Human effector T cells have been difficult to isolate and characterize due to their phenotypic and functional similarity to the memory subset. In this study, a biochemical approach was used to analyze human effector CD4 T cells generated in vitro by activation with anti-CD3 and autologous monocytes for 3 to 5 days. The resultant effector cells expressed the appropriate activation/differentiation markers and secreted high levels of interferon γ (IFN-γ) when restimulated. Biochemically, effector CD4 T cells exhibited increases in total intracellular tyrosine phosphorylation and effector-associated phosphorylated species. Paradoxically, these alterations in tyrosine phosphorylation were concomitant with greatly reduced expression of CD3ζ and CD3ε signaling subunits coincident with a reduction in surface T-cell receptor (TCR) expression. Because loss of CD3ζ has also been detected in T cells isolated ex vivo from individuals with cancer, chronic viral infection, and autoimmune diseases, the requirements and kinetics of CD3ζ down-regulation were examined. The loss of CD3ζ expression persisted throughout the course of effector T-cell differentiation, was reversible on removal from the activating stimulus, and was modulated by activation conditions. These biochemical changes occurred in effector T cells generated from naive or memory CD4 T-cell precursors and distinguished effector from memory T cells. The results suggest that human effector T-cell differentiation is accompanied by alterations in the TCR signal transduction and that loss of CD3ζ expression may be a feature of chronic T-cell activation and effector generation in vivo.
Introduction
The initiation of adaptive immune responses depends on the activation and differentiation of naive T cells into effector cells that migrate to antigenic sites to orchestrate immune-mediated antigen clearance. Effector T cells can also predominate in diseases characterized by chronic immune activation, such as the autoimmune diseases systemic lupus erythematosus (SLE)1 and rheumatoid arthritis,2 and chronic viral infections,3 and during acute rejection of transplanted tissue.4 Thus, characterization of the effector T-cell subset at the mechanistic level is essential to understand its role in normal and pathologic immune states.
Effector T-cell differentiation involves a series of profound cellular and molecular changes, including an increase in size, up-regulation of activation/differentiation markers such as CD69, CD25, and CD45RO,5 transcription of effector cytokine genes such as interferon γ (IFN-γ) and interleukin-4 (IL-4),6 and reconfiguring of chromatin structure in the regions of these differentially expressed genes.7 In contrast to naive CD4 T-cell precursors, the resultant effector CD4 T cells produce high levels of effector cytokines, can be reactivated in the absence of costimulation,8,9 and exhibit an increased susceptibility to apoptosis.8
The disparate functions and activation requirements of effector and naive CD4 T cells suggest that distinct signals are being transduced through the T-cell receptor (TCR) in these subsets; however TCR-mediated signaling in effector CD4 T cells is not well characterized. Studies on TCR-mediated signaling pathways have largely focused on proximal signaling events in T-cell lines and differentiated T-cell clones that occur minutes after TCR triggering.10These studies have revealed that phosphorylation of the TCR-associated CD3ζ subunit by the T-cell–specific p56lckkinase and subsequent recruitment and activation of the CD3ζ-associated kinase ZAP-70, are critical proximal events in T-cell activation.11,12 Although CD3ζ and other proximal phosphorylation events occur minutes after TCR ligation, effector T-cell differentiation occurs over a period of 3 to 5 days of activation,6 and the biochemical events underlying this differentiation process are not known.
Effector T-cell differentiation is not viewed as a terminal event. Although most effector T cells are believed to die after a brief life span, a small proportion is believed to revert to the resting state to become long-lived resting memory T cells.13 This lineage relationship has been difficult to establish due to the phenotypic and functional similarities between effector and memory CD4 T cells.14 We have recently found that mouse effector and memory CD4 T cells can be distinguished based on tyrosine phosphorylation of intracellular substrates,15 suggesting that biochemical analysis of T- cell subsets may be reliable criteria by which to assess their differentiation state.
Although the majority of effector T-cell analyses have been carried out in the mouse, studies with human effector T cells have lagged behind for 2 main reasons. First, human effector T cells are difficult to identify due to their phenotypic and functional similarity to memory T cells14; second, there is no human corollary to the TCR-transgenic mouse, making it virtually impossible to isolate large numbers of antigen-specific human effector T cells. Given these limitations, it is necessary to develop in vitro systems for the generation and characterization of human effector T cells.
In this study, we have generated large numbers of differentiated human effector T cells in vitro from highly purified resting CD4 T cells for subsequent biochemical analysis. The resultant in vitro–activated T cells exhibit the phenotypic and functional properties associated with differentiated effector CD4 T cells. Biochemically, effector CD4 T cells can be distinguished from resting CD4 T cell precursors by quantitative and qualitative differences in total tyrosine phosphorylation and a profound loss of CD3ζ and CD3ε protein expression, whereas expression of the CD3ζ-associated kinase ZAP-70 remains unchanged. This loss of CD3 expression coincided with loss of surface TCR expression, persisted during effector T-cell differentiation, and was reversible once effector cells were removed from the activating stimulus. Our findings strongly suggest that specific changes in the TCR signaling apparatus characterize effector CD4 T-cell differentiation and that loss of CD3ζ may be a feature of chronic T-cell activation.
Materials and methods
Human cells
Heparinized peripheral venous blood was obtained from healthy adult volunteers. Written informed consent was obtained from all participating subjects and the protocol of study was approved by the Health Use Committee of the Walter Reed Army Medical Center. Peripheral blood mononuclear cells (PBMCs) were purified through Ficoll Hypaque and monocytes obtained by elutriation were 90% CD14+.
Antibodies
The following antihuman antibodies were purchased from Pharmingen (San Diego, CA): anti-CD3 (clone UCHT1), phycoerythrin (PE)–conjugated anti-αβTCR (clone T10B9.1A-31), fluorescein isothiocyanate (FITC)–conjugated anti-CD25 (clone M-A251), FITC-conjugated anti-CD14 (clone M5E2), FITC-conjugated anti-CD45RA (clone HI100), PE-conjugated anti-CD45RO (clone UCHL1), FITC-conjugated anti-CD69 (clone FN50), and anti-CD28 (clone CD28.2). PE-conjugated anti-CD4 (clone Q4120) and FITC-conjugated anti-CD3 were purchased from Sigma (St Louis, MO). The monoclonal antiphosphotyrosine antibody (clone 4G10) was purchased from Upstate Biotechnology (Lake Placid, NY). The anti-CD3ζ antiserum was described previously.16Anti–ZAP-70 antiserum16 was previously described. Anti-CD3 antibody (clone OKT3) was obtained from Ortho Biotech (Raritan, NJ) and immunoglobulin M (IgM) anti-CD3 (clone 2Ad2A2) was generously provided by Dr Ellis Reinherz (Dana-Farber Cancer Institute, Boston, MA).
Isolation of naive and memory CD4 T-cell subsets
CD4 T cells were obtained from PBMCs using a CD4 T-cell isolation kit (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. The resultant CD4 T cells were more than 95% pure as determined by fluorescence-activated cell sorter (FACS) analysis. Further fractionation into CD45RA and CD45RO subsets was achieved using anti-CD45RO– or anti-CD45RA–conjugated microbeads (Miltenyi Biotec) to deplete the CD45RO+ and CD45RA+ subsets, respectively. The resulting purity of CD45RA and CD45RO subsets was between 93% and 96%. For antigen-presenting cells (APC), purified monocytes were treated with 100 μg/mL mitomycin C (Boehringer Mannheim, Indianapolis, IN) for 1 hour at 4°C and washed 4 times with RPMI medium before culture.
In vitro generation of effector CD4 T cells
Primary effector CD4 T cells were generated in 24-well plates by incubating 106 CD45RA or whole CD4 T cells with 2 μg/mL anti-CD3 (OKT3 or UCHT1) antibody in soluble form plus 2 × 106 autologous, mitomycin C-treated monocytes for 72 hours at 37°C in complete RPMI media, consisting of RPMI 1640 (Gibco/BRL, Grand Island, NY), 10% fetal calf serum (Gemini Bioproducts, Calabasas, CA), 50 U/mL penicillin/streptomycin (Gibco), 2 mM glutamine (Gibco), and 50μM β-mercaptoethanol (Sigma). The concentration of anti-CD3 antibody used was similar to another study17 and was titrated to be the lowest dose for effector T-cell differentiation as assessed by phenotype (data not shown). Effectors were purified through Ficoll (LSM, ICN/Cappel, San Diego, CA) and washed twice in RPMI, resulting in 95% purity. Residual monocytes were further depleted from effector T-cell preparations using anti-CD14–coupled Dynabeads (Dynal, Lake Success, NY) following the manufacturer's recommendations resulting in 99% purity by FACS analysis.
Cytokine assays
For analysis of IFN-γ production, 105 naive, effector or memory CD4 T cells were cultured on 2 μg/mL anti-CD3 (OKT3) immobilized on plastic. After 24 hours, supernatants were collected, and IFN-γ content was quantitated by specific enzyme-linked immunosorbent assay (ELISA) using matched antibody pairs (Endogen, Cambridge, MA).
Western blotting
Cells (2 × 106 cells in 100μL RPMI) were left untreated or activated for 2 minutes at 37°C with IgM anti-CD3 antibody and immediately lysed in cold 1% NP40 lysis buffer with protease/phosphatase inhibitors.16 Lysates were analyzed by 12% to 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), gels were transferred to nitrocellulose and blots hybridized to antiphosphotyrosine or to anti-CD3ζ or anti–ZAP-70 antiserum followed by horseradish peroxidase (HRP)–conjugated goat antimouse (Bio-Rad, Hercules, CA) or protein A–peroxidase (Sigma) as described.16 Bands were detected using enhanced chemiluminescence (ECL, Amersham, Arlington Heights, IL) and revealed with Hyperfilm ECL (Amersham).
Flow cytometry
For analysis of cell surface phenotype, cells were washed, resuspended in stain buffer (phosphate-buffered saline [PBS]+1% fetal calf serum [FCS]+ 0.05% sodium azide) containing directly conjugated antibodies for 30 minutes at 4°C. Stained cells were analyzed using the FACScalibur (Becton Dickinson, San Jose, CA) with Cellquest software.
Reverse transcriptase–polymerase chain reaction
Total RNA was isolated by using a RNeasy minikit (Qiagen, Valencia, CA) from 5 × 106 whole CD4 and effector CD4 T lymphocytes. Using 400 ng total RNA, complementary DNA (cDNA) was synthesized with AMV reverse transcriptase (RT) (Promega, Madison, WI) and polymerase chain reaction (PCR)–amplified using CD3ζ chain and β-actin specific primers. Primers for PCR were synthesized by Sigma-Genosys (The Woodlands, TX): CD3ζ chain: 5′-AGCCTCTGCCTCCCAGCCTCTTTCTGAG-3′, (nucleotides 34-6218) and 5′-TCAGTGGCTGAGAAGAGTGAACCGGGTTG-3′ (nucleotides 669-64118), ZAP-70: 5′-GACGTGGCCATCAAGGTGCTGAAGCAG-3′ and 5′-GCGCTGCTCCACGGTCAGGAAGTCG-3′, and β-actin: 5′-CATGGGTCAGAAGGATTCCT-3′ and 5′-AGCTGGTAGCTCTTCTCCA-3′. PCR products were electrophoresed on 1.2% SeaKem agarose gel (FMC Bioproducts, Rockland, ME) and visualized with ethidium bromide.
Results
In vitro generation of effector CD4 T cells
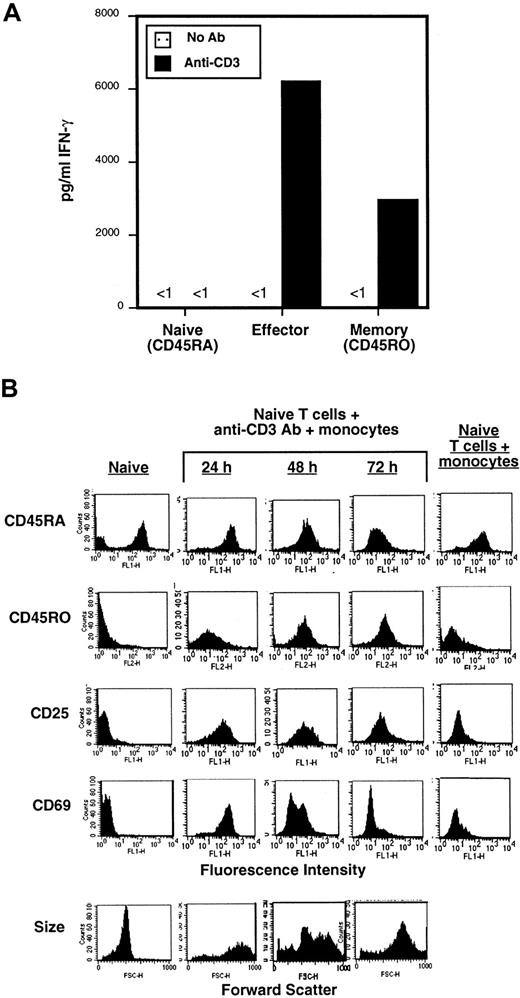
For generation of primary effector CD4 T cells, we isolated highly purified, resting naive (CD45RA) CD4 T cells from human peripheral blood using a 2-step immunodepletion procedure and cultured them with anti-CD3 antibody for 3 to 5 days in the presence of purified autologous peripheral blood monocytes as APC. Monocytes express the costimulatory ligands B7-1/B7-219 and Fcγ receptors for cross-linking anti-CD3 on the T-cell surface. To establish that differentiated effector CD4 T cells were generated using these stimulation conditions (anti-CD3/monocytes), we assessed the ability of the resultant cell population to produce the effector cytokine IFN-γ compared to primary naive (CD45RA) and memory (CD45RO) CD4 T-cell subsets purified from peripheral blood. Consistent with previous studies,20 resting naive CD4 T cells produced no IFN-γ in the resting state and negligible levels when activated with immobilized anti-CD3 (Figure 1A). By contrast, in vitro–generated effector CD4 T cells and ex vivo memory CD4 T cells produced high levels of IFN-γ when restimulated with anti-CD3 (Figure 1A), although the level of IFN-γ produced by effector T cells was consistently higher than memory cells—similar to mouse effector CD4 T cells generated with anti-CD3/APC or antigen/APC and ex vivo mouse memory CD4 T cells.15 21 These data indicate that human effector T cells generated here are functionally differentiated.
In vitro–generated effector CD4 T cells exhibit effector function and an activated/effector phenotype.
Naive (CD45RA) and memory (CD45RO) subsets of CD4 T cells were purified by a 2-step immunomagnetic depletion (see “Materials and methods”). Effector CD4 T cells were generated by coculture of naive CD4 T cells with 2 μg/mL anti-CD3 antibody (OKT3) and twice the number of primary autologous monocytes for 3 days. (A) Functional analysis of naive, effector, and memory CD4 T cells. Freshly isolated ex vivo naive and memory CD4 T cells and in vitro–generated effector CD4 T cells purified by centrifugation through Ficoll and washing, were cultured alone or in wells to which anti-CD3 antibody was immobilized. After 24 hours, culture supernatants were collected and assayed for IFN-γ content by specific ELISA. These data are representative of 3 separate experiments. (B) Phenotypic analysis of effector CD4 T-cell differentiation. The expression of activation/differentiation markers on purified naive (CD45RA) CD4 T cells, naive cells activated for 24 to 72 hours with anti-CD3 and autologous monocytes to generate effectors, and naive cells incubated for 72 hours with monocytes alone as a control was assessed by specific staining with fluorescent-conjugated antibodies and analyzed by FACS. Forward scatter is shown at the bottom. These data from a single donor are representative of 3 separate experiments using T cells derived from 3 different donors.
In vitro–generated effector CD4 T cells exhibit effector function and an activated/effector phenotype.
Naive (CD45RA) and memory (CD45RO) subsets of CD4 T cells were purified by a 2-step immunomagnetic depletion (see “Materials and methods”). Effector CD4 T cells were generated by coculture of naive CD4 T cells with 2 μg/mL anti-CD3 antibody (OKT3) and twice the number of primary autologous monocytes for 3 days. (A) Functional analysis of naive, effector, and memory CD4 T cells. Freshly isolated ex vivo naive and memory CD4 T cells and in vitro–generated effector CD4 T cells purified by centrifugation through Ficoll and washing, were cultured alone or in wells to which anti-CD3 antibody was immobilized. After 24 hours, culture supernatants were collected and assayed for IFN-γ content by specific ELISA. These data are representative of 3 separate experiments. (B) Phenotypic analysis of effector CD4 T-cell differentiation. The expression of activation/differentiation markers on purified naive (CD45RA) CD4 T cells, naive cells activated for 24 to 72 hours with anti-CD3 and autologous monocytes to generate effectors, and naive cells incubated for 72 hours with monocytes alone as a control was assessed by specific staining with fluorescent-conjugated antibodies and analyzed by FACS. Forward scatter is shown at the bottom. These data from a single donor are representative of 3 separate experiments using T cells derived from 3 different donors.
We also analyzed the expression of surface activation and differentiation markers to ensure that activation of naive CD4 T cells with anti-CD3/monocytes resulted in differentiation to effector cells. We found that the activation markers CD25 and CD69 were up-regulated 24 to 48 hours after activation (Figure 1B, rows 3 and 4), whereas up-regulation of the effector/memory differentiation marker CD45RO occurred at later times (between 48 and 72 hours, row 2, Figure 1B). CD69 and CD25 were down-regulated by 72 hours, similar to their down-regulation on mouse effector CD8 T cells,22 whereas CD45RO remained up-regulated during prolonged culture for 120 hours and later (Figure 1B and data not shown). No up-regulation of activation/differentiation markers was observed in the control culture containing naive CD4 T cells incubated with monocytes alone for 72 hours (Figure 1B, last column). Effector T cells are also distinguished from resting counterparts based on large size,22 and as shown in the bottom row of Figure 1B, cell size increased with activation time and the effector cells exhibited a uniform large size after 72 hours activation. When taken together, the functional and phenotypic results presented in Figure 1 indicate that these in vitro activation conditions result in the generation of differentiated effector CD4 T cells.
Tyrosine phosphorylation and CD3ζ expression in effector CD4 T cells
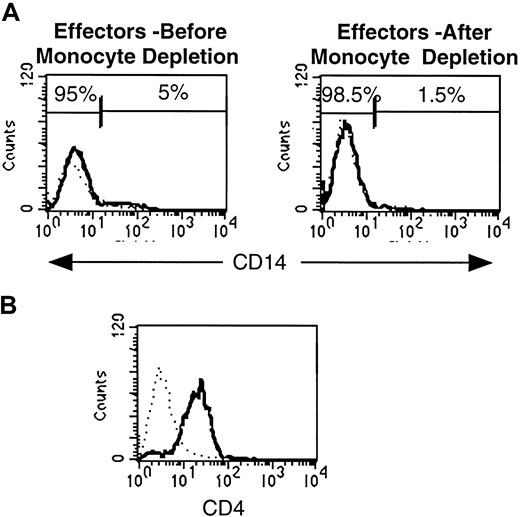
To undertake a biochemical analysis of in vitro–generated effector CD4 T cells, it was first necessary to rigorously establish the purity of the resultant effector T-cell population. Initial purification of effector CD4 T cells by Ficoll centrifugation resulted in approximately 5% residual monocyte contamination as assessed by CD14 staining (Figure 2A, left-most plot). We removed these residual monocytes with anti-CD14–conjugated magnetic beads, resulting in less than 1.5% monocyte contamination (Figure 2A, right-hand plot). This purified effector population consisted almost exclusively of CD4 T cells (Figure 2B).
Purification of in vitro–generated effector cells results in removal of residual monocytic accessory cells.
(A) Extent of monocyte contamination in effector CD4 T-cell preparations before monocyte depletion with anti-CD14 beads and after depletion. (B) CD4 expression profile of purified effector CD4 T cells.
Purification of in vitro–generated effector cells results in removal of residual monocytic accessory cells.
(A) Extent of monocyte contamination in effector CD4 T-cell preparations before monocyte depletion with anti-CD14 beads and after depletion. (B) CD4 expression profile of purified effector CD4 T cells.
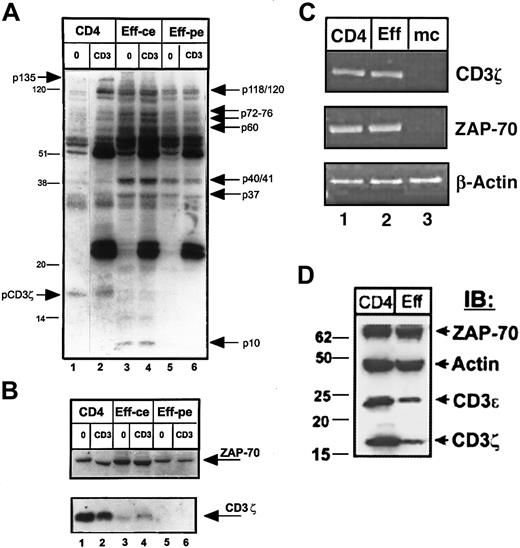
We evaluated the TCR-coupled signaling profile of purified effector T cells relative to resting CD4 T-cell precursors, by analyzing the pattern of total tyrosine phosphorylation before and after TCR/CD3 cross-linking by antiphosphotyrosine immunoblotting of whole cell lysates (Figure 3A). We found that the pattern of tyrosine phosphorylation in resting and effector CD4 T cells differed both quantitatively and qualitatively. Quantitatively, unstimulated effector CD4 T cells exhibited a higher level of phosphorylation overall and a greater number of phosphorylated species compared to unstimulated CD4 T cells apparent when cell or protein equivalents were compared (Figure 3A, compare lanes 3 and 5 to lane 1). Following CD3 cross-linking, the extensive phosphorylation in effector cells persisted, with increased phosphorylation of 37- to 41-kd and 70- to 80-kd proteins (Figure 3A, lanes 4 and 6) and CD3–cross-linked primary CD4 T cells exhibited phosphorylated protein species of 36 kd, 55 kd, 70 to 80 kd, and 120 to 130 kd (Figure 3A, lane 2). Qualitative differences in tyrosine phosphorylation were also apparent in effector versus resting CD4 T cells and included phosphorylated proteins found only in effector cells or present to a much greater level in effector cells. These effector-associated phosphorylated species (indicated by arrows to the right of the blot in Figure 3A) of molecular weights 120, 60 to 80 kd (p60, p72, p76), 36 to 40 kd (p37 and p40/41), and 10 kd (p10, lanes 3 and 4) were consistently present in effector T cells generated from T cells of 20 different donors and are similar in size to phosphorylated proteins we previously detected in mouse effector CD4 T cells.15 Despite these phosphorylation increases in effector cells, there was a notable absence of 2 phosphoproteins present in resting or CD3–cross-linked CD4 T cells—one protein of approximately 18 kd corresponding in size to phosphorylated CD3ζ (Figure 3A, lanes 1 and 2) and a second 135-kd protein.
Biochemical analysis of effector CD4 T cells reveals alterations in tyrosine phosphorylation and loss of CD3ζ and CD3ε protein expression.
Purified CD4 T cells from peripheral blood were used to generate effector cells using OKT3 antibody and autologous monocytes (see “Materials and methods”). CD4 cells and effectors were then left untreated (0) or cross-linked for 2 minutes with IgM anti-CD3 antibody (CD3) before lysis. Lysates from 106 CD4 T cells (lanes 1 and 2), and equivalent numbers of effector CD4 T cells (designated as ce, for cell equivalents, lanes 3 and 4), or equivalent levels of total effector cell protein (designated as pe, for 20μg protein equivalents, lanes 5 and 6) were resolved on reducing 12.0% SDS-PAGE, blotted to nitrocellulose, and probed sequentially with antiphosphotyrosine (A), followed by stripping and reprobing with anti-CD3ζ and anti–ZAP-70 antisera (B). These blots are representative results from 19 individual donors. In panel A, the effector-associated phosphorylated species of molecular weights 120/121, 76, 72, 60, 40/41, 37, and 10 kd are indicated on the right side of the blot. Bands of 135 kd and the phosphorylated CD3ζ band present in CD4 T-cell lysates, but not in effector T cells are indicated on the left side of the blot. (C) RT-PCR analysis of CD3ζ, ZAP-70, and actin transcript amplified from equal amounts of RNA isolated from resting CD4 T cells (CD4), effector CD4 T cells (Eff), and monocytes (mc). This gel is representative of 5 experiments. (D) Expression of ZAP-70, actin, CD3ε, and CD3ζ protein products in lysates of resting and effector CD4 T cells. Protein equivalents (4 μg/lane) from lysates of CD4 T cells or 5-day activated effector CD4 T cells purified as above were resolved by 4% to 20% gradient reducing SDS-PAGE, blotted to nitrocellulose, and blots were cut into strips and hybridized with polyclonal antiserum directed against ZAP-70, actin, CD3ε, and CD3ζ. IB indicates immunoblot.
Biochemical analysis of effector CD4 T cells reveals alterations in tyrosine phosphorylation and loss of CD3ζ and CD3ε protein expression.
Purified CD4 T cells from peripheral blood were used to generate effector cells using OKT3 antibody and autologous monocytes (see “Materials and methods”). CD4 cells and effectors were then left untreated (0) or cross-linked for 2 minutes with IgM anti-CD3 antibody (CD3) before lysis. Lysates from 106 CD4 T cells (lanes 1 and 2), and equivalent numbers of effector CD4 T cells (designated as ce, for cell equivalents, lanes 3 and 4), or equivalent levels of total effector cell protein (designated as pe, for 20μg protein equivalents, lanes 5 and 6) were resolved on reducing 12.0% SDS-PAGE, blotted to nitrocellulose, and probed sequentially with antiphosphotyrosine (A), followed by stripping and reprobing with anti-CD3ζ and anti–ZAP-70 antisera (B). These blots are representative results from 19 individual donors. In panel A, the effector-associated phosphorylated species of molecular weights 120/121, 76, 72, 60, 40/41, 37, and 10 kd are indicated on the right side of the blot. Bands of 135 kd and the phosphorylated CD3ζ band present in CD4 T-cell lysates, but not in effector T cells are indicated on the left side of the blot. (C) RT-PCR analysis of CD3ζ, ZAP-70, and actin transcript amplified from equal amounts of RNA isolated from resting CD4 T cells (CD4), effector CD4 T cells (Eff), and monocytes (mc). This gel is representative of 5 experiments. (D) Expression of ZAP-70, actin, CD3ε, and CD3ζ protein products in lysates of resting and effector CD4 T cells. Protein equivalents (4 μg/lane) from lysates of CD4 T cells or 5-day activated effector CD4 T cells purified as above were resolved by 4% to 20% gradient reducing SDS-PAGE, blotted to nitrocellulose, and blots were cut into strips and hybridized with polyclonal antiserum directed against ZAP-70, actin, CD3ε, and CD3ζ. IB indicates immunoblot.
The apparent lack of phosphorylated CD3ζ in effector T cells was paradoxical given that CD3ζ phosphorylation is a critical event to induce downstream phosphorylation cascades.23 We thus explored CD3ζ protein expression in effector cells by reprobing the phosphotyrosine immunoblot in Figure 3A with anti-CD3ζ antiserum. As shown in Figure 3B, expression of CD3ζ was dramatically decreased in effector CD4 T cells, whereas expression of the CD3ζ-associated ZAP-70 kinase remained unchanged in lysates from both fresh CD4 T cells and in vitro–generated effector T cells (Figure 3B, upper blot). This dramatic loss of CD3ζ expression in effector T-cell lysates ranged from 0% to less than 10% of the level seen in resting T cells in 30 different experiments using purified T cells obtained from 30 different donors. When taken together, the results in Figure 3, panels A and B, demonstrate a profound and specific loss of CD3ζ protein expression in effector CD4 T cells concomitant with an increase in tyrosine phosphorylation of specific proteins.
We asked whether this loss of CD3ζ protein expression occurred at the transcriptional level, by assaying for the presence of CD3ζ and ZAP-70 transcripts using RT-PCR. As shown in Figure 3C, both resting CD4 T cells and purified effector CD4 T cells expressed comparable levels of CD3ζ transcripts, and likewise expressed similar levels of ZAP-70 transcripts (lanes 1 and 2). Monocytes, as expected, did not express either CD3ζ or ZAP-70 transcripts (Figure 3C, lane 3). These results demonstrate that the loss of CD3ζ expression in effector CD4 T cells occurs after transcription.
We also asked whether the loss of CD3ζ expression in effector cell lysates was specific for the ζ chain of the CD3 complex or also included other CD3 chains. To address this question we probed lysates from resting and effector CD4 T cells with antiserum directed against CD3ζ, CD3ε, and as controls, ZAP-70 and actin. As shown in Figure3D, effector CD4 T cells exhibited dramatic decreases in the expression of both CD3ζ and CD3ε chains, whereas ZAP-70 and actin expression remained constant. These results indicate that expression of CD3ζ and CD3ε are comodulated in effector CD4 T cells.
CD3ζ down-regulation in differentially activated CD4 T cells
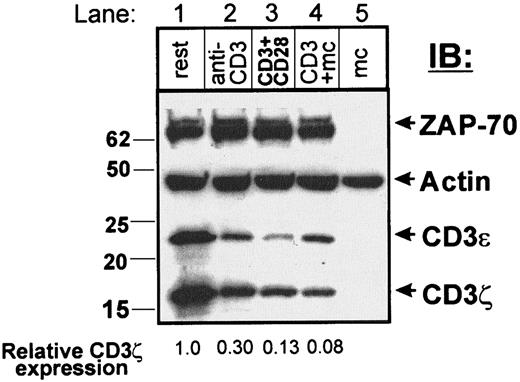
We asked whether this dramatic loss of CD3ζ and CD3ε protein expression was due exclusively to prolonged contact with anti-CD3 as the activating stimulus. We thus compared the expression of CD3ζ, CD3ε, and ZAP-70 and actin in fresh primary resting CD4 T cells before and after 72 hours of continuous culture in the presence of anti-CD3 immobilized on plastic, immobilized anti-CD3 and anti-CD28, or anti-CD3/monocytes (Figure 4). Although substantial down-regulation of CD3ζ was observed in effector cells generated with anti-CD3/monocytes to 8% of the level observed in resting cells (Figure 4, lane 4), there was a gradation of CD3ζ loss in anti-CD3 and anti-CD3/anti-CD28–stimulated T cells to 30% and 13%, respectively, of the level seen in resting T cells (Figure 4, compare lanes 2 and 3 to lane 1). CD3ε expression, by contrast, was reduced to similar extents for all stimulation conditions. All cells exhibited comparable levels of ZAP-70 and actin protein expression (Figure 4), and stimulation with soluble anti-CD3 alone (data not shown) or monocytes alone (Figure 5A) did not affect CD3ζ or CD3ε expression.
Varying activation conditions results in differential loss of CD3ζ.
Resting CD4 T cells (lane 1) were cultured for 72 hours in the presence of immobilized anti-CD3 (lane 2, anti-CD3), immobilized anti-CD3 and anti-CD28 (lane 3, CD3+CD28), or with anti-CD3 and autologous monocytes (lane 4, CD3+mc). Cells were purified through Ficoll, and residual monocytes were depleted with anti-CD14 coupled Dynabeads before lysis. Lysates containing equal protein levels (4.1 μg/ lane) were resolved on a 4% to 20% gradient reducing SDS-PAGE and blotted to nitrocellulose, cut into strips, and probed with anti–ZAP-70, antiactin, anti-CD3ε, and anti-CD3ζ antiserum. Lysates of purified monocytes (lane 5, mc) were run as blotting controls. The relative expression of CD3ζ determined by dividing the band densities in activated cells by the band density in resting CD4 T cells is indicated at the bottom of each lane. This partial reduction of CD3ζ expression with CD3 and CD3/CD28-stimulated T cells was observed in 5 different experiments.
Varying activation conditions results in differential loss of CD3ζ.
Resting CD4 T cells (lane 1) were cultured for 72 hours in the presence of immobilized anti-CD3 (lane 2, anti-CD3), immobilized anti-CD3 and anti-CD28 (lane 3, CD3+CD28), or with anti-CD3 and autologous monocytes (lane 4, CD3+mc). Cells were purified through Ficoll, and residual monocytes were depleted with anti-CD14 coupled Dynabeads before lysis. Lysates containing equal protein levels (4.1 μg/ lane) were resolved on a 4% to 20% gradient reducing SDS-PAGE and blotted to nitrocellulose, cut into strips, and probed with anti–ZAP-70, antiactin, anti-CD3ε, and anti-CD3ζ antiserum. Lysates of purified monocytes (lane 5, mc) were run as blotting controls. The relative expression of CD3ζ determined by dividing the band densities in activated cells by the band density in resting CD4 T cells is indicated at the bottom of each lane. This partial reduction of CD3ζ expression with CD3 and CD3/CD28-stimulated T cells was observed in 5 different experiments.
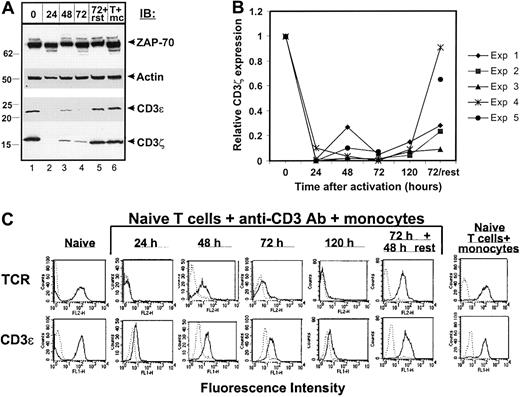
Kinetics of CD3 and TCR down-regulation during effector CD4 T cell differentiation.
CD4 T cells were cultured with anti-CD3/monocytes, harvested 24 to 120 hours, and either lysed for immunoblotting or stained and analyzed by FACS. (A) Kinetics of differentiation-induced CD3ζ and CD3ε down-regulation. Anti–ZAP-70, actin, CD3ε, and CD3ζ immunoblot of lysates derived from CD4 T cells before activation (lane 1), CD4 T cells harvested and purified 24 to 72 hours after culture with anti-CD3/monocytes (lanes 2-4, 24, 48, 72), 72 hours purified effector CD4 T cells rested in media alone for 2 days (lane 5, 72+rst), or purified CD4 T cells cultured with monocytes alone for 72 hours (lane 6, T+mc). Molecular weight markers are indicated at left. (B) Change in CD3ζ expression over time of activation. A compilation of the densitometric analysis of 5 separate kinetic experiments (Exp 1-5) performed as in panel A with purified T cells and monocytes from 5 different donors. Relative CD3ζ expression is defined as in Figure 4. In Exp 5 (shown in panel A), the 120-hour time point was not done. (C) TCR/CD3 and surface marker expression during effector differentiation. Histograms show surface expression of αβ TCR and CD3ε compared to control staining.
Kinetics of CD3 and TCR down-regulation during effector CD4 T cell differentiation.
CD4 T cells were cultured with anti-CD3/monocytes, harvested 24 to 120 hours, and either lysed for immunoblotting or stained and analyzed by FACS. (A) Kinetics of differentiation-induced CD3ζ and CD3ε down-regulation. Anti–ZAP-70, actin, CD3ε, and CD3ζ immunoblot of lysates derived from CD4 T cells before activation (lane 1), CD4 T cells harvested and purified 24 to 72 hours after culture with anti-CD3/monocytes (lanes 2-4, 24, 48, 72), 72 hours purified effector CD4 T cells rested in media alone for 2 days (lane 5, 72+rst), or purified CD4 T cells cultured with monocytes alone for 72 hours (lane 6, T+mc). Molecular weight markers are indicated at left. (B) Change in CD3ζ expression over time of activation. A compilation of the densitometric analysis of 5 separate kinetic experiments (Exp 1-5) performed as in panel A with purified T cells and monocytes from 5 different donors. Relative CD3ζ expression is defined as in Figure 4. In Exp 5 (shown in panel A), the 120-hour time point was not done. (C) TCR/CD3 and surface marker expression during effector differentiation. Histograms show surface expression of αβ TCR and CD3ε compared to control staining.
Alterations in CD3ζ and TCR expression during effector T-cell differentiation
Our findings of a reduction in expression of CD3ζ in effector CD4 T cells bore striking parallels to the loss of CD3ζ identified in human T cells associated with chronic diseases such as SLE,24 cancer,25 and chronic viral infection.26 We reasoned that our system provided a means to analyze the kinetics of CD3ζ down-regulation, to determine how CD3ζ down-regulation affected surface TCR and CD3ε expression, and to make a direct comparison of CD3ζ expression in naive, effector and memory CD4 T cells.
To analyze the kinetics of CD3ζ down-regulation during effector T-cell differentiation, we cultured naive CD4 T cells with anti-CD3 and monocytes for periods of time between 24 and 120 hours, and assessed the expression of CD3ζ (Figure 5). As shown in Figure 5A, both CD3ζ and CD3ε expression were substantially reduced 24 hours after activation (lane 2) and remained low for 48 to 72 hours (lanes 3 and 4). There was some fluctuation in CD3ζ expression at 48 to 72 hours, which occurred in 3 of the 5 kinetic experiments performed (Figure 5B). When 72-hour effector CD4 T cells were removed from the activating stimulus and recultured in media alone for 2 days, CD3ζ was re-expressed although at a lower level than originally present in resting CD4 T cell lysates (Figure 5A, compare lane 5 to lane 1). As a control, CD4 T cells cultured for 72 hours with monocytes maintained high levels of CD3ζ expression (Figure 5A, lane 6), and all cells examined expressed comparable levels of actin and ZAP-70 protein (Figure 5A, upper blots). We compiled densitometric analysis of CD3ζ expression from 5 different kinetic experiments (5 different donors) and these results are presented in Figure 5B. As seen in this graph, CD3ζ expression is drastically reduced in all cases after 24 hours of activation, is re-expressed in some cases at a low level at 48 hours, and is uniformly re-expressed to levels 40% to 100% of resting cells after effector cells are removed from the activating stimulus. When taken together, the results from these kinetic experiments indicate that loss of CD3ζ occurs early in effector CD4 T-cell differentiation and persists while effectors remain in the presence of the activating stimulus.
Because expression of CD3ζ is required for optimal surface expression of the TCR,27 and TCR down-regulation has also been established as an early feature of T-cell activation,28 we examined whether loss of surface TCR correlated with loss of CD3ζ during effector cell differentiation. As shown in Figure 5C (first row), surface TCR expression was dramatically reduced early in the differentiation process (within 24 hours) and remained down-regulated throughout the culture period with low level surface expression between 48 and 72 hours. Consistent with loss of CD3ε expression in cell lysates, CD3ε surface expression was also reduced after 24 hours and partially restored at 48 to 72 hours (Figure 5C, second row). No down-regulation of TCR/CD3 expression was observed when naive CD4 T cells were incubated with monocytes alone (Figure 5C, column 7), and both TCR and CD3ε were re-expressed when 72-hour effector cells were washed and recultured in media. These data demonstrate that loss of CD3ζ occurs with the same kinetics as the reduction in TCR/CD3ε surface expression, and that both TCR and CD3 components are re-expressed in “rested” effector T cells removed from the activating stimulus.
Comparison of naive, effector, and memory CD4 T-cell subsets
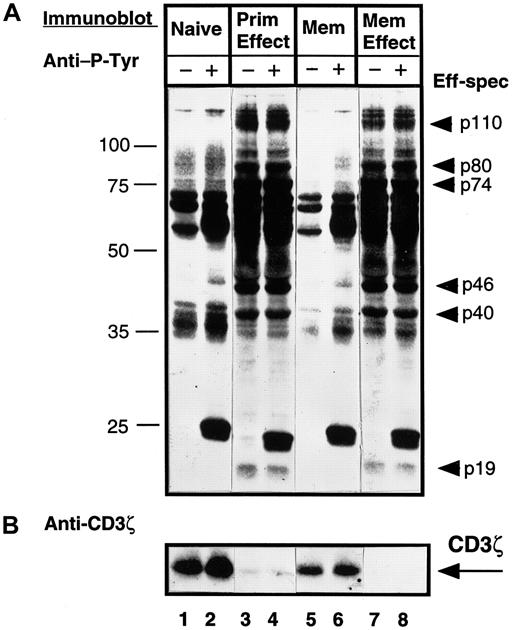
Because effector CD4 T cells bear phenotypic and functional similarities to the memory subset, we asked whether the changes in CD3ζ and tyrosine phosphorylation observed in effector T-cell lysates could likewise be observed in memory CD4 T cells. To address this question, we compared the pattern of tyrosine phosphorylation and CD3ζ expression in cell equivalents of purified naive (CD45RA) and memory (CD45RO) CD4 T cells compared to effector CD4 T cells generated in vitro from these subsets by activation with anti-CD3/monocytes. As shown in Figure 6A, when comparing resting (lanes 1 versus 5), or CD3–cross-linked (lanes 2 and 6) naive and memory subsets, the qualitative pattern of tyrosine phosphorylation is similar, although quantitatively, memory CD4 T cells exhibit a lower level of tyrosine phosphorylation as also shown by others.29 Effector CD4 T cells derived either from naive T cells (primary effectors) or memory T cells (memory effectors) exhibited a high level of tyrosine phosphorylation of effector-associated bands identified in Figure 3A (Figure 6A, lanes 3, 4, 7, and 8). CD3ζ was expressed at high levels in both naive and memory T cells, yet was virtually absent in lysates from primary or memory effectors (Figure 6B). These data indicate that the increased tyrosine phosphorylation and loss of CD3ζ expression distinguishes activated effector T cells from memory T cells and that effector T cells derived from either naive or memory precursors exhibit similar biochemical profiles.
Both primary and memory effectors exhibit similar tyrosine phosphorylation and loss of CD3ζ expression, whereas memory CD4 T cells are more similar to the naive subset.
Purified naive (CD45RA) and memory (CD45RO) CD4 T cells were isolated from PBMCs from an individual donor. Primary effector cells were generated from the CD45RA subset and memory effectors were generated from the CD45RO subset by activation with OKT3 antibody and autologous monocytes for 3 days. T-cell subsets were left untreated (−) or cross-linked for 2 minutes with IgM anti-CD3 antibody (+) before lysis. Lysates from 106 cell equivalents of naive (lanes 1 and 2), primary effector (lanes 3 and 4), memory (lanes 5 and 6), and memory effector (lanes 7 and 8) CD4 T cells were resolved on 12.0% SDS-PAGE, blotted to nitrocellulose, and probed sequentially with antiphosphotyrosine (A), followed by stripping and reprobing with anti-CD3ζ antiserum (B). These blots are representative results from 12 different donors. Eff-spec indicates effector-specific bands.
Both primary and memory effectors exhibit similar tyrosine phosphorylation and loss of CD3ζ expression, whereas memory CD4 T cells are more similar to the naive subset.
Purified naive (CD45RA) and memory (CD45RO) CD4 T cells were isolated from PBMCs from an individual donor. Primary effector cells were generated from the CD45RA subset and memory effectors were generated from the CD45RO subset by activation with OKT3 antibody and autologous monocytes for 3 days. T-cell subsets were left untreated (−) or cross-linked for 2 minutes with IgM anti-CD3 antibody (+) before lysis. Lysates from 106 cell equivalents of naive (lanes 1 and 2), primary effector (lanes 3 and 4), memory (lanes 5 and 6), and memory effector (lanes 7 and 8) CD4 T cells were resolved on 12.0% SDS-PAGE, blotted to nitrocellulose, and probed sequentially with antiphosphotyrosine (A), followed by stripping and reprobing with anti-CD3ζ antiserum (B). These blots are representative results from 12 different donors. Eff-spec indicates effector-specific bands.
Discussion
Effector CD4 T cells are central to both cellular and humoral immunity and predominate in many types of chronic diseases. In this study, we have undertaken a biochemical analysis of differentiated human effector CD4 T cells to determine whether alterations in TCR-mediated signaling accompany differentiation to effector T cells and distinguish effector and memory CD4 T-cell subsets. We generated human effector CD4 T cells that bore activation and differentiation markers such as CD25 and CD45RO and were functionally differentiated in their ability to produce effector cytokines. Biochemically, effector T cells exhibited distinct patterns of tyrosine phosphorylation and extended down-regulation of CD3ζ and CD3ε protein expression concomitant with a reduction of surface TCR/CD3 expression. These biochemical changes were specific to the effector subset and did not occur in memory CD4 T cells, suggesting that TCR-mediated signal transduction in effector CD4 T cells is distinct from naive and memory subsets.
We generated primary human effector CD4 T cells by in vitro activation of freshly isolated naive (CD45RA) or whole CD4 T cells with anti-CD3 antibody and primary autologous monocytes. We designate these cells effectors based on established criteria such as increased cell size, increased expression of differentiation markers, and the ability to secrete large amounts of effector cytokines when restimulated. In vitro differentiation of human naive T cells has been accomplished in other studies using anti-CD3 and exogeneously added IL-2 in the absence of accessory cells,20,30 and polarized human Th1 and Th2 lines have been generated by activation of human PBMCs with mitogens and exogneous cytokines,31 or with allogeneic monocytic-derived, lipopolysaccharide-activated dendritic cells in the presence of IL-2 and anticytokine antibodies.32 The in vitro generation of effector T cells as described here occurs in nonpolarizing conditions in the presence of untreated primary autologous accessory cells without exogeneously added cytokines.
Our results reveal several interesting and novel features of TCR-mediated signaling in differentiated effector CD4 T cells that have implications for effector T-cell function and survival. First, effector CD4 T cells exhibit a high level of tyrosine phosphorylation of TCR signaling intermediates consistent with their ability to produce high levels of the effector cytokine IFN-γ when restimulated. Second, despite this biochemical hyperactivity, there is a profound loss of TCR/CD3 expression at the surface and in cell lysates while expression of CD3-associated kinase ZAP-70 remains constant. Third, these changes in TCR/CD3 expression occur during the course of effector T-cell differentiation and in effector T cells generated from naive or memory precursors. Below we discuss these findings in the context of other studies on TCR signaling and TCR/CD3 down-regulation.
The effector CD4 T cells examined here exhibit a high level of tyrosine phosphorylation, particularly proteins of 120/121 kd, which correspond in size to p120cbl33 and Fyb/SLAP,34 37 to 41 kd, which correspond in approximate size to p38 and erk mitogen-activated protein kinases,10,35 and 72 to 80 kd, which may include the p72syk tyrosine kinase36 or the linker/adaptor molecule SLP-76.37 We have found effector-associated tyrosine phosphorylated species of comparable size expressed by mouse effector CD4 T cells generated either by antigen or anti-CD3 activation,15 strongly suggesting that phosphorylation of these proteins is a general feature of differentiated effector T cells. We are currently investigating the activation state of these effector-specific tyrosine phosphorylated species to elucidate the precise signaling pathways operative in effector T cells.
The second main TCR-coupled signaling alteration identified here is a substantial reduction in the expression of TCR and CD3 signaling components both on the effector T-cell surface and in detergent lysates. Loss of either surface TCR expression and CD3ζ expression has been previously associated in a number of studies with short-term activation (minutes to hours) of mouse and human T-cell clones, lines, and primary T cells. In human T cells, TCR stimulation for 1 to 2 hours resulted in loss of CD3ζ expression in human T cell clones38,39 and loss of surface TCR and/or CD3 expression in human T-cell clones and primary human T cells.38,40 In mouse T cells, transient loss of CD3ζ expression was demonstrated in lysates of mouse primary T cells activated with anti-CD3ε for several hours41 and surface TCR expression was down-modulated in primary mouse T cells 1 to 12 hours after stimulation with anti-CD3ε antibody41 or antigen/APC.42 Recently, surface TCR and CD3ε were found to be down-modulated in primary mouse T cells activated with antigen/APC for 10 to 60 minutes, yet the total amount of CD3ζ protein in cell lysates was unchanged from the level in resting T cells.43 When taken together, these published studies demonstrate a more pronounced activation-induced decrease in CD3ζ expression in human T cells compared to mouse T cells. In our results, we found that expression of CD3ζ was dramatically reduced during the course of human effector CD4 T-cell differentiation up to 5 days. By contrast, mouse effector CD4 T cells maintain expression of CD3ζ protein (data not shown), suggesting that modulation of CD3ζ expression may be a unique feature of human effector T cells.
We found that the extent of CD3ζ loss could be modulated by changing the activation condition. We observed a greater loss of CD3ζ expression when anti-CD3 stimulation was augmented by cocross-linking CD28 or adding monocytic APC, suggesting that the extent of CD3ζ down-regulation may be regulated by signal strength as shown in studies of TCR down-regulation in T-cell clones treated with peptide agonists and antagonists.44 Because it is difficult to isolate antigen-specific effector T cells without defined major histocompatibility complex (MHC) class II-tetramer reagents, we are currently examining whether CD3ζ loss occurs in human effector CD4 T cells generated by alloantigen stimulation.
There are at least 3 possibilities to account for the apparent loss of CD3ζ expression in effector T cells; first, CD3ζ may partition to another portion of the cell; second, CD3ζ may be degraded, and finally, CD3ζ may be continuously and rapidly recycled through the endocytic compartment. For the first possibility, it has been shown that as much as 40% of total cellular CD3ζ can associate to the actin cytoskeleton in resting T cells and partitions to the triton-insoluble pellet,45,46 suggesting that loss of CD3ζ expression in NP40 lysates of effector T cells may be due to partitioning to the cytoskeletal fraction. We think this possibility unlikely, however, because we did not detect CD3ζ protein expression in the NP40-insoluble pellet of effector T cells, but did detect CD3ζ in the insoluble fraction of resting CD4 T cells (data not shown). For the latter 2 possibilities, it has been demonstrated in T-cell clones that CD3ζ is lost due to proteolytic degradation,38 and TCR internalization mediated through 2 different pathways—either through CD3γ/PKC or p56lck/p59fyn—has been demonstrated in the human Jurkat T-cell line.47 48 Whether these processes occur in effector CD4 T cells is currently being investigated.
Although the dramatic loss of CD3ζ expression also led to the coordinate reduction in TCR/CD3 expression, the intracellular CD3ζ-associated kinase, ZAP-70, was expressed at comparable levels in resting and effector CD4 T cells. These findings contrast a previous study that demonstrated a loss of ZAP-70 expression with the same kinetics as CD3ζ down-regulation in a human T cell clone.39 We are currently examining whether the ZAP-70 kinase is active in human effector T cells, or whether another ZAP-70-related kinase such as p72syk may function in place of ZAP-70 when CD3ζ and CD3ε expression is decreased.
When taken together, the signaling alterations identified here in human effector T cells appear paradoxical. Whereas an increase in tyrosine phosphorylation and IFN-γ production suggests an augmentation of intracellular signals consistent with potent effector cell functions and increased activation kinetics,8 down-regulation of TCR and CD3ζ suggests a diminution of TCR-mediated proximal signaling. Signal extinction due to TCR down-regulation has been demonstrated in T-cell clones in which TCR/CD3 down-regulation occurred.49We propose that signaling in effector CD4 T cells may occur in the absence of CD3ζ expression, either through another TCR-signaling molecule or via activated kinases such as ZAP-70. Alternately, the low level of CD3ζ re-expression observed during effector T-cell differentiation (Figure 5A-B) coupled with the enhanced phosphorylation may enable propagation of signals in effector T cells. Because effector T cells are susceptible to apoptosis triggered by prolonged TCR occupancy,8 TCR/CD3ζ down-regulation may also serve to protect differentiating effector T cells from apoptosis. It was found that CD3ζ-deficient mouse primary T cells, or Jurkat cells, exhibited decreased susceptibility to activation-induced cell death but were not impaired in activation parameters such as up-regulation of activation markers.50 51 Maintaining or increasing intracellular proximal signals while down-regulating surface TCR and CD3ζ expression may thus enable effector cell turnover or cytokine production in the presence of prolonged TCR triggering that would otherwise have led to apoptosis.
Our findings that effector CD4 T cells derived from naive or memory precursors exhibit similar changes in tyrosine phosphorylation and CD3ζ expression suggest that examination of signaling parameters in ex vivo human T cells may serve to reliably assess their activation or differentiation state. In support of this idea, significant correlation can be drawn between our findings and biochemical analyses of ex vivo–derived T cells from certain disease states. For example, both an increase in total tyrosine phosphorylation and loss of CD3ζ has been found in human T cells isolated from patients with SLE.24Moreover, T cells exhibiting a reduction of CD3ζ expression have also been found in ex vivo isolated tumor-infiltrating lymphocytes from human renal carcinoma,52 synovial fluid T cells in rheumatoid arthritis,53 and peripheral T cells from individuals with active human immunodeficiency virus infection54 and Epstein-Barr virus and cytomegalovirus infection.26 In light of our findings, it would be interesting to examine the possibility that these disease-associated cells may represent effector T cells generated as a result of chronic immune activation. Indeed, the CD3ζ− fraction of CD8 T cells associated with chronic viral infections was found to express an activated/effector phenotype and could likewise be stimulated to produce IFN-γ.26 Our findings that CD3ζ is re-expressed in effector T cells following removal of the activating stimulus are also consistent with the ability of CD3ζ− T cells from chronic viral infection to re-express CD3ζ when cultured in vitro26 or following antiviral therapy.55
In summary, the alterations in overall TCR-mediated signaling and TCR/CD3 expression in effector CD4 T cells suggest mechanisms for the control of effector T-cell function and survival, and can likewise be used to identify this subset in vivo.
We wish to thank Dr David Hoover and his staff (WRAIR) for blood cell preparations and Dr Kristin Abraham and Dr Gregg Hadley (University of Maryland, Baltimore) for critical reading of this article.
Supported by National Institutes of Health grants AI42269 (to G.C.T.) and AI42092 (to D.L.F.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Donna L. Farber, Department of Surgery, University of Maryland Baltimore, MSTF Bldg, Rm 400, 685 W Baltimore St, Baltimore, MD 21201; e-mail: dfarber@smail.umaryland.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal