The glycoprotein (GP)–Ib-IX-V receptor complex has recently been reported to signal through a pathway similar to that used by the collagen receptor GPVI, with a critical role described for the Fc receptor γ-chain. The evidence for this was based in part on studies with the GPIbα-selective snake venom toxin, alboaggregin-A. In the present study, it is reported that alboaggregin-A has activity at the collagen receptor GPVI in addition to GPIbα, and evidence is provided that this contributes to protein tyrosine phosphorylation, shape change, and GPIIb-IIIa–dependent aggregation. This may explain why responses to alboaggregin-A are distinct from those to von Willebrand factor– ristocetin.
Introduction
The snake venom toxin alboaggregin-A is a 50-kd tetrameric C-type lectin consisting of 2 α and 2 β subunits, isolated from Trimeresurus albolabris. Alboaggregin-A has been reported to bind specifically to the platelet receptor glycoprotein (GP)–Ibα.1,2 In contrast to other toxins that bind to GPIbα, eg, echicetin, crotalus HH-A, and HH-B, alboaggregin-A promotes increases in cytosolic Ca++, dense granule secretion, and GPIIb-IIIa–dependent aggregation.3 4
In a recent study, alboaggregin-A was observed to promote tyrosine phosphorylation of the Fc receptor (FcR) γ-chain leading to phosphorylation and activation of Syk, events that are critical in signaling by the collagen receptor GPVI.5 6 Partly on the basis of these observations, it was proposed that GPIbα signals through a GPVI-like pathway. Although alboaggregin-A binds to GPIbα, we show in this study that the toxin is capable of binding GPVI and that this is sufficient to stimulate activation.
Study design
Materials
Alboaggregin-A was purified from the venom of Trimeresurus albolabris according to 2 different published procedures.3,5 The anti-GPIbα monoclonal antibody (mAb) 6D1 was a kind gift from Dr B. Coller.7 The construct for human GPVI was a kind gift from Dr M. Moroi.8 Other reagents were from previously described sources.3,6 9-11
Platelet preparation
Human platelets were isolated from drug-free donors on the day of the experiment as described.6,9 Platelets from 3 Bernard-Soulier syndrome patients (deficient in GPIb-IX-V as determined by flow cytometry) were obtained through University College Hospital, London, United Kingdom, and were isolated from other cells by allowing them to stand at room temperature for 3 hours. Following isolation in this way, they were handled as described.6 9Ethylene-glycol-bis (βaminoethyether)-N,N,N,N′,N′tetraacetic acid (EGTA) (1 mM) and indomethacin (10 μM) were present in phosphorylation studies, but absent in aggregation studies.
Precipitation studies
Cell culture and transfection
K562 human erythroleukemia cells11 were stably transfected with plasmids containing human GPVI and human FcR γ-chain or the FcR γ-chain alone (O.B. and S.P.W., unpublished data, 2000).
Results and discussion
Platelet-platelet interactions are mediated via GPIbα-mediated agglutination and GPIIb-IIIa–dependent aggregation. Agglutination represents the passive cross-linking of adjacent platelets by the multimeric ligand von Willebrand factor (vWF), whereas aggregation via GPIIb-IIIa requires activation of the integrin through inside-out signaling events. Since GPIbα activates GPIIb-IIIa,12-16an increase in light transmission in an aggregometer in response to GPIbα ligands could represent agglutination, aggregation, or a combination of these events.
Platelet agglutination/aggregation in response to alboaggregin-A (10 μg/mL) is preceded by an increase in optical density, denoting a change in platelet morphology known as shape change. This is a classical pattern of aggregation, which is also observed in response to the GPVI-specific snake venom toxin convulxin (1 μg/mL) (Figure1A). In contrast, vWF in the presence of ristocetin promotes agglutination/aggregation without a preceding shape change (Figure 1A).
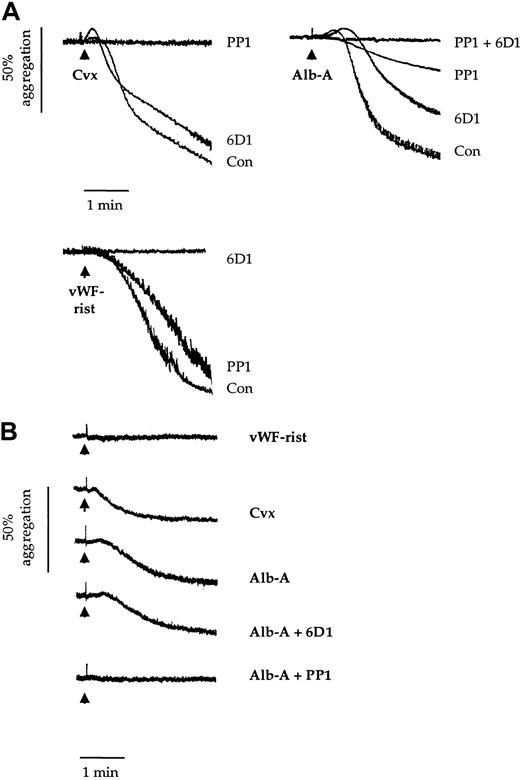
Effects of 6D1 and PP1 on platelet agglutination/aggregation.
Human washed platelets (2 × 108/mL) (panel A) and Bernard-Soulier syndrome washed platelets (1.5 × 108/mL) (panel B) were preincubated for 5 minutes with vehicle (dimethyl sulfoxide 0.1%); anti-GPIbα mAb 6D1 (10 μg/mL); the Src family kinase inhibitor PP1 (10 μM); or mAb 6D1 and PP1 in combination. This was followed by stimulation with convulxin (Cvx; 1 μg/mL); alboaggregin-A (Alb-A; 1 μg/mL); and vWF ristocetin (vWF-rist; 10 μg/mL and 1 mg/mL, respectively). Experiments were conducted in aggregometer cuvettes with stirring at 37°C. Results are representative of 3 to 8 experiments.
Effects of 6D1 and PP1 on platelet agglutination/aggregation.
Human washed platelets (2 × 108/mL) (panel A) and Bernard-Soulier syndrome washed platelets (1.5 × 108/mL) (panel B) were preincubated for 5 minutes with vehicle (dimethyl sulfoxide 0.1%); anti-GPIbα mAb 6D1 (10 μg/mL); the Src family kinase inhibitor PP1 (10 μM); or mAb 6D1 and PP1 in combination. This was followed by stimulation with convulxin (Cvx; 1 μg/mL); alboaggregin-A (Alb-A; 1 μg/mL); and vWF ristocetin (vWF-rist; 10 μg/mL and 1 mg/mL, respectively). Experiments were conducted in aggregometer cuvettes with stirring at 37°C. Results are representative of 3 to 8 experiments.
In confirmation of previous reports, the anti-GPIbα mAb 6D1 (10 μg/mL) completely blocked agglutination/aggregation in response to vWF ristocetin7 (Figure 1A). In contrast, it had a negligible effect on aggregation to convulxin and only partially inhibited the response to alboaggregin-A (38.2% ± 5.7%; n = 4) (Figure 1A). A partial inhibition of alboaggregin-A–induced aggregation by mAb 6D1 was also reported by Falati et al.5Shape change induced by convulxin and alboaggregin-A was maintained in the presence of mAb 6D1 (Figure 1A).
The Src family kinase inhibitor PP1 has been reported to inhibit shape change and aggregation to convulxin. PP1 also blocked shape change and reduced the agglutination/aggregation response to alboaggregin-A by 82.6% ± 3.7% (n = 3) (Figure 1A). In contrast, it had a minimal effect on the vWF-ristocetin response. Complete inhibition of aggregation to alboaggregin-A was achieved by a combination of mAb 6D1 and PP1. These results demonstrate that the alboaggregin-A response is made up of 2 distinct components, one of which is Src kinase–dependent.
The above results suggest that alboaggregin-A binds either to a second site on GPIbα that is insensitive to mAb 6D1 or to another platelet receptor. This was addressed in 2 ways. First, we treated platelets with the snake venom toxin mocarhagin, which cleaves the GPIbα receptor at E282 (single-letter amino acid code), thereby removing the major extracellular portion of the glycoprotein.17 Although this abolished agglutination/aggregation to vWf ristocetin, the response to alboaggregin-A was slightly delayed (not shown). Second, we investigated whether alboaggregin-A promotes platelet aggregation in Bernard-Soulier syndrome platelets. Alboaggregin-A and convulxin induced similar levels of aggregation of Bernard-Soulier syndrome platelets, whereas vWf ristocetin was inactive (Figure 1B). Furthermore, PP1, but not mAb 6D1, completely inhibited the response to alboaggregin-A (Figure 1B). This confirms that alboaggregin-A has an activity at another receptor distinct from GPIbα and demonstrates that this receptor promotes Src kinase activity.
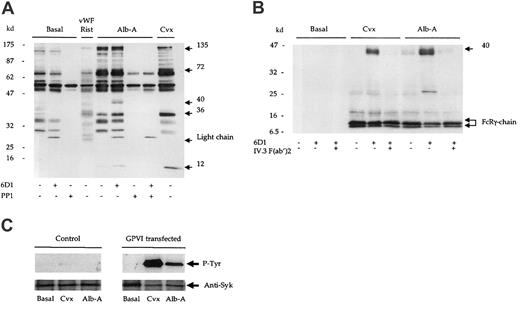
We next investigated the ability of alboaggregin-A to stimulate protein tyrosine phosphorylation in the presence of the mAb 6D1 and PP1. Alboaggregin-A stimulated a significant increase in tyrosine phosphorylation, which was comparable in pattern and magnitude to that induced by convulxin (Figure 2A). PP1, but not mAb 6D1, completely inhibited alboaggregin-A–mediated whole-cell tyrosine phosphorylation. In contrast, vWF ristocetin did not induce a detectable increase in protein tyrosine phosphorylation (Figure 2A). Since it has been proposed that alboaggregin-A signaling is analogous to that of GPVI,5 we examined tyrosine phosphorylation of a number of proteins in the GPVI pathway including the FcR γ-chain. Convulxin and alboaggregin-A stimulated phosphorylation of the FcR γ-chain (Figure 2B), Syk, and phospholipase Cγ2 (not shown), confirming that alboaggregin-A is able to stimulate a GPVI-like pathway. In addition, a 40-kd band was precipitated in response to alboaggregin-A and convulxin in the presence of mAb 6D1. A protein of the same molecular weight was also observed in whole-cell lysates (Figure 2A). We showed the 40-kd protein to be the low-affinity immunoreceptor FcγRIIA18by using F(ab′)2 fragments of the mAb IV.3 to block the phosphorylation mediated by the interaction of the Fc portion of mAb 6D1 with the low-affinity immunoreceptor (Figure 2B).
Alboaggregin-A binds and signals through GPVI.
(A) Human washed platelets (5 × 108/mL) in the presence of EGTA (1 mM) and indomethacin (10 μM) were stimulated by convulxin (Cvx; 1 μg/mL); alboaggregin-A (Alb-A; 1 μg/mL); or vWF ristocetin (vWF; 10 μg/mL; rist; 1mg/mL). (A) Reactions were stopped by the addition of equal volumes of Laemmli sample buffer. (B) Experiments were stopped by addition of an equal volume of cold lysis buffer, and the FcR γ-chain was precipitated from lysates by means of the tandem SH2 domains of Syk expressed as a glutathione-S-transferase fusion protein. Proteins were separated by 8% to 18% gradient SDS-PAGE and Western blotted for phosphotyrosine with mAb 4G10. (C) K562 cells (2 × 106/mL) transfected with either GPVI/FcR γ-chain (FID10/γ) or FcR γ-chain alone (PRC/γ) were stimulated with alboaggregin-A (Alb-A; 1 μg/mL) or convulxin (Cvx; 1 μg/mL). Following separation by SDS-PAGE, blots were probed for phosphotyrosine with mAb, 4G10.
Alboaggregin-A binds and signals through GPVI.
(A) Human washed platelets (5 × 108/mL) in the presence of EGTA (1 mM) and indomethacin (10 μM) were stimulated by convulxin (Cvx; 1 μg/mL); alboaggregin-A (Alb-A; 1 μg/mL); or vWF ristocetin (vWF; 10 μg/mL; rist; 1mg/mL). (A) Reactions were stopped by the addition of equal volumes of Laemmli sample buffer. (B) Experiments were stopped by addition of an equal volume of cold lysis buffer, and the FcR γ-chain was precipitated from lysates by means of the tandem SH2 domains of Syk expressed as a glutathione-S-transferase fusion protein. Proteins were separated by 8% to 18% gradient SDS-PAGE and Western blotted for phosphotyrosine with mAb 4G10. (C) K562 cells (2 × 106/mL) transfected with either GPVI/FcR γ-chain (FID10/γ) or FcR γ-chain alone (PRC/γ) were stimulated with alboaggregin-A (Alb-A; 1 μg/mL) or convulxin (Cvx; 1 μg/mL). Following separation by SDS-PAGE, blots were probed for phosphotyrosine with mAb, 4G10.
We proceeded to investigate the possibility that alboaggregin-A binds to GPVI using the erythroleukemia cell line K562, stably transfected with GPVI and the FcR γ-chain. The K562 cells did not express the GPIb-IX-V receptor complex as assessed by flow cytometry. Convulxin and alboaggregin-A stimulated tyrosine phosphorylation of Syk in GPVI/FcR γ-chain–transfected cells but not in control cells (Figure 2C), demonstrating that alboaggregin-A binds directly to GPVI. Furthermore, Jurkat cells transfected with GPVI but not control cells bound convulxin and alboaggregin-A as assessed by flow cytometry (David Tulasne and S.J.M., unpublished data, February 2001).
These observations demonstrate that alboaggregin-A binds to GPVI. To eliminate the possible action of a contaminant, we repeated the study with 3 different samples of alboaggregin-A, purified by 2 different procedures.3 5 All 3 samples yielded equivalent results. This provides indirect support for the activity residing in alboaggregin-A itself and is supported by the delay in aggregation resulting from treatment with mocarhagin.
In summary, responses to alboaggregin-A are made up of the combined effects of GPIbα, giving rise to agglutination, and a second receptor, GPVI, causing aggregation. (See note.) Thus, this venom cannot be used to identify signaling pathways linked to GPIbα in cells that express GPVI.
We thank Dr P Harrison for help in obtaining the Bernard-Soulier syndrome platelets, Drs M Leduc and C Bon for supply of convulxin, Dr B Coller for the gift of mAb 6D1, and Dr M Moroi for the GPVI construct.
Supported by the British Heart Foundation, Wellcome Trust, and Biotechnology and Biological Sciences Research Council (BBSRC). S.J.M. is the recipient of a BBSRC Co-operative Awards in Science and Engineering studentship; S.P.W. is a British Heart Foundation Senior Research Fellow.
N.A. and S.J.M. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Note added in proof
This is in agreement with Dörmann et al, who recently reported that alboaggregin-A binds to GPVI.19
Author notes
Stuart J. Marshall, University Department of Pharmacology, Mansfield Rd, Oxford, OX1 3QT, United Kingdom; e-mail:stuart.marshall@pharm.ox.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal