Derivatives of the Edmonston-B strain of measles virus (MV-Ed) are safe, live attenuated measles virus (MV) vaccines that have been used worldwide for more than 30 years. The cytoreductive potential of MV-Ed has been investigated in murine models of both aggressive and indolent B-cell lymphoma in severe combined immunodeficient (SCID) mice. The rationale for these studies was generated by experience with viral fusogenic membrane glycoproteins as cytotoxic genes and the recognition of the potential of replicating viruses in the treatment of human malignancy. Intratumoral injection of both unmodified MV-Ed and a strain of MV-Ed genetically modified by the addition of a β-galactosidase reporter gene (MVlacZ) induced regression of large established human lymphoma xenografts, in contrast to control therapy with UV-inactivated virus, in which all tumors progressed. The antitumor effect still occurred in the presence of passively transferred anti-MV antibody. Intravenous administration of MV also resulted in considerable slowing of tumor progression. Analysis of sections of residual tumor confirmed replication of MV within the tumors. Thus, the vaccine strain of MV mediates regression of large, established human B-cell lymphoma xenografts in SCID mice, and proof of principle is established that MV is oncolytic for lymphomas in vivo. Attenuated MVs may have value as a novel replicating-virus therapy for this group of disorders.
Introduction
Standard gene-therapy approaches to cancer treatment, such as transfer of suicide genes that confer sensitivity to prodrugs, have limitations as cytoreductive strategies owing to insufficient bystander effects of the therapeutic gene combined with suboptimal transduction efficiency of currently available gene delivery vectors. A more compelling approach in this situation is the use of a vector or virus that is able to replicate within the tumor tissue, resulting in direct cell death through cytolysis or toxicity of viral proteins. Ideally, such an agent should also be capable of stimulating a potent immune response to the tumor within which it can replicate.
Studies throughout the twentieth century have documented the lytic effects of various viruses on many types of human cancer,1 and systematic study of candidate oncolytic viruses is intensifying. Viruses under investigation as oncolytic agents include human adenoviruses, ONYX-015,2,3reovirus,4 herpes viruses5,6 and vesicular stomatitis virus.7 All of these viruses have shown promise in preclinical studies, and clinical studies of some of the agents are now in progress.8 Viruses of theParamyxoviridae family are also oncolytic. Almost 30 years ago, the human paramyxovirus, mumps, was administered to 90 patients with advanced malignancy,9 resulting in significant (although mostly short-lived) responses. Toxicity was minimal. More recently, Newcastle disease virus, an avian paramyxovirus, has also shown promising results in preclinical studies,10-12 and clinical trials in human subjects have begun.
In this study, we have investigated another human paramyxovirus, measles, as a potential antitumor agent for lymphoid malignancies. Measles virus (MV) may be particularly promising as an oncolytic virus for the treatment of lymphoid malignancy for a number of reasons. First, a nonpathogenic strain of MV is available, well characterized, and safe. Live attenuated MV vaccines, derived from the Edmonston-B strain (MV-Ed),13 have been used worldwide for more than 30 years, and in excess of 160 million doses have been administered in the United States alone with an excellent safety record. Second, although many human cell types are permissive for MV infection in vitro, in the presence of an intact immune system, virus replication after natural infection is limited to a few cell types in vivo. Lymphoid organs are prominent sites of MV replication; indeed, multinucleated giant cells develop during infection in lymph nodes as a result of gross cell-cell fusion.14 Third, we have recently shown that expression of virally derived fusogenic membrane glycoproteins in tumor cells, including MV fusion (F) and hemagglutinin (H) glycoproteins,15-17 results in a potent cytopathic effect mediated by massive cell-cell fusion. The considerable local bystander effect implies that transduction of all tumor cells would not be necessary to achieve significant tumor cell kill. However, the use of MV as a replicating vector with which to deliver the F and H glycoproteins is an attractive option. To confirm the potential clinical relevance of the study of MV in this context, there are several reported cases of regression of Hodgkin disease and of non-Hodgkin lymphoma (NHL) after natural MV infection.18 19 On the basis of these laboratory and clinical observations, we began studies with the aim of developing MV as a therapy for B-cell NHL.
MV is a negative-strand RNA virus whose genome encodes 6 protein products. Three of these proteins participate in the formation of the viral envelope. The 2 MV proteins of specific interest to this study are the H and F proteins. The H protein is the surface glycoprotein that mediates the attachment of MV-Ed to its receptor, the ubiquitously expressed regulator of complement activation, CD46.20,21The F protein is responsible for virus entry and cell-cell fusion. MV-infected cells express the F and H proteins at the cell surface and become highly fusogenic with neighboring, uninfected cells.22
In this study we have used the MV-Ed and a derivative thereof, expressing β-galactosidase, for therapy of human lymphoma xenografts in immunodeficient mice. We show that these live attenuated viruses are capable of causing regression of large established lymphoma tumors and that this effect is not abrogated by the presence of passively transferred anti-MV antibodies.
Materials and methods
Cells
Vero cells (ATCC CCL-81) (ATCC, Manassas, VA) were grown in Dulbecco's modified Eagle's medium supplemented with 5% fetal calf serum (FCS). Raji cells (ATCC CCL-86) were grown in RPMI supplemented with 10% FCS (Gibco BRL, Rockville, MD). DoHH2 cells were grown in RPMI supplemented with 10% FCS, 10 mM Hepes, 2.5 mM glucose, and 1 mM sodium pyruvate.
MV production
MV was inoculated onto 106 Vero (African green monkey) cells in T75 tissue-culture flasks at a multiplicity of infection (MOI) of 0.01 in 2 mL Optimem (Gibco BRL) at 37°C for 2 hours. The viral inoculum was removed and replaced by normal medium. The cultures were then observed until all cells were in syncytia, whereupon the cells were harvested in 2 mL Optimem and the virus was released by 2 cycles of freeze-thawing. Virus was titrated on Vero cells, and the 50% tissue-culture infectious dose (TCID50) was calculated according to the method of Spearman and Kärber.23 MVlacZ reaches a maximum titer of about 1 logarithm less than MV-Ed.24 25 Stocks of MV-Ed with a titer of 4 × 107 plaque-forming units (pfu)/mL and MVlacZ stocks with a titer of 1 × 106 pfu/mL were obtained and stored at −70°C in aliquots, ready for injection.
Animals
Four-week-old Balb/C severe combined immunodeficient (SCID) mice (Jackson Laboratories, Bar Harbor, ME) were housed in a barrier facility and cared for according to standards set by the Institutional Animal Care and Use Committee. The experimental protocol was approved by the Mayo Clinic Institutional Animal Care and Use Committee.
Tumorigenicity experiments
DoHH2 and Raji cells were infected with MV at an MOI of 0.01. The infection was allowed to proceed for 4 days (Raji) or 7 days (DoHH2). At this time, 107 viable infected tumor cells or noninfected controls were implanted subcutaneously into the flank region of the mice. The viability of cells before implantation was confirmed by trypan blue exclusion.
MV therapy
Mice were injected subcutaneously in the flank region with 107 viable tumor cells. For intratumoral administration, after the tumors reached a volume of approximately 0.4 cm3, they were injected daily with MV in a total volume of 100 μL for 10 days. As controls, tumors were injected daily with the same volume of UV-inactivated virus. Another control group of tumors was left unmanipulated. For intravenous administration, the mice were injected with 1 × 107 pfu MV via the tail vein on 4 occasions. Tumor measurements were made daily in 2 diameters, and the tumor volume was calculated according to the formula V = a2b/2 where a is the shortest and b the longest diameter. Mice whose tumors reached a volume of 2.5 cm3 or had begun to invade surrounding tissues were euthanized.
MV therapy in the presence of anti-MV antibodies
We obtained serum with an anti-MV titer of 195 IU/mL, as determined by the Mayo Clinic virology laboratory (Rochester, MN). We administered 850 μL (168 IU) anti-MV serum intraperitoneally to mice with established Raji tumors 1 day before beginning intratumoral injections of MV. A further 250 μL (49 IU) serum was administered at 7-day intervals to ensure the presence of MV antibody throughout the experiment.
Tissue preparation
Residual tumors were carefully dissected and, if possible, divided into thirds. One third was embedded in OCT tissue compound (Sakura Finetek, Torrance, CA) and snap-frozen in liquid nitrogen–cooled isopentane before storage at −70°C. One third was fixed in 0.5% glutaraldehyde for 2 hours. This aliquot of tissue was then stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal). The final third was fixed in 10% neutral buffered formalin and sectioned in 0.5-μm sections.
Detection of MV H protein
Detection of MV H proteins was carried out in paraffin-embedded tissue sections by means of a primary monoclonal anti-MV H antibody (Chemicon International, Temecula, CA) and a secondary biotinylated goat antimouse immunoglobulin (Ig) G antibody (Dako, Carpenteria, CA). Stains were developed by the addition of horseradish peroxidase–conjugated streptavidin followed by the addition of AEC (Dako). Counterstaining was carried out with Gill's hematoxylin (Surgipath Medical Industries, Richmond, IL).
In situ hybridization for MV RNA
MV nucleocapsid (N)–specific messenger RNA (mRNA) was detected in tissue sections with digoxigenin (DIG)–labeled N RNA of negative polarity.26 After deparaffinization, 5-μm–thick sections were processed as instructed by the manufacturer (Boehringer) with the following modifications: Prehybridization was carried out at 37°C for 2 hours after proteinase K pretreatment. We added 150 μL DIG-labeled N RNA probe to each section at a concentration of 30 pg/μL (in hybridization buffer with Denhardt's solution), and incubated the sections at 68°C overnight in a humid chamber. Immunological detection was carried out by means of the DIG–nucleic acid detection kit (Boehringer, Indianapolis, IN). The sections were developed in the dark at room temperature for 1.5 hours.
Detection and quantification of replication-competent virus in tumors
Thin slices of tumor tissue and organs were incubated with subconfluent Vero cells in individual wells of 6-well plates overnight at 37°C. The cells were then fixed and examined microscopically. In order to quantify the amount of virus recovered from tumors, a portion of tumor of known weight was subjected to mechanical pulverization and 2 cycles of freeze-thawing to release the virus. The virus-containing supernatant was then titrated on Vero cells, as described above.
Flow cytometric detection of CD46 expression
One million DoHH2 or Raji cells were incubated with monoclonal anti-CD46 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or IgG2A isotype control followed by a fluorescein isothiocyanate (FITC)–conjugated secondary antibody. Flow cytometry was performed with a FACSCalibur cytometer (Becton Dickinson, San Diego, CA), and data were analyzed with the Cellquest program.
Enzyme-linked immunosorbent assay for detection of anti-MV antibody
Enzyme-linked immunosorbent assay (ELISA) plates were coated overnight at 4°C with MV vaccine (Attenuvax) (Merck, West Point, PA) at a concentration of 2.6 mg/mL. The residual binding capacity of the plate was blocked with buffer containing 1% bovine serum albumin. Appropriate dilutions of serum were added to the plate in 100-μL aliquots and incubated overnight. Goat antihuman IgG conjugated to alkaline phosphatase (Accurate Chemicals, Westbury, NY) was added for 2 hours at a 1:500 dilution. The ELISA was developed for 30 minutes by means of a p-nitrophenylphosphate substrate (Sigma, St Louis, MO). A standard curve was generated by means of the second international standard antimeasles serum (NIBSC, Potters Bar, United Kingdom).
Heat shock protein detection with reverse transcriptase–polymerase chain reaction
DoHH2 and Raji cells were infected with MV at an MOI of 0.01. Control cells were left uninfected. On the fourth day following infection, RNA was obtained from the cells by means of TRIzol (Gibco BRL). Then, 1 μg RNA was reverse-transcribed by means of oligodeoxythymidine (oligo-dT) primers. Complementary DNA was amplified by polymerase chain reaction (PCR) with primers specific for human heat shock proteins hsp70 and gp96, and glyceraldehyde phosphate dehydrogenase (GAPDH) control.16 The products were subjected to agarose gel electrophoresis.
Results
MV replicates lytically in DoHH2 and Raji cells
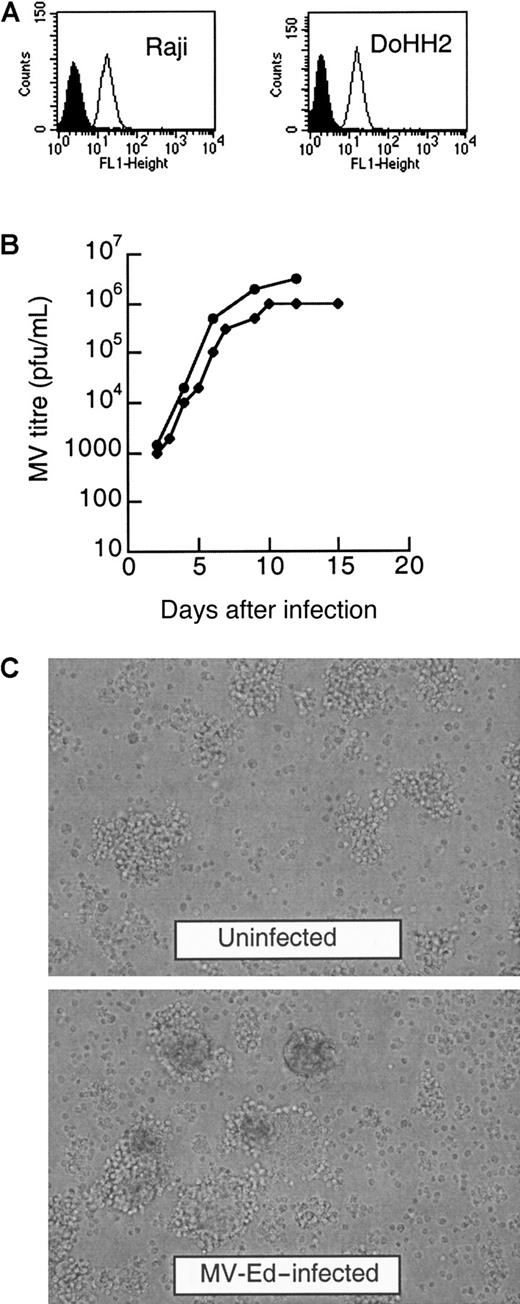
We first determined if MV-Ed and MVlacZ (an MV genetically modified by the addition of a β-galactosidase reporter gene) were able to replicate and lyse the lymphoma cell lines DoHH2 and Raji. The expression of CD46, the receptor for MV-Ed, was quantified and found to be similar on both cell lines (Figure1A). We determined that both MV strains were able to efficiently infect both these lymphoma cell lines in vitro. After inoculation at the low MOI of 0.001, the Raji and the DoHH2 cells were lytically infected by both MV-Ed and MVlacZ. Figure 1B shows that both cell lines can be infected by MVlacZ in suspension culture and that the titers of virus reached a maximum of 106 pfu/mL (DoHH2) and 3 × 106 pfu/mL (Raji). The viruses propagated more quickly and were more rapidly lytic in Raji cells. All the cells in the MVlacZ-infected Raji culture were lysed after 15 days of infection, whereas the equivalent DoHH2 culture did not lyse until 25 days after infection. Considerable cytopathic effect occurred after 4 to 7 days of infection, with readily observable multinucleated giant cells, as shown for MV-Ed in Figure 1C.
DoHH2 and Raji lymphoma cell lines express CD46 and are lytically infected by MV-Ed and MVlacZ.
(A) Quantification of CD46 expression by fluorescence-activated cell sorter analysis by means of an anti-CD46 antibody and a secondary antibody conjugated to FITC. The shaded histograms represent cells incubated with isotype controls; the line histograms represent the fluorescence intensity of cells after incubation with anti-CD46 antibody. (B) Infection of DoHH2 and Raji cells in suspension culture with MVlacZ. The circles represent the titer on Raji cells; the diamonds represent the titer on DoHH2 cells. (C) Infection of Raji cells by MV-Ed results in a characteristic cytopathic effect, with the formation of multinucleated cells in suspension culture. Noninfected Raji cells are shown in comparison with MV-Ed–infected cells 4 days after infection.
DoHH2 and Raji lymphoma cell lines express CD46 and are lytically infected by MV-Ed and MVlacZ.
(A) Quantification of CD46 expression by fluorescence-activated cell sorter analysis by means of an anti-CD46 antibody and a secondary antibody conjugated to FITC. The shaded histograms represent cells incubated with isotype controls; the line histograms represent the fluorescence intensity of cells after incubation with anti-CD46 antibody. (B) Infection of DoHH2 and Raji cells in suspension culture with MVlacZ. The circles represent the titer on Raji cells; the diamonds represent the titer on DoHH2 cells. (C) Infection of Raji cells by MV-Ed results in a characteristic cytopathic effect, with the formation of multinucleated cells in suspension culture. Noninfected Raji cells are shown in comparison with MV-Ed–infected cells 4 days after infection.
MV infection abolishes the tumorigenicity of both DoHH2 and Raji cells
To investigate if this in vitro cytopathic effect could translate to in vivo antitumor activity, we first determined if MV infection had any effect on the tumorigenicitiy of these cell lines in SCID mice. DoHH2 and Raji cells were infected in vitro with MV-Ed. At the first appearance of multinucleated cells in the suspension cell culture, 107 viable infected DoHH2 or Raji cells were injected subcutaneously into the flank region of each of 10 mice. The same number of viable noninfected cells were injected into control animals. Preinfection of cells with MV prevented DoHH2 tumor growth. One of 10 mice injected with MV-infected DoHH2 cells developed tumors, whereas 9 of 10 animals injected with control DoHH2 cells developed tumors. Similarly, MV preinfection prevented Raji tumor growth: none of 10 mice injected with MVinfected Raji cells developed tumors, whereas tumors developed in all 10 mice injected with control Raji cells. Thus, preinfection with MV efficiently prevents tumor seeding of both DoHH2 and Raji cells after subcutaneous implantation in SCID mice.
Intratumoral MV injection causes regression of established lymphoma xenografts
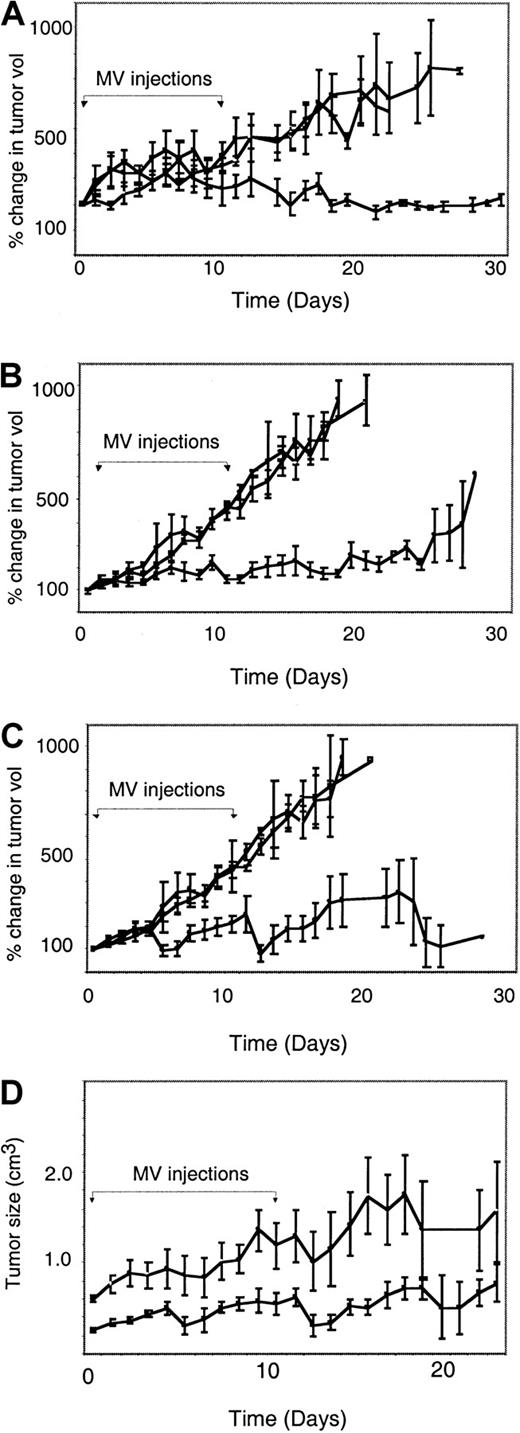
Next, we determined if intratumoral injections of MV results in regression of established DoHH2 and Raji lymphoma xenografts. Mice bearing large established DoHH2 tumors at a median volume of 0.87 cm3 (range, 0.23-1.63 cm) were injected intratumorally with 10 daily doses of either 105 pfu MVlacZ (n = 5) or UV-inactivated MVlacZ (n = 5). Another control group was left entirely untreated (n = 5). After 10 injections of MVlacZ, the mean volume of the DoHH2 tumors had not changed, whereas all of the control tumors progressed, as shown in Figure 2A. Injection with MVlacZ resulted in a significant difference in the progression rate of DoHH2 tumors compared with noninjected and inactivated virus–injected controls. In addition, injection with MVlacZ resulted in complete tumor regression of 1 of 5 of these large established DoHH2 tumors, although at the end of the observation period the tumor had begun to regrow. In a separate experiment, the same total dose of virus (106 pfu) was injected into DoHH2 tumors in a single aliquot, as opposed to 10 divided doses. No therapeutic effect was seen in this experiment (data not shown). This implies that delivery of the virus in divided doses is necessary to achieve a therapeutic effect.
Intratumoral injection with MV-Ed or MVlacZ into both DoHH2 and Raji tumors results in significant retardation or regression of tumor growth as compared with controls.
(A-C) Percentage change in tumor volumes after injection with MV (●) or UV-inactivated MV (■) and in noninjected controls (○). The day of injection was designated day 0. The x-axis represents time after intratumoral injection; the y-axis represents tumor volume relative to size at initial MV injection. The 10 days on which MV injections were given are indicated by the brackets with arrows. Panel A shows DoHH2 tumors injected with a total dose of 106 pfu MVlacZ. Panel B shows Raji tumors injected with a total dose of 106 pfu MV-Ed. Panel C shows Raji tumors injected with a total dose of 107 pfu MV-Ed. (D) A comparison between small (< 0.4 cm3) and large (> 0.4 cm3) Raji in their response to MV therapy. All MV-treated Raji tumors, regardless of virus dose, were categorized as large or small (greater or smaller than the mean). The x-axis represents time after intratumoral injection; the y-axis represents tumor size. Tumors that were small at the time of injection (▪) responded significantly better to MV therapy than those that were large at the time of injection (○).
Intratumoral injection with MV-Ed or MVlacZ into both DoHH2 and Raji tumors results in significant retardation or regression of tumor growth as compared with controls.
(A-C) Percentage change in tumor volumes after injection with MV (●) or UV-inactivated MV (■) and in noninjected controls (○). The day of injection was designated day 0. The x-axis represents time after intratumoral injection; the y-axis represents tumor volume relative to size at initial MV injection. The 10 days on which MV injections were given are indicated by the brackets with arrows. Panel A shows DoHH2 tumors injected with a total dose of 106 pfu MVlacZ. Panel B shows Raji tumors injected with a total dose of 106 pfu MV-Ed. Panel C shows Raji tumors injected with a total dose of 107 pfu MV-Ed. (D) A comparison between small (< 0.4 cm3) and large (> 0.4 cm3) Raji in their response to MV therapy. All MV-treated Raji tumors, regardless of virus dose, were categorized as large or small (greater or smaller than the mean). The x-axis represents time after intratumoral injection; the y-axis represents tumor size. Tumors that were small at the time of injection (▪) responded significantly better to MV therapy than those that were large at the time of injection (○).
Next, 7 mice bearing established Raji tumors were injected with 105 pfu MVlacZ daily for 10 days. Tumors in control mice were injected with inactivated MVlacZ (n = 6) or left untreated (n = 9). As with the DoHH2 tumors, there was a significant difference in the rate of progression of the MVlacZ-injected Raji tumors compared with that of the controls injected with UV-inactivated virus or the no-therapy controls (Figure 2B), with tumor regression in 3 of 7 Raji tumors. To determine if a higher dose of MV would lead to a greater response rate, 8 mice bearing established Raji tumors received 10 injections of 4 × 106 pfu MV-Ed (total dose, 4 × 107 pfu), with 10 mice receiving UV-inactivated MV-Ed control. As expected, at this higher dose, we also observed a significant difference in the rate of tumor progression as compared with controls (Figure 2C). In addition, we observed 4 substantial tumor regressions, with 2 tumors remaining undetectable, even after postmortem histological examination of the former tumor-bearing area.
We determined some of the correlates of the response to MV. While all of the MV-injected tumors demonstrated considerable slowing of growth compared with controls, there were substantial differences in the magnitude of response among Raji tumors. The mean tumor size of the Raji tumors injected with 106 pfu MVlacZ was 0.41 cm3 (range, 0.19-0.91), and the mean size of tumors injected with 4 × 107 pfu MV-Ed was 0.45 (range, 0.21-0.67 cm3). We therefore compared the effect of MV injection at either dose level into small (less than 0.4 cm3) or large (greater than 0.4 cm3) tumors. There was a significant difference in the response of small and large Raji tumors to MV injection, as shown in Figure 2D; thus, small tumors were more responsive than large tumors to intratumoral injection of MV, suggesting that physical limits to MV propagation after intratumoral injection are present.
The presence of anti-MV antibodies does not alter the antitumor effect of intratumoral injection of MV
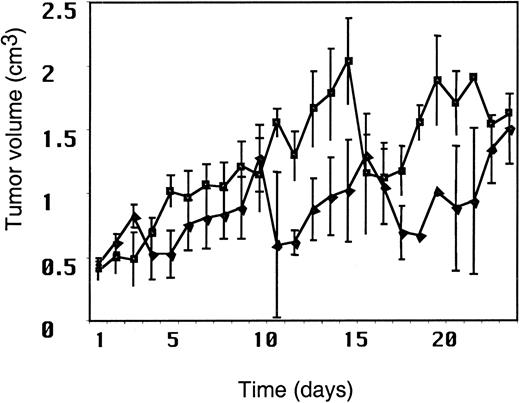
Since most adults are immune to MV, we wished to determine whether pre-existing anti-MV immunity would abrogate or abolish the therapeutic efficacy of intratumoral injection of MV. We used adoptive transfer of human MV hyperimmune serum in the SCID/Raji xenograft model of NHL. We based our approach on a recently described MV vaccine model in the cotton rat, where this method was successfully used to evoke the presence of maternally derived anti-MV antibody.27 The anti-MV antibody levels generally accepted to confer MV immunity in humans is 20 IU/mL. To establish high antibody levels of anti-MV antibody in the mice, we administered anti-MV antiserum intraperitoneally to mice with established Raji tumors 1 day before beginning MV therapy. We confirmed the presence of anti-MV antibodies in the mice by ELISA. At the beginning of MV injections, the mean human anti-MV antibody titer in the mice was 86 IU/mL (SEM ± 27 IU/mL). Figure 3 shows that the antitumor response in mice with anti-MV antibodies (n = 9) was very similar to that in nonimmune mice (n = 7). Thus, in this model, the administration of large amounts of anti-MV antibody does not compromise the antitumor efficacy of intratumoral injection of MV.
The presence of humoral immunity to MV does not compromise the antitumor response to MV.
Tumor volume after intratumoral injection of MV in the presence of anti-MV antibodies (▪) as compared with that in the absence of MV antibodies (▴). The first dose of serum was administered on day −1; MV injections began on day 0. The x-axis represents time after MV injection. The y-axis represents tumor volume (cm3).
The presence of humoral immunity to MV does not compromise the antitumor response to MV.
Tumor volume after intratumoral injection of MV in the presence of anti-MV antibodies (▪) as compared with that in the absence of MV antibodies (▴). The first dose of serum was administered on day −1; MV injections began on day 0. The x-axis represents time after MV injection. The y-axis represents tumor volume (cm3).
Pathological effects related to MV intratumoral injection
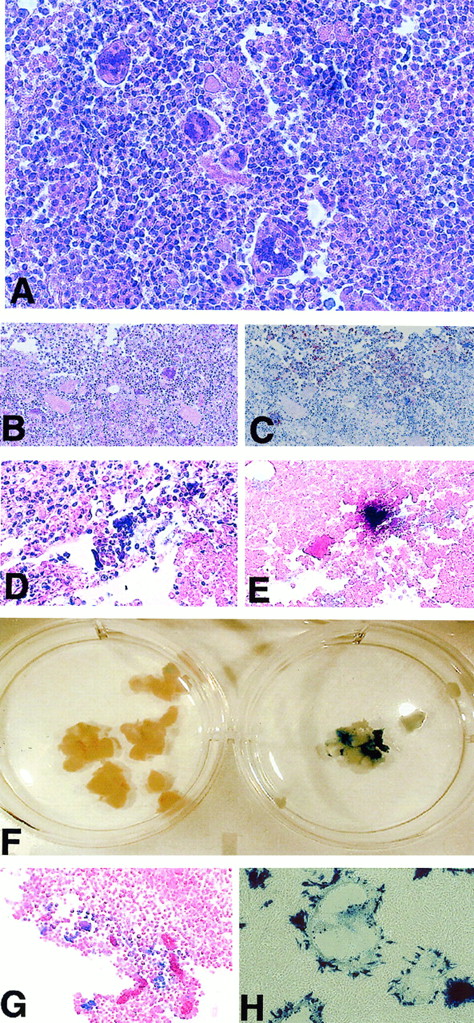
We examined histological sections of those tumors that remained after MV therapy and compared them with control tumors. Hematoxylin and eosin staining of tumor sections revealed multinucleated giant cells typical of MV infection although, upon microscopic examination of sections of tumors that had undergone significant regression, it was not always possible to detect any specific histological changes. Figure4A shows a section of Raji tumor in which multiple multinucleated syncytia were seen. Confirmation that these syncytia resulted from infection with MV was provided by detection of MV H protein expression and detection of MV N mRNA by in situ hybridization. Figure 4B-C shows consecutive tumor sections stained with hematoxylin and eosin and anti–MV H antibody, and Figure 4D-E shows consecutive tumor sections stained with hematoxylin and eosin and subjected to in situ hybridization for MV N-specific mRNA. Portions of tumor that had been infected with MVlacZ were stained in X-gal and then embedded and paraffin-sectioned. We observed macroscopic blue staining consistent with β-galactosidase activity (a representative MVlacZ-injected DoHH2 tumor is shown with noninjected control in Figure 4F) in all MVlacZ-injected tumors. No blue staining was observed in noninjected control tumors. Faint blue macroscopic staining was observed in a proportion of tumors that had been injected with UV-inactivated MVlacZ. The most likely explanation for this is passive transfer of β-galactosidase protein from the virus-producer cells. Microscopic examination of blue-stained MVlacZ-injected tumor sections revealed positive β-galactosidase staining. This staining was mostly along the edges of the tumor section, coinciding with the cytopathological effect of MV where such an effect was seen (Figure4G). The histological analysis thus confirms that the tumor regressions we observed after MV injection are due to the specific cytopathic effects of this virus on the tumors. These cytopathological effects were not evenly distributed throughout the tumors and were not apparent in all sections examined. Taken together with the tumor response data, in which the larger tumors showed a less pronounced response to MV therapy than the smaller tumors, these results suggested that virus propagation within the tumor was subject to certain physical limitations or that conditions within some of the tumors may have been inadequate to sustain viral replication or spread.
Pathological analysis of MV-injected tumors shows the characteristic cytopathic effect of MV as demonstrated by anti–MV H staining, in situ hybridization for MV mRNA, and X-gal staining.
Replicating MV can be rescued from tumors up to 20 days after the final injection. (A) Hematoxylin and eosin staining of a section of Raji tumor showing multiple multinucleated syncytia. (B-C) Consecutive tissue sections stained with hematoxylin and eosin and with an anti–MV H antibody. Anti–MV H staining, which appears as brown staining upon a background of blue counterstain, is coincident with the MV-induced cytopathic effect. (D-E) Consecutive tissue sections stained with hematoxylin and eosin and subjected to in situ hybridization for MV N-specific mRNA. MV N mRNA appears as a dark brown–stained region upon a background counterstained pink with eosin. (F) Macroscopic X-gal–stained DoHH2 tumors injected with MVlacZ or control UV-inactivated MVlacZ. (G) A section from a DoHH2 tumor injected with MVlacZ and stained with X-gal. (H) X-gal–stained Vero cells 24 hours after coculture with a small tumor section. Large β-galactosidase–expressing syncytia are seen, a result of infection with replicating MVlacZ recovered from the tumor.
Pathological analysis of MV-injected tumors shows the characteristic cytopathic effect of MV as demonstrated by anti–MV H staining, in situ hybridization for MV mRNA, and X-gal staining.
Replicating MV can be rescued from tumors up to 20 days after the final injection. (A) Hematoxylin and eosin staining of a section of Raji tumor showing multiple multinucleated syncytia. (B-C) Consecutive tissue sections stained with hematoxylin and eosin and with an anti–MV H antibody. Anti–MV H staining, which appears as brown staining upon a background of blue counterstain, is coincident with the MV-induced cytopathic effect. (D-E) Consecutive tissue sections stained with hematoxylin and eosin and subjected to in situ hybridization for MV N-specific mRNA. MV N mRNA appears as a dark brown–stained region upon a background counterstained pink with eosin. (F) Macroscopic X-gal–stained DoHH2 tumors injected with MVlacZ or control UV-inactivated MVlacZ. (G) A section from a DoHH2 tumor injected with MVlacZ and stained with X-gal. (H) X-gal–stained Vero cells 24 hours after coculture with a small tumor section. Large β-galactosidase–expressing syncytia are seen, a result of infection with replicating MVlacZ recovered from the tumor.
Replicating MV can be recovered from injected tumors
To examine the possibility that viral replication was compromised under in vivo conditions in some of the tumors, we determined whether or not replicating MV could be recovered from the injected lesions. We therefore excised small portions of tumor that remained at the end of the experiment at 20 days following the final injection of virus and cocultured these with Vero cells for 24 hours. We then examined the Vero cells for syncytia formation and by X-gal staining. As negative controls, slices of excised noninjected tumors were cocultured with Vero cells. As assessed by the presence of syncytia on Vero cells, virus was recovered from all MV-injected tumors tested. A photomicrograph of X-gal–stained Vero cells 24 hours after coculture with an actively treated tumor slice is shown in Figure 4H. No syncytia were present after culture with noninjected control tumors.
The titer of residual MV within the tumor 20 days after the final MV injection was determined in 2 Raji tumors. After physical disruption of the tumor, the cells were subjected to 2 cycles of freeze-thawing, and the supernatant was subjected to TCID50 determination on Vero cells. The titer of virus recovered from the tumor tissue was similar in both cases: 3.5 and 5 × 105 pfu/g of tumor tissue.
Thus, in this immunodeficient murine model, replication-competent MV can be recovered from injected tumors for at least 20 days following injection, indicating that the tumor xenografts can sustain in vivo viral replication, with continued depression of tumor growth in many cases.
Intravenous injection of MV slows tumor progression
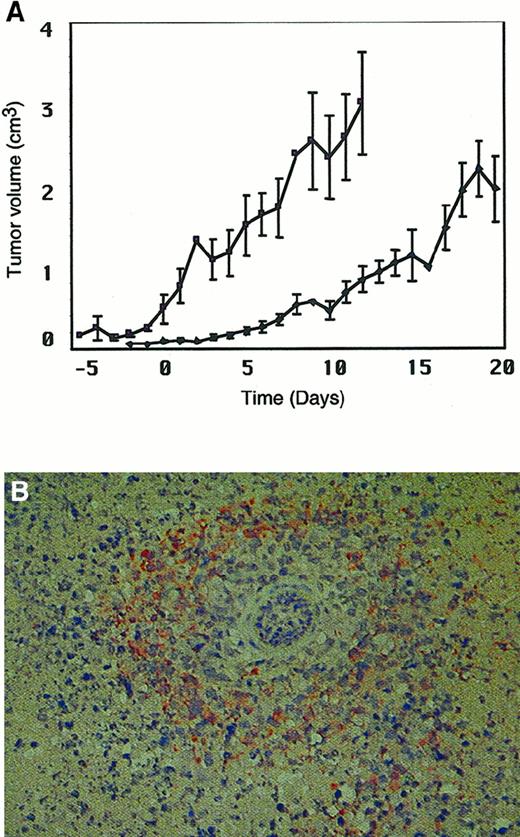
Since lymphoma is a systemic disease, we were interested in determining if systemic administration of MV could affect tumor progression in this model. Using the Raji model, we administered 4 doses of 1 × 107 pfu MV intravenously, via the tail vein, to 10 mice with established tumor xenografts. Control mice were injected intravenously with UV-inactivated MV (n = 4). Figure5A shows that progression of large established Raji tumors was halted after systemic injection of MV, in contrast to control mice, in which tumors progressed rapidly. We recovered replicating MV from the tumors in all mice treated with active virus. Hematoxylin and eosin staining of sections of residual tumors from the MV-treated mice revealed multinucleated syncytia and widespread immunoreactivity with anti–MV H antibody. Figure 5B shows prominent perivascular anti-H staining after intravenous injection of MV.
Intravenous injection of MV slows progression of Raji tumors.
Prominent perivascular anti–MV H staining is observed in tumor sections after systemic injection of MV. (A) Mice bearing established Raji xenografts were injected intravenously with 107 pfu MV on 4 occasions (days 0, 3, 5, and 7) (●) or UV-inactivated control virus (♦). The x-axis represents time after MV injection. The y-axis represents tumor volume (cm3). (B) A section of Raji tumor after intravenous injection of MV. Immunoreactivity with anti–MV H antibody appears as orange-brown staining around a blood vessel.
Intravenous injection of MV slows progression of Raji tumors.
Prominent perivascular anti–MV H staining is observed in tumor sections after systemic injection of MV. (A) Mice bearing established Raji xenografts were injected intravenously with 107 pfu MV on 4 occasions (days 0, 3, 5, and 7) (●) or UV-inactivated control virus (♦). The x-axis represents time after MV injection. The y-axis represents tumor volume (cm3). (B) A section of Raji tumor after intravenous injection of MV. Immunoreactivity with anti–MV H antibody appears as orange-brown staining around a blood vessel.
MV infection of DoHH2 and Raji cells induces expression of immunostimulatory heat shock proteins in vitro
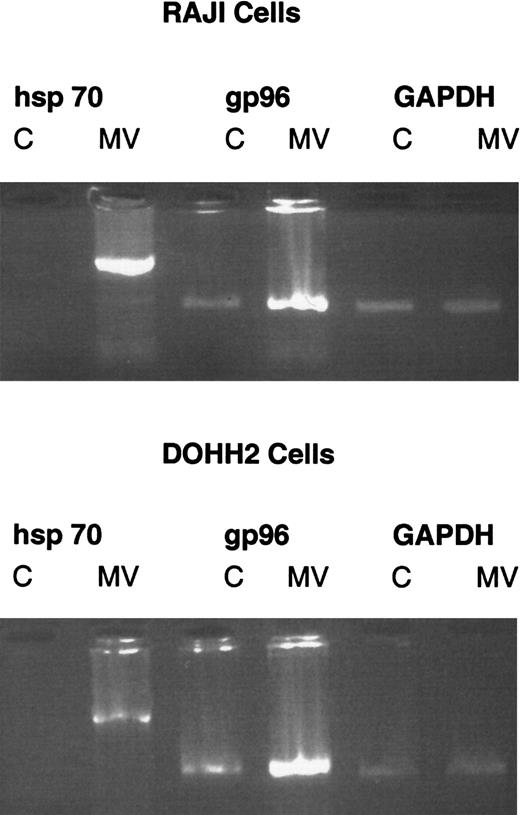
Since it is not possible to use the SCID model to examine whether local injection of MV can generate a systemic immune response, we sought a surrogate indicator of the immunostimulatory properties of MV infection in lymphoid cell lines. We therefore investigated in vitro whether infection of DoHH2 and Raji cells with MV resulted in up-regulation of highly immunostimulatory hsp expression. Both cell lines were infected with MV at an MOI of 0.01. Control cells were left uninfected. On the fourth day following infection, RNA was obtained from the cells by means of RNAzol. We reverse-transcribed 1 μg RNA using oligo-dT primers. Complementary DNA was amplified by PCR by means of primers specific for human hsp70 and gp96 and a GAPDH control. The PCR products were subjected to agarose gel electrophoresis. Figure6 shows that both hsp70 and gp96 are induced by MV infection. Uninfected Raji and DoHH2 cells do not express hsp70. Upon MV infection, expression is induced. There is constitutive expression of gp96 in both cell lines. Upon infection with MV, expression is up-regulated. These data suggest that MV infection of these DoHH2 and Raji cells will result in increased immunogenicity of these cells through induction of immunostimulatory hsps.
Infection with MV induces expression of immunostimulatory hsps in DoHH2 and Raji cells.
Reverse transcriptase PCR for hsp70, gp96, and GAPDH 4 days after infection of DoHH2 and Raji cells with MV. C denotes control, uninfected cells. MV denotes MV-infected cells. Each pair of lanes is labeled with the specific primers used.
Infection with MV induces expression of immunostimulatory hsps in DoHH2 and Raji cells.
Reverse transcriptase PCR for hsp70, gp96, and GAPDH 4 days after infection of DoHH2 and Raji cells with MV. C denotes control, uninfected cells. MV denotes MV-infected cells. Each pair of lanes is labeled with the specific primers used.
Discussion
In this study, we have shown that a vaccine strain of MV causes regression of large established human lymphoma xenografts in immunodeficient mice. This study provides proof of the principle that an attenuated measles virus has the capacity to lyse lymphoma tumors in vivo and opens the ways for further studies of this approach. Models representing both a very aggressive and a less aggressive histological subtype of B-cell lymphoma have been included in these studies. Raji cells, derived from a patient with Burkitt lymphoma,28readily establish rapidly growing subcutaneous tumors. Their growth rate is markedly faster than tumors composed of DoHH2 cells, derived from a patient with follicular lymphoma29 (data not shown). In both models, tumor growth is significantly impaired by intratumoral injections of MV compared with various controls, as shown in Figure 2A-C. Indeed, some of the tumors underwent total regression.
Our data show, in line with other published data on therapy with replicating viruses,2,4,11 that a dose on the order of 107 pfu MV is needed to achieve tumor regression. In addition, divided dosing is necessary. Taken together with the findings that preinfection of tumorigenic cells with MV abolishes tumorigenicity and that histological analysis reveals a patchy distribution of cytopathic effect, this indicates that intratumoral injection results in limited spread of MV within the tumor. This is not unexpected, since similar findings have recently been reported in relation to the ONYX-015 replicating adenovirus, where divided intratumoral injections of virus were more efficacious than a single injection of the same total dose.30 This observation has implications for dose scheduling and virus delivery for future studies of intratumoral injection of MV.
Most adults are immune to MV. To extend the clinical relevance of MV therapy for lymphoma strategy, we investigated whether tumor regressions could still take place in the presence of anti-MV antibodies. We used passive transfer of anti-MV antibodies to achieve protective levels of humoral immunity against MV in the SCID mouse model. Under these conditions, tumor regressions still occurred after MV injection, confirming that the presence of anti-MV antibodies does not abrogate the oncolytic effect of MV in this model. Our findings add to the increasing body of evidence that antiviral immunity does not necessarily compromise the efficacy of replicating-virus therapy.4 Indeed, a recent clinical study of intratumoral injection of a selectively replicating adenovirus in patients with head and neck cancer showed no correlation between the presence of neutralizing antibodies and response to therapy.8
Lymphoma is usually a systemic disease. The future clinical potential of our studies is enhanced by our finding that intravenous administration of MV can halt progression of Raji tumors in this model. The concept of systemic administration of this virus for therapeutic purposes may require the possibility of limiting virus entry and replication to lymphoma cells. Encouraging steps in the targeting of MV entry31 32 have recently been made, opening further possibilities for the future systemic use of attenuated strains of MV for therapy of lymphoma.
Complete eradication of all tumor cells by any therapy is likely to depend at least in part on the active involvement of the immune system. Our data demonstrate that infection of lymphoma cells with MV induces up-regulation of hsps. Several studies have shown a positive correlation between hsp expression and tumor immunogenicity in rodent models.33,34 Proteins initially defined biochemically as tumor antigens are now known to correspond to the cytosolic hsp70 and endoplasmic gp96 whose up-regulation we have confirmed.35 There are a number of mechanisms by which hsps might influence tumor cell immunogenicity, including the sending of an immunological danger signal,36 transfer of antigenic peptides to professional antigen-presenting cells to activate tumor-specific T cells, and enhancement of the ability of tumor cells to process and present endogenous tumor antigens.37 Our data suggest at least one mechanism by which MV infection of lymphoma cells will result in increased tumor immunogenicity.
Our studies allow us to conclude definitively that direct cytolysis by MV or cytotoxicity of MV proteins plays a significant role in the antitumor activity of MV after both local and systemic administration of the virus. We find that the presence of anti-MV antibodies does not compromise the oncolytic effect of MV. In addition, we suggest a mechanism by which MV infection of lymphoma cells can make these cells much more immunostimulatory. In ongoing studies, we plan to evaluate the role of the immune system more fully, using a syngeneic model of lymphoma in an immune-competent mouse that expresses an MV receptor, CD46, with humanlike tissue specificity.26 A phase 1 clinical study of intratumoral administration of the MV vaccine to patients with NHL has been approved. This will provide further insight into this novel therapeutic approach to the treatment of lymphoma. The potential of MV therapy for myeloma is also under investigation (Peng et al, unpublished data).
We wish to thank Becky Sanford for excellent secretarial assistance; Anthea Hammond, Richard Plemper, and Eric Poeschla for useful comments; and David Phelan and Inna Ovsyannikova for advice regarding MV-ELISA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Adele Fielding, Mayo Clinic, Guggenheim 18, 200 1st St, SW, Rochester, MN 55905; e-mail: adele.fielding@mayo.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal