Abstract
Monocyte chemoattractant protein-1 (MCP-1) is a major chemoattractant for monocytes and T lymphocytes. The MonoMac6 cell line was used to examine MCP-1 receptor-mediated signal transduction events in relation to MCP-1–mediated monocytic transendothelial migration. MCP-1 stimulates, with distinct time courses, extracellular signal-related kinases (ERK1 and ERK2) and stress-activated protein kinases (SAPK1/JNK1 and SAPK2/p38). SAPK1/JNK1 activation was blocked by piceatannol, indicating that it is regulated by Syk kinase, whereas SAPK2/p38 activation was inhibited by PP2, revealing an upstream regulation by Src-like kinases. In contrast, ERK activation was insensitive to PP2 and piceatannol. Pertussis toxin, a blocker of Go/Gi proteins, abrogated MCP-1–induced ERK activation, but was without any effect on SAPK1/JNK1 and SAPK2/p38 activation. These results underscore the major implication of Go/Gi proteins and nonreceptor tyrosine kinases in the early MCP-1 signaling. Furthermore, MCP-1–mediated chemotaxis and transendothelial migration were significantly diminished by a high concentration of SB202190, a broad SAPK inhibitor, or by SB203580, a specific inhibitor of SAPK2/p38, and abolished by pertussis toxin treatment. Altogether, these data suggest that coordinated action of distinct signal pathways is required to produce a full response to MCP-1 in terms of monocytic locomotion.
Introduction
Leukocyte migration toward inflammatory or injured tissues is a multistep process mediated by a series of sequential cellular interactions in which the generation of chemotactic gradients plays a key role. A superfamily of chemotactic polypeptides, known as chemokines, selectively induces endothelial cell adhesion and transmigration of leukocyte subsets. Chemokines are structurally related proteins characterized by the presence, on their primary amino acid sequence, of 4 conserved cysteines. Chemokines are grouped into 4 families as designated CXC, CC, C, and CX3C, depending on the number and spacing of the first 2 conserved cysteines.1-5
Schematically, CXC (or α) chemokines are preferentially active on neutrophils, T cells, and nonhemopoietic cells, whereas CC (or β) chemokines exert their action on multiple leukocyte subtypes, including monocytes, eosinophils, basophils, natural killer (NK), dendritic, and T cells, but they do not target neutrophils. The C (or γ) chemokine is specific for T and NK lymphocytes,6 and the CX3C (or δ), like the CC chemokines, appears to be a potent chemoattractant for monocytes and lymphocytes but not for neutrophils.2All chemokines activate their function through interaction with 7-transmembrane spanning G protein-coupled receptors. Five receptors for CXC chemokines (CXCR1-5) and 9 for CC chemokines (CCR1-9) have been cloned to date. The receptor for the CX3C fractalkine has been recently characterized,7 whereas that for the C lymphotactin is still unknown. These receptors are generally, but not exclusively, coupled to pertussis toxin-sensitive Gαi proteins. It has been demonstrated that the association of the chemokine receptor to different G proteins depends on the receptor and the cell line studied.8
Monocyte chemoattacant protein-1 (MCP-1) was the first CC chemokine identified, from culture supernatants of human malignant tumor cell lines, as a tumor-derived chemotactic factor with the characteristic properties of inducing migration of monocytes.9 MCP-1 was predominantly expressed in tumor epithelial areas and correlated with the number of infiltrate macrophages.10-12 Many other activities have been assigned to MCP-1, including induction of migration of memory/activated T cells13 and NK cells14 and activation of mast cells.15 MCP-1 mediates its cellular effects primarily through its binding to CCR2,16 which exists in A and B forms that arise via alternative splicing of the carboxyl-terminal tail.17Analysis of the signal transduction pathways triggered by MCP-1 has revealed that it induces a pertussis toxin (PTX)-sensitive rise of intracellular calcium,18,19 inhibition of adenyl cyclase,19 phospholipase C activation,20activation of extracellular signal-related kinases (ERKs),21 stimulation of 2 separate PI 3-kinase isoforms, namely p85/p110 PI3-kinase (PI3-K) and PI3K-C2α.22Recently, analysis of signaling events in human monocytic cells has shown that MCP-1 triggers tyrosine phosphorylation and activation of the JAK2/STAT3 pathway in a PTX-independent manner.23Importantly, the signaling pathways through which MCP-1 triggers cell migration are still poorly documented. The exact relationships between ERKs, PI3-K, and transient calcium influx in inducing leukocyte migration in response to MCP-1 remains elusive.
In this study, we report on the activation of different mitogen-activated protein kinases (MAPKs) in a human monocytic cell line, MonoMac6, by the CC chemokine MCP-1. We show that although ERK activation is swift and transient, the activation of the 2 stress-activated protein kinases (SAPKs), JNK1 and P38, is delayed and more sustained. Specific inhibitors of these serine/threonine kinases have been used to study their implication in the chemotactic process on acellular filters as well as during the transendothelial migration of MonoMac6 induced by MCP-1.
Materials and methods
Cell culture
MonoMac6 cells (DSM ACC124) originally established from a patient with monoblastic leukemia24 were obtained from the German Collection of Microorganisms. The cell cultures (Braunschweig, Germany) were grown in suspension in RPMI 1640 (Gibco-BRL Life Technologies, Cergy-Pontoise, France) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Gibco),l-glutamine (2 mM), penicillin (100 U/mL), and streptomycin (100 μg/mL). Cells were passaged every 3 to 6 days using a split ratio of 1:3.
Products
Human rMCP-1 was procured from R&D Systems (Abington, UK). Fibronectin, pertussis, and cholera toxins were obtained from Sigma (St Louis, MO). PP2, piceatannol, PD98059, SB202190, and SB203580 were purchased from Calbiochem (Meudon, France), and tumor necrosis factor (TNF)-α from Pepro Tech Inc (Rocky Hill, NJ).
Cell stimulation and lysis
MonoMac6 cells (106 cells/mL) were starved 16 hours in RPMI 1640 medium and harvested by centrifugation for 5 minutes at 1000g before being resuspended in RPMI at a concentration of 2 × 107 cells/mL. Cells (5 × 106) were treated at 37°C with or without the effectors for the indicated times and lysed 30 minutes at 4°C in a buffer containing 150 mM NaCl, 0.8 mM MgCl2, 5 mM EGTA, 1% NP-40, 1 mM phenylmethylsulfonyl fluoride (PMSF), 15 μg/mL leupeptin, 1 μM pepstatine and 1 mM Na3VO4, and 50 mmol/L Hepes at pH 7.5. The crude lysates were centrifuged at 18 000g for 10 minutes at 4°C, the supernatants (lysates) were removed, and the protein concentration was assayed using the Bradford method (Biorad, Hercules, CA).
Immune-complex kinase assays
JNK1 activity.
The lysates were precleared with rabbit nonimmune serum prebound to protein A-Sepharose (Pharmacia-LKB Biotechnologies, Uppsala, Sweden) and JNK1 kinase was immunoprecipitated from precleared lysates by incubation at 4°C for 16 hours with 1 μg polyclonal anti-JNK1 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) bound to protein A-Sepharose. Immnopellets were washed twice with lysis buffer and twice with MAPK buffer (30 mM NaCl, 0.1% NP-40, 10% glycerol, 200 μM Na3VO4, 30 mM Hepes at pH 7.5) before being resuspended in 50 μL MAPK buffer containing 30 mM Mg-acetate in the presence of 0.5 mg/mL GST-ATF2, which was used as exogenous substrate. The kinase assay was initiated by addition of 25 μM ATP and 20 μCi/mL γ[32P]ATP (370 MBq/mL, ICN). After incubation at 30°C for 30 minutes the reactions were stopped by addition of 9 × Laemmli sample buffer and boiling for 3 minutes.
Src kinase activity.
Precleared lysates were incubated at 4°C for 4 hours with 2 μg polyclonal anti-c-Src antibodies (Argène Biosof, Varilhes, France) followed by the addition of protein A/G Sepharose (Santa Cruz Biotechnology), and then incubated for an additional hour at 4°C. The immunopellets were washed twice with lysis buffer and twice with tyrosine kinase buffer (10 mM MnCl2, 20 mM Hepes at pH 7.5). Samples were then resuspended in 50 μL tyrosine kinase buffer supplemented with 1 mM DTT and 0.1 mg/mL acid-denatured enolase, which was used as an exogenous substrate. The kinase assay was started by addition of 3.75 μM ATP and 20 μCi/mL γ[32P]ATP. After 15 minutes at 30°C the reactions were stopped by addition of 25 μL 9 × Laemmli sample buffer and boiling for 3 minutes.
For JNK and c-Src kinase activities, immunocomplex reactions were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 10%-15% gel) followed by blotting onto Immobilon-P-membrane (Millipore, Bedford, MA) and autoradiography using Biomax ML (Kodak, Rochester, NY).
Syk activity.
Syk kinases were immunoprecipitated from precleared lysates by incubation at 4°C for 3 hours with polyclonal anti-Syk antibodies (Santa Cruz Biotechnology) bound to protein A-Sepharose. Immunopellets were washed twice with lysis buffer, twice with lysis buffer supplemented with 0.25% sodium deoxycholate, and resuspended in 50 μL 3 × Laemmli sample buffer. Proteins resolved by SDS-PAGE were electrophoretically transferred to Immobilon-P-membrane and tyrosine phosphorylated Syk kinases were detected using 4G10 monoclonal antiphosphotyrosine antibodies (Upstate Biotechnology, Lake Placid, NY).
The amount of immunoprecipitated ERKs, JNK, and Syk was evaluated on Western blots using specific antibodies revealed by chemiluminescence using Enhanced ChemiLuminescence (ECL) kit (Amersham, Arlington Heights, IL) and hyperfilms MP (Amersham).
ERKs and p38 MAPK activities.
Cell lysates (100 μg) were boiled after addition of 9 × Laemmli sample buffer prior to being separated by 12% SDS-PAGE and transferred onto Immobilon-P membranes (Millipore) as detailed previously.25 The immunoblots were incubated overnight at 4°C with rabbit polyclonal antiactive ERK antibodies (Santa Cruz Biotechnology) or antiactive p38 antibodies (Promega, Madison, WI). After 3 washes with buffer (10 mM Tris at pH 7.4, 150 mM NaCl, 1% NP-40), the antibody binding was detected using horseradish peroxidase (HRP)-conjugated goat antirabbit (GAR) antibodies (Dako, Botany, Australia) and ECL system with autoradiography hyperfilms MP. The immunoblots were stripped for 30 minutes at 50°C in 67 mM Tris at pH 6.7, 2% SDS, 100 mM 2-ME and reprobed with rabbit polyclonal anti-ERK2 or anti-p38 antibodies (Santa Cruz Biotechnology) as described above.
Chemotaxis assays
Chemotactic responses of MonoMac6 cells were evaluated by using 24-well chemotaxis chambers and polyethylene terephtalate (PET) inserts with 8 μm pores (Becton Dickinson, San Jose, CA) coated with 6.5 μg/mL fibronectin (Sigma) on both sides. MonoMac6 cells, incubated for 16 hours with [3H]-methyl thymidine (ICN, 2.5 μCi/mL) were preincubated or not at 37°C with the different effectors and then were placed in the upper well (0.5 × 106 cells/100 μL) and 5 nM MCP-1 was added to the lower well. Plates were incubated for different time courses at 37°C in 5% CO2 atmosphere, and the migrated cells collected in the lower well were evaluated by the measure of incorporated [3H]-methyl thymidine by scintillation spectroscopy.
Transendothelial migration assays
Isolation and culture of human umbilical vein endothelial cells (HUVECs) was performed as described.26 27 Transendothelial migration assays were performed by using 24-well chemotaxis chambers. HUVECs were grown on PET inserts with 8-μm pores (Becton Dickinson) and treated with TNF-α at 10 ng/mL for 48 hours before use. Transendothelial migration assays were performed by using 24-well chemotaxis chambers. [3H]-methyl thymidine–labeled MonoMac6 cells (ICN, 2.5 μCi/mL), preincubated or not at 37°C with the different effectors, were placed in the upper well (0.5 × 106 cells/100 μL) and 6 nM MCP-1 was added to the lower well. Plates were incubated for different time courses at 37°C in 5% CO2 atmosphere, and the migrated cells collected in the lower well were evaluated by scintillation spectroscopy.
Results
MCP-1 stimulates ERK and SAPK activities in human MonoMac6 cells following distinct time courses
Monocyte chemoattractant protein-1 has been reported to modulate ERK activities in murine T-cell hybrids,21 in human monocytes, and in Chinese hamster ovary cells expressing CCR2B.28 On the basis of this observation, we became interested in studying the effect of MCP-1 on the activity of the different members of endogenous MAP kinases in the human monocytic cell line, MonoMac6.
To study the effects of MCP-1 on ERK activities, MonoMac6 cells were treated with MCP-1 (20 nM) for various periods of time before being lysed and subsequently analyzed for the activation status of ERK1 and ERK2 by immunodetection with a specific antiphospho p42/p44 ERKs antibody. As shown in Figure 1A, MCP-1 stimulated rapidly and transiently both ERK1 and ERK2 activities with a peak between 5 and 10 minutes and a basal level recovered within 30 minutes.
MCP-1 stimulates with distinct time courses ERKs, JNK1, and p38 activities in MonoMac6 cells.
MonoMac6 cells incubated with 20 nM MCP-1 for the indicated times and proteins extracted from lysates, resolved by SDS-PAGE, and electrophoretically transferred to Immobilon-P membrane were detected by immunoblotting with antiactive ERKs (A) or p38 (C) antibodies. JNK1 activity (B) was performed, from cell lysates, by in vitro kinase assay using GST-ATF2 as substrate after immunoprecipitation (IP) with specific antibodies as described in “Materials and methods.” The amounts of ERKs, JNK1, or p38 in immunoprecipitates were assessed by Western blotting analysis with the appropriate antibodies (WB). The results are representative of 5 experiments.
MCP-1 stimulates with distinct time courses ERKs, JNK1, and p38 activities in MonoMac6 cells.
MonoMac6 cells incubated with 20 nM MCP-1 for the indicated times and proteins extracted from lysates, resolved by SDS-PAGE, and electrophoretically transferred to Immobilon-P membrane were detected by immunoblotting with antiactive ERKs (A) or p38 (C) antibodies. JNK1 activity (B) was performed, from cell lysates, by in vitro kinase assay using GST-ATF2 as substrate after immunoprecipitation (IP) with specific antibodies as described in “Materials and methods.” The amounts of ERKs, JNK1, or p38 in immunoprecipitates were assessed by Western blotting analysis with the appropriate antibodies (WB). The results are representative of 5 experiments.
To further characterize the different signaling pathways implicated in response to MCP-1, we investigated the possible involvement of the 2 SAPKs, JNK1 and p38. SAPK1/JNK1 activity was assayed in JNK1 immunoprecipitates from lysates of MCP-1–treated-MonoMac6 cells, by testing their ability to phosphorylate GST-ATF2 as substrate. As can be seen in Figure 1B, MCP-1 stimulates JNK1 activity with a kinetic profile markedly delayed compared to the time course observed for ERKs, starting by 1 hour, peaking at 2 hours before decreasing to the basal level by 3 hours.
To evaluate the response of the SAPK2/p38 activity in response to MCP-1, MonoMac6 cell lysates were analyzed by immunodetection with a specific antiphosphorylated p38 antibody. MCP-1 stimulated p38 activity within 1 hour but at the difference with JNK1, this activity persisted for up to 4 hours.
Syk- and Src-like protein tyrosine kinases are involved in MCP-1–induced MAPK activation in MonoMac6 cells
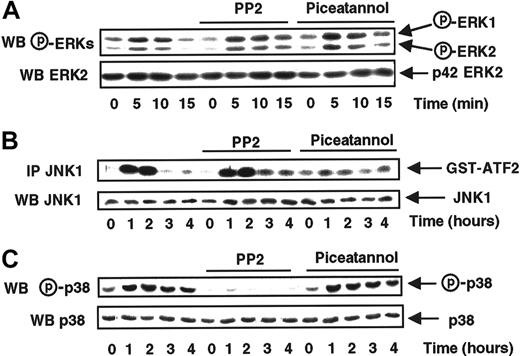
To better identify signaling pathways involved in MCP-1–induced ERK and SAPK activation, we examined the possible involvement of protein tyrosine kinases, often described to be implicated in signal transduction from 7 transmembrane receptors. To this aim, MonoMac6 cells were preincubated in the presence of either PP2, a potent inhibitor of the Src kinase family,29 or piceatannol, a specific inhibitor of the Syk tyrosine kinase family30before being exposed to MCP-1.
The data presented in Figure 2A show that treatment with these 2 tyrosine kinase inhibitors did not affect ERK1 and ERK2 activation in response to MCP-1, revealing that neither Syk nor Src tyrosine kinases were involved in the regulation of those kinases.
Syk and Src-like tyrosine kinases are, respectively, involved in the MCP-1–induced JNK1 and p38 activations.
MonoMac6 cells, untreated or preincubated 2 hours with 10 μM PP2 or 5 μg/mL piceatannol, were exposed following different time courses to 20 nM MCP-1. The activity and the amount of each MAPK were evaluated as previously described in Figure 1 and in “Materials and methods.” The results are representative of 2 experiments.
Syk and Src-like tyrosine kinases are, respectively, involved in the MCP-1–induced JNK1 and p38 activations.
MonoMac6 cells, untreated or preincubated 2 hours with 10 μM PP2 or 5 μg/mL piceatannol, were exposed following different time courses to 20 nM MCP-1. The activity and the amount of each MAPK were evaluated as previously described in Figure 1 and in “Materials and methods.” The results are representative of 2 experiments.
Instead, MCP-1–induced JNK1 activation was abrogated by piceatannol but not by PP2 suggesting that Syk, but not Src, controlled the JNK1 activation mediated by MCP-1 (Figure 2B).
The inhibitory profile of the 2 tyrosine kinase inhibitors on p38 activation (Figure 2C) mirrored that observed with JNK1, because only PP2 was efficient, revealing a specific involvement of Src-like kinases, but not Syk, in the control of MCP-1–induced p38 activation.
MCP-1 activates Syk and Src tyrosine kinases in MonoMac6 cells
On the basis of the above data suggesting a possible involvement of Src in the control of p38, we sought to verify that Src activity was really activated in response to MCP-1. To this end, Src activity present in anti-Src immunoprecipitates from lysates of cells exposed to MCP-1 was tested. Figure 3B shows that Src kinase was transiently activated, peaking at 2 minutes before declining to the basal level by 5 minutes. Syk was immunoprecipitated from MCP-1–treated MonoMac6 cells and examined for its phosphotyrosine profile. Stimulation of MonoMac6 cells by MCP-1 induced a rapid increase in the level of Syk phosphorylation culminating after 15 minutes (Figure 3A), revealing a marked lag in the activation of the 2 tyrosine kinase activities.
MCP-1 activates Syk and Src tyrosine kinases in MonoMac6 cells.
MonoMac6 cells were incubated with 20 nM MCP-1 for the indicated times. Syk (A) and Src (B) kinases were immunoprecipitated (IP) with specific antibodies from cell lysates, resolved by SDS-PAGE, and electrophoretically transferred to Immobilon-P membrane. Syk activity was assessed by immunoblotting using antiphosphotyrosine 4G10 Mabs and Src activity by in vitro kinase assay using enolase as substrate. The amounts of Syk and c-Src immunoprecipitated were evaluated by Western blotting analysis with the appropriate antibodies (WB). The results are representative of 2 experiments.
MCP-1 activates Syk and Src tyrosine kinases in MonoMac6 cells.
MonoMac6 cells were incubated with 20 nM MCP-1 for the indicated times. Syk (A) and Src (B) kinases were immunoprecipitated (IP) with specific antibodies from cell lysates, resolved by SDS-PAGE, and electrophoretically transferred to Immobilon-P membrane. Syk activity was assessed by immunoblotting using antiphosphotyrosine 4G10 Mabs and Src activity by in vitro kinase assay using enolase as substrate. The amounts of Syk and c-Src immunoprecipitated were evaluated by Western blotting analysis with the appropriate antibodies (WB). The results are representative of 2 experiments.
MCP-1–induced ERKs, JNK1, and p38 pathways display distinctive sensitivities to cholera and pertussis toxins
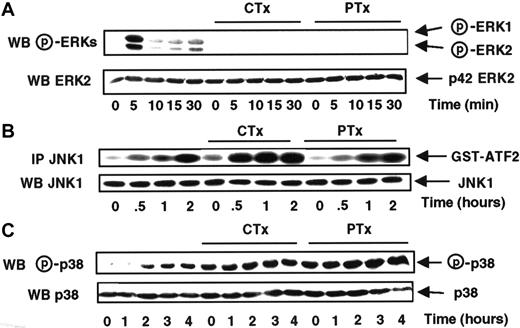
Chemokine receptors have long been known to be coupled to G-proteins sensitive to bacterial toxins.31 Previous studies have shown that several MCP-1 downstream signals in monocytic and T cells are coupled to PTX-sensitive G proteins8 and cholera toxin (CTX)-sensitive G proteins.32 To precisely determine whether Go/Gi and Gs proteins were implicated in the differential control of ERKs, JNK1, and p38, MonoMac6 cells were first treated for 4 hours, prior to the stimulation by MCP-1, with PTX, an inhibitor of αo/i G proteins, or CTX, an activator of αs G proteins.
Figure 4A shows that MCP-1–mediated ERK1 and ERK2 activations are abrogated by the treatment of MonoMac6 cells with both CTX and PTX, suggesting that those pathways did require the coordinated action of Gs and Go/Gi proteins.
CTX and PTX stimulate the MCP-1–induced JNK1 and p38 activations, but not the ERK activation.
MonoMac6 cells, untreated or preincubated 4 hours with 250 ng/mL pertussis toxin (PTX) or 10 μg/ml cholera toxin (CTX), were exposed following different time courses to 20 nM MCP-1. The activity and the amount of each MAPK were evaluated as previously described in Figure 1and in “Materials and methods.” The results are representative of 3 experiments.
CTX and PTX stimulate the MCP-1–induced JNK1 and p38 activations, but not the ERK activation.
MonoMac6 cells, untreated or preincubated 4 hours with 250 ng/mL pertussis toxin (PTX) or 10 μg/ml cholera toxin (CTX), were exposed following different time courses to 20 nM MCP-1. The activity and the amount of each MAPK were evaluated as previously described in Figure 1and in “Materials and methods.” The results are representative of 3 experiments.
At variance with ERKs, JNK1 activity was enhanced by treatment with CTX and remained unaffected when the cells were treated either for 4 hours with 250 ng/mL PTX (Figure 4B) or for 16 hours with 100 ng/mL PTX (data not shown), implying the exclusive involvement of Gs protein in the control of JNK1 activation in response to MCP-1. In the case of p38, we failed to detect any significant variation of the MCP-1–stimulated level of p38 activity in cells exposed to CTX or PTX, although those 2 toxins significantly increased the basal level of p38 activity (Figure 4C).
The various kinase activities stimulated in response to MCP-1 are abrogated when MonoMac6 cells are incubated with their respective inhibitors
To define the implication of each pathway in the MCP-1–induced monocyte locomotion, we used different pharmacologic drugs. We thus verified that each specific inhibitor was fully efficient on its respective target when applied to intact cells. As shown in Figure5, MonoMac6 cells were pretreated with the Src kinase family inhibitor PP2,29 the Syk tyrosine kinase inhibitor piceatannol,30 the MAPK/ERK kinase (MEK) inhibitor PD98059,33 the P38/SAPK2 inhibitor SB203580,34 or the SAPK1/SAPK2 inhibitor SB202190.35 The activity of c-Src (panel A), Syk (panel B), ERKs (panel C), or SAPK1/JNK1 (panel D) was then tested after different periods of incubation. The inhibitory effect of SB203580 was assayed by following inhibition of the phosphorylation status of CREB (panel E), a downstream substrate of the SAPK2/P38 pathway.36
MCP-1–induced kinase activities are abrogated by their respective specific inhibitors.
MonoMac6 cells, untreated or preincubated 2 hours either with 10 μM PP2, 5 μg/mL piceatannol, 40 μM PD98059, 40 μM SB202190, or 40 μM SB202580, were exposed following different time courses to 20 nM MCP-1. The activity and the amount of Src (A), Syk (B), ERKs (C), and SAPK1/JNK1 (D) kinases were evaluated as described in Figure 1 and in “Materials and methods.” (E) CREB phosphorylation was analyzed by Western blot using antibodies (New England Biolabs, Beverly MA) specific for phosphorylated Ser133 of CREB (P-CREB) or phosphorylated Ser63 of ATF1 (P-ATF1). CREB expression was analyzed by Western blot using anti-CREB antibodies (Santa Cruz Biotechnology). Results are representative of 2 experiments.
MCP-1–induced kinase activities are abrogated by their respective specific inhibitors.
MonoMac6 cells, untreated or preincubated 2 hours either with 10 μM PP2, 5 μg/mL piceatannol, 40 μM PD98059, 40 μM SB202190, or 40 μM SB202580, were exposed following different time courses to 20 nM MCP-1. The activity and the amount of Src (A), Syk (B), ERKs (C), and SAPK1/JNK1 (D) kinases were evaluated as described in Figure 1 and in “Materials and methods.” (E) CREB phosphorylation was analyzed by Western blot using antibodies (New England Biolabs, Beverly MA) specific for phosphorylated Ser133 of CREB (P-CREB) or phosphorylated Ser63 of ATF1 (P-ATF1). CREB expression was analyzed by Western blot using anti-CREB antibodies (Santa Cruz Biotechnology). Results are representative of 2 experiments.
MonoMac6 cells display different kinetic migratory profiles during chemotaxis or transmigration through HUVEC monolayers
In the chemotaxis experiments, MonoMac6 cells were placed in migration chambers fitted out with fibronectin-coated filters, whereas MonoMac6 transmigration was tested across filters covered with endothelial monolayers (HUVEC).37
Although both types of experiments, chemotaxis and transmigration, were performed with an identical gradient of MCP-1 chemokine, migratory responses were significantly different. Although the simple coating of filters with fibronectin allowed a significant mobilization (6.8% ± 0.4%, at 6 hours) of the MonoMac6 cells in response to MCP-1 (Figure 6B), the migratory response through a monolayer of HUVEC cells after a lag period of 1 hour was roughly 4-fold higher than that observed through fibronectin-coated filters (Figure 6C), underlying the importance of the presence of matrix protein components to ensure an efficient migration.
MonoMac6 cell migration across fibronectin- or HUVEC-coated filters in response to MCP-1 gradient.
[3H]-methyl thymidine-labeled MonoMac6 cells were washed and loaded in the upper well of the chemotaxis chamber. The lower wells contained either serum-free medium (○) or MCP-1 (●) at different concentrations (A) or 6 nM MCP-1 (B,C). Cell chemotaxis was tested by the ability of MonoMac6 cells to migrate across a fibronectin-coated filter for 4 hours (A) or for different periods of time (B). Transmigration was assayed through a TNF-α–activated monolayer of HUVECs (C) for indicated periods at 37°C. The amount of migrated cells, collected in the lower wells, was evaluated by liquid scintillation β counts as described in “Materials and methods.” Results are expressed as the mean ± SD of triplicates from 3 separate experiments.
MonoMac6 cell migration across fibronectin- or HUVEC-coated filters in response to MCP-1 gradient.
[3H]-methyl thymidine-labeled MonoMac6 cells were washed and loaded in the upper well of the chemotaxis chamber. The lower wells contained either serum-free medium (○) or MCP-1 (●) at different concentrations (A) or 6 nM MCP-1 (B,C). Cell chemotaxis was tested by the ability of MonoMac6 cells to migrate across a fibronectin-coated filter for 4 hours (A) or for different periods of time (B). Transmigration was assayed through a TNF-α–activated monolayer of HUVECs (C) for indicated periods at 37°C. The amount of migrated cells, collected in the lower wells, was evaluated by liquid scintillation β counts as described in “Materials and methods.” Results are expressed as the mean ± SD of triplicates from 3 separate experiments.
SAPKs, but not ERKs, are implicated in MCP-1–induced MonoMac6 cell locomotion
To investigate the respective importance of each type of MAPK in the mediation of MCP-1 chemoattractant effects, MonoMac6 cells were preincubated with different specific MAPK inhibitors before being tested for chemotaxis and transmigration. As shown in Figure7, pretreatment of MonoMac6 cells with 40 μM PD98059 (lane 2),33 an inhibitor of MAPK/ERK kinase (MEK) activation, did not affect either chemotaxis or transendothelial migration induced by MCP-1, indicating that the ERK1/ERK2 pathways were not involved in those 2 processes.
MCP-1–induced MonoMac6 cells chemotaxis or transendothelial migration are sensitive to SAPK pharmacologic inhibitors and to PTX.
[3H]-methyl thymidine labeled MonoMac6 cells were washed and pretreated for 2 hours with 40 μM of PD98059, SB203580, or SB202190, 4 hours with 10 μg/mL cholera toxin (CTX) or 16 hours with 100 ng/mL pertussis toxin (PTX) before being tested for their ability to migrate across fibronectin (▩) or TNF-α–activated HUVECs (▨) toward 6 nM MCP-1. Migrated cells, collected after 4 hours in the lower reservoir, were counted by liquid scintillation as described in “Materials and methods.” Results are presented as means ± SD of triplicate samples from 5 separate experiments.
MCP-1–induced MonoMac6 cells chemotaxis or transendothelial migration are sensitive to SAPK pharmacologic inhibitors and to PTX.
[3H]-methyl thymidine labeled MonoMac6 cells were washed and pretreated for 2 hours with 40 μM of PD98059, SB203580, or SB202190, 4 hours with 10 μg/mL cholera toxin (CTX) or 16 hours with 100 ng/mL pertussis toxin (PTX) before being tested for their ability to migrate across fibronectin (▩) or TNF-α–activated HUVECs (▨) toward 6 nM MCP-1. Migrated cells, collected after 4 hours in the lower reservoir, were counted by liquid scintillation as described in “Materials and methods.” Results are presented as means ± SD of triplicate samples from 5 separate experiments.
Conversely, when MonoMac6 cells were preincubated with SB203580 (lane 3), a SAPK2/p38 specific inhibitor,34 chemotaxis as well as transmigration were reduced by 35% and 44%, respectively. Furthermore, treatment with SB202190 (lane 4), which exerts an inhibitory effect on both p38 and JNK1 activities,35resulted in a 60% decrease for chemotaxis and 82% for transmigration. We verified that neither SB203580 nor SB202190 displays a cross-reactivity toward the ERK pathway (data not shown) These data underscore the coordinate action that the 2 SAPKs pathways play during MCP-1–mediated monocytes locomotion.
MCP-1–induced MonoMac6 cell migration across acellular or HUVEC-coated filters is sensitive to CTX and PTX
Considering the dramatic effect of CTX and PTX on ERK activity, we were interested in determining the implication of G proteins in the mediation of the MCP-1 chemoattractant effect. MonoMac6 cells were pretreated either with PTX or CTX and then tested for their ability to migrate in response to MCP-1. As seen in Figure 7, the data demonstrated that MCP-1–induced chemotaxis as well as transmigration were sensitive to both toxins. CTX, an activator of Gs proteins, slightly stimulated both acellular and transendothelial migration with respective rates of 114% and 120% (lane 5). Conversely, treatment of MonoMac6 cells with PTX, which inactivates Go/Gi proteins, abrogated the MCP-1–induced migration of monocytic cells through acellular filters as well as HUVEC monolayers (lane 6).
Discussion
Monocyte emigration is known to require dynamic regulation of integrin adhesiveness for endothelial and extracellular matrix ligands.37 38 However, the signaling pathways involved in the regulation of these processes in monocytic cells are still ill-defined.
Analysis of signal transduction revealed that MCP-1 binds to G protein-coupled receptor(s) (GPCR) promoting Ca++mobilization and cellular transmigration; both processes are blocked by treatment with PTX but not with CTX.39 Recently, several signaling pathways have been more precisely characterized in monocytic cells such as stimulation of at least 2 separate PI3-K isoforms in THP1 cells22 and an activation of the JAK2/STAT3 pathway in MonoMac1 cells.23
Concerning MAPK pathways, experiments performed on human monocytes have reported a stimulation of ERK activities by MCP-1 and the involvement of the ERK cascade in MCP-1–mediated chemotaxis.28However, to date, no other MAPK family member such as SAPKs (stress MAPKs) has been examined in response to MCP-1. It thus seemed critical to determine the nature of the MAPK triggered by MCP-1 and to elucidate precisely how each of these pathways may contribute to the monocytic cell locomotion.
We present evidence here that, in the MonoMac6 cell line, MCP-1 not only stimulated ERK1/ERK2 but also promoted an important increase in the JNK1 and p38 activities. Interestingly, the kinetic profiles appeared very different for these various members of the MAPK family with a rapid and transient activation for both ERKs, whereas JNK1 and p38 SAPKs exhibited an unusual kinetic profile with a delay followed by a sustained activation of, respectively, 2 hours and 4 hours for JNK1 and p38. Those results suggested that different regulatory mechanisms could be triggered by MCP-1 interaction with its receptor, leading to an independent control of the different MAPK pathways.
Receptors for MCP-1 belong to a large family of 7 transmembrane-spanning, heterotrimeric GPCRs.17,40 Ligands interacting GPCR have been demonstrated to activate different tyrosine kinases, which may be a way to bridge the G proteins to the ERK pathway.41-44 To better delineate the GPCR-coupled mechanisms responsible for MAPK and SAPK activation in the case of MCP-1, we tested the possible involvement of tyrosine kinases. Various nonreceptor tyrosine kinases of the Src family have recently been shown to participate in myeloid cell signaling responses to growth factors and to IgG binding to Fc receptors, including Lyn, Yes, Hck, Fgr, and c-Src45-49 as well as the non-Src kinase, Syk.50-53 Syk has been reported to be activated in monocytes stimulated by the engagement of integrins.54-56Furthermore, this tyrosine kinase has been shown to control JNK activation during CD28-mediated T-cell stimulation.35
In our model, we demonstrated that tyrosine kinases exerted a complex control on the different congeners of the MAPK family. Although ERK1 and ERK2 activation seemed to be resistant to piceatannol and PP2, respective inhibitors of Syk and Src-like tyrosine kinases, the activities of the 2 SAPKs were positively controlled by these nonreceptor protein tyrosine kinases. More precisely, JNK1 activation was dramatically diminished by piceatannol but remained unaffected in the presence of PP2, implying that Syk exerts a positive control on the JNK1 pathway. MCP-1–mediated p38 activation presented a susceptibility to these inhibitors, which mirrored that observed with JNK1, showing a total inhibition in the presence of PP2 and no effect of piceatannol. We verified that MCP-1 was effectively capable of activating both Src and Syk activities following a rapid and sharp profile of stimulation, occurring within minutes, compatible with a subsequent activation of JNK1 and p38 that was not detectable before 1 hour.
Taken collectively, our data lend support to a model in which MCP-1 activates Syk and Src independently, which in turn stimulate, respectively, the JNK1 and the p38 pathways, whereas the ERK pathway seems to be independent of these nonreceptor kinases.
Seven transmembrane domain receptors are known to be coupled to heterotrimeric G proteins for eliciting a wide spectrum of cellular responses. Analysis of the signal transduction pathways has revealed that MCP-1 activates phospholipase C activation,20intracellular calcium,17,18 and inhibition of adenylate cyclase19 in a PTX-dependent manner suggesting a coupling of MCP-1 receptors to Go/Gi proteins. However, it has recently been demonstrated that the association of chemokine receptors to different G proteins depends on both the receptor and the cell line studied.8 57 To evaluate the coupling mechanism(s) of the MCP-1 receptor and the nature of possible G proteins involved in the signaling events activated by this chemokine, MonoMac6 cells were treated with different bacterial toxins.
Interestingly, we found that ERKs and SAPKs exhibited differential sensitivity to PTX and CTX. Although MCP-1–mediated ERK1 and ERK2 activation was abrogated by both types of toxins, suggesting that ERKs are under the control of Gs and Go/Gi proteins, p38 activity was insensitive to both kinds of toxins. Furthermore, MCP-1–induced JNK1 activation exhibited a slight stimulation in response to CTX and no response to PTX, implying specific involvement of Gs proteins to couple MCP-1 receptor to the JNK pathway.
To date, scarce information is available on the mechanisms implicated during monocyte migration except that it is a PTX-sensitive process.32 58 The above data prompted us to investigate the relationships that might exist between the different MAPK pathways and the migration of MonoMac6 cells in response to MCP-1. To address this question, 2 types of migratory conditions were tested corresponding to a transendothelial migration or a simple chemotaxis through an acellular filter. In the first model, transmigration of MonoMac6 cells in response to an MCP-1 gradient was carried out through a monolayer of endothelial cells. Even though the efficacy to cross the barrier was higher than that observed on an acellular filter coated with fibronectin, we preferred to also use this second model, owing to possible interference of pharmacologic drugs on the permeability of endothelial barrier.
Incubation of the cells with PD98059, a specific inhibitor of the MEK/ERK pathway, did not alter the migration of the monocytic cells in the inner reservoir in either migration conditions. This is in contradiction with a previous study28 reporting that abrogation of the ERK pathway was sufficient to prevent MCP-1–mediated mobilization. Explanation of this discrepancy likely lies on the transformed status of MonoMac6 cells compared to peripheral blood monocytes used in this study, but more work is needed to clarify this point.
Interestingly, when MonoMac6 cells were treated with SB203580, a specific inhibitor of p38 pathway, mobilization of the cells was reduced by 56% in chemotaxis and 43% in transmigration, highlighting the crucial role that this pathway plays in the control of MCP-1–induced monocytic locomotion. However, the use of SB202190, which displays an equal inhibitory effect on both JNK1 and p38, resulted in an even more marked inhibition of the MCP-1–mediated migration, revealing that JNK1 was also implicated in the control of the migratory process in response to a preestablished MCP-1 gradient. This idea was strengthened by the effect of CTX that stimulated both JNK1 activation and monocytic migration in response to MCP-1. Furthermore, we found that PTX abrogated both MCP-1–induced chemotaxis and transendothelilal migration, confirming that the activation of Go/Gi proteins is an absolute prerequisite for monocytic locomotion.
In conclusion, we provide evidence that JNK1 and p38 act in concert for controlling the MCP-1–induced monocyte locomotion. These SAPK activities are, respectively, upstream regulated by Syk and c-Src activities. The kinetic profile of both SAPK activities that starts after about 1 hour and lasts between 2 and 4 hours is fairly in accord with the MCP-1–induced migration time course of MonoMac6 cells, especially in the case of transendothelial migration.
Supported by the Institut National de la Santé et de la Recherche Médicale, the Ligue Nationale Contre le Cancer, ComitéDépartemental des Alpes Maritimes (grant 5417), and the Association pour la Recherche sur le Cancer (subvention 2000).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bernard Rossi, INSERM U364, Faculté de Médecine, Avenue de Valombrose, 06107 Nice Cedex 02, France; e-mail: rossi@taloa.unice.fr.




![Fig. 6. MonoMac6 cell migration across fibronectin- or HUVEC-coated filters in response to MCP-1 gradient. / [3H]-methyl thymidine-labeled MonoMac6 cells were washed and loaded in the upper well of the chemotaxis chamber. The lower wells contained either serum-free medium (○) or MCP-1 (●) at different concentrations (A) or 6 nM MCP-1 (B,C). Cell chemotaxis was tested by the ability of MonoMac6 cells to migrate across a fibronectin-coated filter for 4 hours (A) or for different periods of time (B). Transmigration was assayed through a TNF-α–activated monolayer of HUVECs (C) for indicated periods at 37°C. The amount of migrated cells, collected in the lower wells, was evaluated by liquid scintillation β counts as described in “Materials and methods.” Results are expressed as the mean ± SD of triplicates from 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/2/10.1182_blood.v97.2.359/5/m_h80210590006.jpeg?Expires=1767715962&Signature=cueJgKlcP5lEpNbh-EYCQF-HqnjApLxRtvVVoYk2m4qhSgP1IvuVKfTVbbv8gKQwaISx2spMyeKdjYXIanx0VwhBeN4KXfWiiz37gTEVOYJIWCujBGmZL1ZhxwagblBE~dQaNQ3m~xQKSrvEyknYrE9uIzr5PmFhM378ZRV1ovq0Zso1k752Ga5OSB6q64l-e9VjL-Q-XwAxMMvOsBlfBQ~9Ukm6Uphpq9-A9ba6kSI90tT20CKsf5PVwlG9tc72oaWthb97rQhA6OjPkm7oXqrYdkJB~yHcrwdXy4zTOFOWeyBGfmTTvd0v2m8FNMs1dpH4wJeJ0mVNzn15ixaKjA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. MCP-1–induced MonoMac6 cells chemotaxis or transendothelial migration are sensitive to SAPK pharmacologic inhibitors and to PTX. / [3H]-methyl thymidine labeled MonoMac6 cells were washed and pretreated for 2 hours with 40 μM of PD98059, SB203580, or SB202190, 4 hours with 10 μg/mL cholera toxin (CTX) or 16 hours with 100 ng/mL pertussis toxin (PTX) before being tested for their ability to migrate across fibronectin (▩) or TNF-α–activated HUVECs (▨) toward 6 nM MCP-1. Migrated cells, collected after 4 hours in the lower reservoir, were counted by liquid scintillation as described in “Materials and methods.” Results are presented as means ± SD of triplicate samples from 5 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/2/10.1182_blood.v97.2.359/5/m_h80210590007.jpeg?Expires=1767715962&Signature=WcyTz4mbwvPDY2r3buxcc4BXJppwXrsu8pzMGGm0Hl6bdj8xQ9bpGG4k~G5JNvNyVih1Ek~P5m7Fi~Rj~nUNB434dr2gWj~iS~FzEbeqQuOVXULGYBfwpSCgYN7Y2vLXkQdVaqzpWUZGP38GFpu9ffGfoNJMqjRQgDXDY4RIW6~vremhlPkfyppcSFmOulmNQ1vvh9ngVpeNPzvjeS2rMx5kV~4PDrLbCjw2ebLSeUYQsK7~ent~aXYnxwSTk7AljE0hZFfOOOD7nvKpkrIQGntCJCJYA9A2nVymNvKSa2x9-NO5X10RzL1N9WZQ5VaLEZTtkbQIo0tbDbIkfrqkyA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal