|---|---|---|---|---|
| Healthy controls (n = 0) | < 1.0 | 1.7-3.5 | ||
| Family V | ||||
| Father | not tested | 1.5 | G757A heterozygote | V252M |
| Mother | not tested | 1.3 | G757A heterozygote | V252M |
| Child 1 | not tested | 0.1 | G757A homozygote | V252M |
| Child 2 | not tested | 3.0 | G757G normal | |
| Child 3 | not tested | 0 | G757A homozygote | V252M |
| Family A | ||||
| Father | 0.4 | 0.7 | G757A heterozygote | V252M |
| Mother | 0.4 | 1.0 | G757A heterozygote | V252M |
| Child 1 | 10.7 | 0.1 | G757A homozygote | V252M |
| Child 2 | 10.0 | 0.1 | G757A homozygote | V252M |
| Child 3 | 0.5 | 1.5 | G757A heterozygote | V252M |
| Family T | ||||
| Mr T | 0.3 | 0.2 | G757A homozygote | V252M |
| Family W | ||||
| II.1 | 0.4 | 1.2 | C434T heterozygote | P144L |
| II.3 | not tested | 0 | not tested | |
| II.4 | not tested | 1.2 | not tested | |
| III.2 | 0.7 | 0.7 | C434T heterozygote | P144L |
| III.3 | not tested | 0.5 | not tested | |
| III.5 | 0.4 | 3.0 | C434C normal | |
| IV.1 | not tested | 1.7 | not tested | |
| IV.2 | not tested | 1.9 | not tested | |
| IV.3 | 0.3 | 1.1 | C434T heterozygote | P144L |
| IV.4 | 0.4 | 2.0 | C434C normal | |
| IV.5 | not tested | 1.0 | not tested | |
| Family Am | ||||
| Father | 0.7 | 2.3 | G149A heterozygote | R49Q |
| Mother | 0.1 | 1.6 | C194T heterozygote | P64L |
| Child 1 | 6.1 | 0.4 | G149A heterozygote | R49Q |
| C194T heterozygote | P64L | |||
| Child 2 | 2.0 | 0.8 | G149A heterozygote | R49Q |
| C194T heterozygote | P64L | |||
| Child 3 | 0.7 | 1.3 | G149A heterozygote | R49Q |
| Child 4 | not tested | 2.6 | normal | |
| Family L | ||||
| Father | 2.8 | 1.1 | T716G heterozygote | L238R |
| Mother | 1.7 | 1.1 | intron 4 − 1g → a heterozygote | Splice defect |
| Child | 9.1 | 0.2 | T716G heterozygote | L238R |
| Intron 4 − 1g → a heterozygote | Splice defect | |||
| Family O | ||||
| Father | 1.0 | 1.5 | G535A heterozygote | A178T |
| Mother | 2.0 | 1.1 | G535A heterozygote | A178T |
| Child | 31 | 0.1 | G535A homozygote | A178T |
Single-letter amino acid codes used.
Family A had the same mutation as found in family V. Both parents as well as one child were heterozygotes. These 3 individuals showed decreased enzyme activity. The 2 patients in the family were homozygotes for the mutation, showed very low metHb reductase activity and had about 10% metHb in their red cells (Figure 1A, Table 2).
Mr T, whose relatives were not available for investigation, was a homozygote for the G757A mutation and had almost no metHb reductase activity (Figure 1A, Table 2). As far as we know, Mr T and the families V and A are not related to each other. In all 3 of these families, the mutation was confirmed by restriction analysis and was excluded as a polymorphism in 50 control DNA samples (100 alleles).
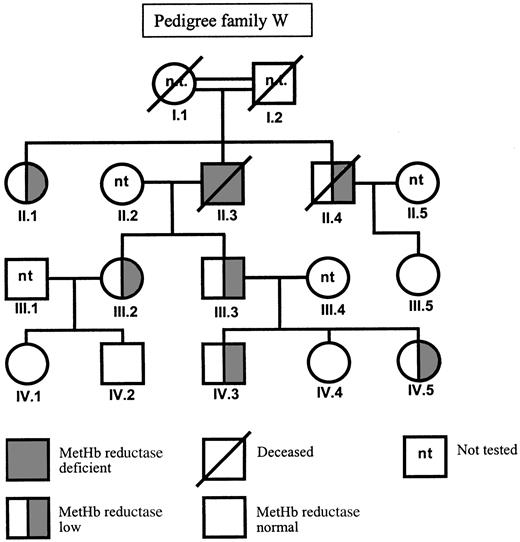
In family W, a C to T mutation in exon 5 was found by sequencing (Figure 1B). This C434T mutation predicts the substitution of a proline to leucine at position 144. Also this mutation was confirmed by restriction analysis. Unfortunately, we could not investigate the DNA of the presumably homozygous patient II.3 (Figure2), because he died between the time of enzyme analysis and the time of DNA analysis. In his family members, the presence of the heterozygous C434T mutation correlated with a metHb reductase activity of half the control value (Table 2). The family members who lacked the mutation showed a normal metHb reductase activity, indicating that the C434T mutation was the cause of the enzyme deficiency.
Mutation C434T was excluded as a polymorphism in 50 control DNA samples (100 alleles). Only in one control sample, the exon 5 PCR product was not totally digested by ApaI. When this sample was further investigated by sequencing, it became clear that the enzyme was not capable of digesting one allele because a polymorphic C432T was present. The normal sequence is 429GGGCCC434; the C434T mutation leads to 429GGGCCT434 and the polymorphism to 429GGGTCC434. The metHb reductase activity of this variant sample was normal.
In family Am from Spain, we found (Figure 1C) the parents to be heterozygotes, each for a different mutation, viz. G149A (predicting substitution of arginine for glutamine at position 49 in exon 2) and C194T (predicting substitution of proline for leucine at position 64 in exon 3). Two of their children proved to be compound heterozygotes for these mutations. Only in child 1, a girl, this situation was manifested by 6% metHb, low metHb reductase activity, and slight cyanosis in her lips (Table 2). Child 2, her brother, had 2% metHb and half-normal metHb reductase activity, without clinical signs. The third child, a girl, proved to be a heterozygote for the G149A mutation and had low normal metHb reductase activity. The fourth child was enzymatically and genetically unaffected. These mutations were confirmed by restriction analysis and excluded as polymorphisms by analysis of 50 control DNA samples.
Family L from Hong Kong had an almost 2-month-old baby with severe cyanosis. This patient had 9% metHb in her erythrocytes and very low metHb reductase activity (Table 2). The parents had half-normal metHb reductase activity. In exon 8 we found a T716G mutation heterozygous in the father and in the child (Figure 1D). This mutation predicts the substitution of a leucine to an arginine at position 238. In 50 control DNA samples, the T716G mutation was excluded as a polymorphism. In the child and in the mother, another mutation was found at the 3′ end of intron 4, where the normal sequence cagGTTTAC was replaced on one allele by caaGTTTAC (Figure 1D). This acceptor splice site mutation predicts skipping of exon 5 during mRNA processing. Indeed, in the mother's cDNA the sequence of exon 4 was followed by a mixture of exon 5 and exon 6 sequences (not shown), indicating that she was heterozygous for a mutation that caused skipping of exon 5. From the child, no RNA or cDNA was available for these investigations. Both mutations in this family were confirmed by restriction analysis. The child showed no neurologic abnormalities at the age of 1 year.
In family O, of North African origin, a newborn girl presented with severe cyanosis. This patient had 31% metHb in her erythrocytes and very low metHb reductase activity (Table 2). In her DNA we found an apparently homozygous G535A mutation in exon 6 of the DIA1gene (Figure 1B), predicting an A178T substitution. Both parents proved to be heterozygous for this mutation; their metHb percentage was low and their metHb reductase activity was below the normal range (Figure 1B, Table 2). The G535A mutation was confirmed by restriction analysis and excluded as a polymorphism in 50 control DNA samples. The child showed no signs of neurologic abnormalities at the age of 9 months.
Structural properties
Figure 3 shows the sequence alignment of human b5R and the solubilized form of pig liver b5R. From this alignment it is obvious that both structures are highly homologous. The amino acid differences between both enzymes are spread throughout the structure and are mainly localized in flexible regions near the protein surface. None of the variable residues are directly involved in FAD or NADH binding.
Sequence alignment of membrane-bound human b5R with the solubilized form of pig liver b5R.
Differences between the human and the pig enzyme are indicated by an asterisk. Secondary structure elements are underlined, the fingerprint sequence for binding the pyrophosphate moiety of NADH56 is depicted in bold, and type I mutated residues are indicated by a shadowed box.
Sequence alignment of membrane-bound human b5R with the solubilized form of pig liver b5R.
Differences between the human and the pig enzyme are indicated by an asterisk. Secondary structure elements are underlined, the fingerprint sequence for binding the pyrophosphate moiety of NADH56 is depicted in bold, and type I mutated residues are indicated by a shadowed box.
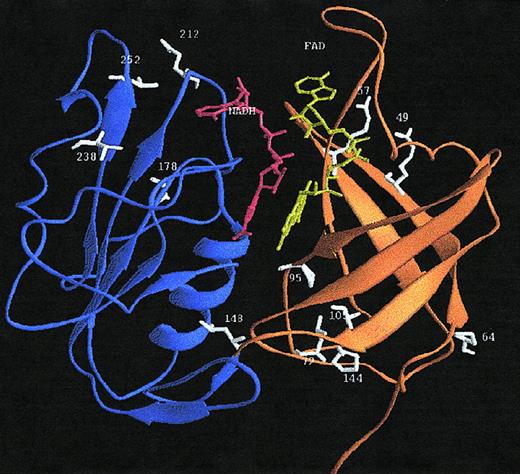
A 3-dimensional model of the structure of human b5R was constructed, based on the crystal structure of the solubilized form of1ndh. A ribbon diagram of the modeled structure of human b5R is presented in Figure 4. From this structural model and the sequence alignment, the location of the amino acid substitutions in b5R of the methemoglobinemia patients can be deduced (see “Discussion”).
Ribbon diagram of the 3-dimensional model of the soluble form of human b5R.
The crystal structure of the solubilized form of pig liver b5R11 served as the template. The FAD-binding domain is drawn in orange, the NADPH-binding domain in blue, FAD in yellow, NADPH in red, and the mutated residues giving rise to methemoglobinemia type I in white. The schematic ribbon diagram was generated with RIBBONS.57
Ribbon diagram of the 3-dimensional model of the soluble form of human b5R.
The crystal structure of the solubilized form of pig liver b5R11 served as the template. The FAD-binding domain is drawn in orange, the NADPH-binding domain in blue, FAD in yellow, NADPH in red, and the mutated residues giving rise to methemoglobinemia type I in white. The schematic ribbon diagram was generated with RIBBONS.57
The NADH cofactor was modeled in an extended conformation, highly similar to the mode of binding of NADP(H) in the related ferredoxin NADP+ reductase.26 The NADH molecule is situated in the interdomain cleft at the re side of the FAD isoalloxazine ring (Figure 4). The nicotinamide ring was modeled in theanti conformation, consistent with the proposed stereochemistry of A-hydrogen transfer in the FNR family.26 The pyrophosphate moiety is located near the 175 to 190 sequence of the single βα unit of the dinucleotide-binding fold, whereas the 2-OH ribose of NADH interacts in the model with the negatively charged D214. NADH-dependent proteins with a typical dinucleotide fold58 carry also a negatively charged residue at a similar position to contact the 2-OH of NADH.
Discussion
In 7 families with methemoglobinemia type I, characterized by cyanosis as the only clinical symptom, we found 6 different missense mutations in DIA1, the gene encoding human cytochromeb5 reductase. These mutations predict amino acid substitutions at various sites in the protein (Table3). In addition, we found one splice site mutation, which caused skipping of exon 5. This out-of-frame exon skipping in itself leads to enzyme inactivation and type II methemoglobinemia,42 but in our patient, this mutation was present in heterozygous form, in combination with a L238R substitution. We presume, therefore, that this last amino acid substitution does not have a strong deleterious effect on the b5R activity, because the patient did not suffer from type II symptoms (developmental and neurologic aberrations). Likewise, in family Am, we found 2 compound heterozygous individuals for R49Q and P64L substitutions. Because these patients had very mild cyanotic symptoms, we presume again that these mutations, too, should be classified as type I mutations. The other 3 amino acid substitutions found by us were expressed in homozygous form in type I patients.
Mutations in cytochrome b5reductase
| Type I mutations . | |
|---|---|
| Amino acid substitution . | Reference . |
| R49Q | This article |
| R57Q | 33,35 |
| P64L | This article |
| L72P | 37 |
| P95H | 43 |
| V105M | 33 |
| P144L | This article |
| L148P | 34, 35 |
| A178V | 38 |
| A178T | This article |
| C203Y | 39 |
| E212K | 36 |
| L238R | This article |
| V252M | This article |
| Type I mutations . | |
|---|---|
| Amino acid substitution . | Reference . |
| R49Q | This article |
| R57Q | 33,35 |
| P64L | This article |
| L72P | 37 |
| P95H | 43 |
| V105M | 33 |
| P144L | This article |
| L148P | 34, 35 |
| A178V | 38 |
| A178T | This article |
| C203Y | 39 |
| E212K | 36 |
| L238R | This article |
| V252M | This article |
| Type II mutations . | |
|---|---|
| Y42stop | 43 |
| R83stop | 38 |
| Intron 4 g − 1 → a (skip exon 5) | This article |
| S127P | 31-33 |
| Intron 5 g + 8 → c (skip exon 5) | 42 |
| Intron 5 a − 2 → c (skip exon 6) | 44 |
| C203R | 42 |
| R218stop | 42 |
| Intron 8 g − 1 → t (skip exon 9) | 41 |
| Deletion M272 | 42 |
| Deletion F298 | 40 |
| Type II mutations . | |
|---|---|
| Y42stop | 43 |
| R83stop | 38 |
| Intron 4 g − 1 → a (skip exon 5) | This article |
| S127P | 31-33 |
| Intron 5 g + 8 → c (skip exon 5) | 42 |
| Intron 5 a − 2 → c (skip exon 6) | 44 |
| C203R | 42 |
| R218stop | 42 |
| Intron 8 g − 1 → t (skip exon 9) | 41 |
| Deletion M272 | 42 |
| Deletion F298 | 40 |
Single-letter amino acid codes used.
In the literature, 7 additional amino acid substitutions have been reported in patients with type I methemoglobinemia33-39(Table 3). Some of these mutant enzymes have been expressed in bacterial systems, purified and studied for kinetic properties, heat stability, and trypsin sensitivity.33,34,38,39 In general, the recombinant type I mutant enzymes were found to have retained about 60% to 70% of the catalytic activity expressed by the recombinant wild-type enzyme, but to be more heat labile and trypsin sensitive. Similar experiments have been carried out with mutant enzymes from patients with type II methemoglobinemia.32,33,43,59 These enzymes were found to express very low catalytic activity and to be heat labile as well. According to these principles, the P95H mutation studied by Manabe and coworkers,43 although found in a type II patient, should be classified as a type I mutation, because the recombinant enzyme showed about 60% of the wild-type activity. The patient was a compound heterozygote for this mutation in combination with a Y42stop mutation. Apparently, this combination did not leave enough enzyme activity in the tissues to prevent psychomotor and neurologic deficiencies. Arbitrarily, we have listed this mutation in Table 3 as a type I mutation.
Localization and implication of amino acid substitutions in b5R
Inspection of the 3-dimensional model of b5R (Figure 4) revealed that the amino acid substitutions in b5R of the methemoglobinemia type I patients described in this paper are spread throughout the structure and not directly involved in FAD or NADH binding. The R49Q replacement identified in family Am from Spain concerns a nonconservative mutation located near the protein surface in the first β-strand of the FAD domain. In 1ndh, the Nζof the corresponding K21 forms a strong hydrogen bond with the carbonyl oxygen of the conserved E103.11 From this observation and the low levels of b5R in family Am we conclude that a positively charged residue at position 49 in human b5R is important for the structural integrity of the FAD-binding domain by forming optimal intradomain contacts.
The P64L substitution, also found in family Am, is localized in a surface loop connecting the first 2 β-strands of the FAD-binding domain. P64 is conserved in 1ndh (P36) and is responsible for the sharp turn of the surface loop comprising residues 62 to 76.11 On this basis and the very mild cyanotic symptoms in these patients we propose that substitution of P64 influences the folding properties of the FAD-binding domain. As reviewed recently,60 differences in loop flexibility may lead to a significantly decreased protein stability.
The P144L substitution present in b5R of some members of family W is situated at the C-terminal end of the final β-strand of the FAD-binding domain. This strand is connected by a short loop to the NADH-binding domain. P144 is conserved in 1ndh (P116), solvent accessible and rather far away from the active site.11 We conclude that the P144L replacement may lead to more conformational freedom impairing optimal interdomain contacts.
The A178T substitution in family O is localized at the C-terminal end of strand β8 of the NADH domain and fairly close to the adenosine moiety of the NADH coenzyme (Figure 4). Although not directly involved in coenzyme recognition, introduction of a bulkier and polar residue at position 178 might influence NADH binding, resulting in decreased catalytic activity.
The L238R substitution in family L from Hong Kong is localized at the C-terminal end of strand 10 of the NADH binding domain, somewhat remote from the protein surface and relatively far away from the domain interface. In 1ndh, L238 is conservatively replaced by a less bulky hydrophobic residue (V210). In the structure of the pig liver enzyme, V210 is relatively close (4 Å) to W217, which is conserved in the human enzyme (W245). Introduction of the polar, bulky arginine side chain at position 238 in human b5R might lead to an electrostatic interaction with E212 (b5R numbering), which is located in the surface loop, connecting strand β9 and helix α3 of the NADH domain. It is conceivable that such an interaction would lead to local structural perturbations, decreasing the overall protein stability.
The V252M substitution found in family V, family A, and Mr T is localized near the protein surface at the end of the large surface loop connecting strand β10 and the short helix α5 of the NADH domain. The side chain of Val 252 is rather close to the amide oxygen of the conserved Asn258 of helix α5 (4.1 Å). Replacement of valine with the bulkier methionine at position 252 might lead to some steric constraints, impairing optimal intradomain contacts.
Previously found amino acid substitutions
As noted above, 8 other type I disease mutations have been found in the b5R gene (Table 3). These mutations are also spread throughout the protein structure and are mostly localized in surface loops. Based on our 3-dimensional model, new insights about some of these mutations are discussed below.
The L72P replacement identified in a Chinese patient37 is localized in a loop connecting strands β2 and β3 of the FAD domain. This is in disagreement with Wu and colleagues,37 who concluded that the conserved L72 is part of a β-sheet. The side chain of L72 points toward the inner part of the protein into a hydrophobic environment. Therefore, the L72P replacement might distort the rigidity of the loop, resulting in a decreased stability of the folded protein.
Based on sequence alignments, it was predicted that V105 is part of a β-barrel structure.33 However, our 3-dimensional model suggests that the V105M replacement identified in a patient from Italy33 is localized in a loop that connects strands β4 and β5 of the FAD domain. The side chain of V105 is shielded from solvent and is likely involved in maintaining local hydrophobic contacts.
Based on secondary structure analysis, it was predicted that the E212K replacement identified in an African American patient disrupts an α-helix peptide structure.36 However, our 3-dimensional model suggests that E212 is located in a short surface loop connecting strand β9 and helix α3 of the NADH domain and that its side chain interacts with the conserved R218. This ionic interaction likely determines the rigidity of the loop.
In conclusion, we have shown that the novel point mutations in the b5R gene of patients with methemoglobinemia result in amino acid replacements that are distributed throughout the protein structure and not in positions directly involved in FAD and NADH binding. This is in agreement with the mild cyanotic symptoms of these patients and supports the idea that enzyme inactivation is a cause of the type II disease, whereas enzyme instability may lead to the type I form. Moreover, from our 3-dimensional model of human b5R, which was constructed on the basis of the crystal structure of the pig liver enzyme,11 the effects of some other point mutations could be reinterpreted in a rational way. This knowledge, together with additional information on the interaction between b5R and its physiologic electron acceptor cytochromeb561-63 sheds more light on the molecular aspects of methemoglobin reductase deficiency.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dirk Roos, Central Laboratory Netherlands Blood Transfusion Service, Plesmanlaan 125, 1066CX Amsterdam, The Netherlands; e-mail: d_roos@clb.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal