In acute myocardial infarction (AMI), monocyte procoagulant activity is increased and may contribute to the risk for recurrence and other thrombotic events. This study sought to investigate the role tissue factor (TF) and tissue factor pathway inhibitor-1 (TFPI-1) in the regulation of monocyte procoagulant activity in AMI. Serial venous blood samples were obtained from 40 patients with AMI undergoing revascularization by stent placement. Twenty patients with elective stenting for stable angina served as control subjects. TF proteolytic activity was measured with spectrozyme factor Xa (FXa), TF and TFPI-1 surface expression on monocytes by flow cytometry, RNA expression in whole blood by reverse transcription–polymerase chain reaction, and concentrations of plasma prothrombin fragments F1 + 2 by immunoassay. Forty-eight hours after AMI, an increase was found in TF RNA, followed by an increase in TF surface expression by 24% ± 4% and in plasma concentration of F1 + 2 by 103% ± 17% (P < .05). These changes could not be attributed to the intervention because they did not occur in the control group. TFPI-1 RNA and binding to the monocyte surface remained unchanged. FXa generation by monocytes of patients with AMI increased 53.6% ± 9% in the presence of polyclonal antibodies to TFPI-1, indicating that cell-associated TFPI-1 inhibits monocyte TF activity. The increased monocyte procoagulant activity in AMI was caused by an up-regulation of TF that was partially inhibited by surface-bound TFPI-1. Anticoagulant therapy by direct inhibition of TF activity may, thus, be particularly effective in AMI.
Introduction
In patients with acute myocardial infarction (AMI), we have shown an increase in procoagulant activity in circulating leukocytes that could promote resistance to thrombolytic therapy, reocclusion, and peripheral thrombotic complications.1Although it may be speculated that this increase in leukocyte procoagulant activity is attributed to tissue factor (TF) expression, the exact mechanism remains obscure. An integral membrane protein, TF initiates the extrinsic coagulation cascade by allosterically enhancing the enzyme activity of the plasma serine protease factor VIIa (FVIIa). The TF-FVIIa complex then activates coagulation factors IX and X by limited proteolysis and thereby induces thrombin generation.2 TF is normally absent from cells in contact with circulating blood, but several inflammatory mediators such as interleukin 6 (IL-6), IL-8, monocyte-chemoattractant protein 1 (MCP-1), and tumor necrosis factor-α can induce TF on monocytes.3,4 The endogenous inhibitor of TF, tissue factor pathway inhibitor-1 (TFPI-1), is also expressed on monocytic cells and may inhibit procoagulant activity of circulating leukocytes.5
Thus far, only a TF degradation pathway through binding of the endocytic receptor low-density lipoprotein-related protein to exogenously added TFPI-1 and FVIIa has been described as a mechanism to inhibit TF proteolytic activity.6 On endothelial cells, we have identified an alternative inhibitory pathway for TF-FVIIa proteolytic function that is based on cell-associated glycosyl-phosphatidyl-inositol (GPI)–anchored TFPI-1.7 On association of TFPI-1 with TF-FVIIa-FXa, the resultant quaternary complex translocates to caveolae and becomes proteolytically inactive.8 GPI-anchored TFPI-1 only transiently down-regulates TF-VIIa–dependent initiation of coagulation on endothelial cells.
The relevance of these in vitro findings for pathophysiological conditions in vivo is still unknown. We, therefore, investigated the mechanism of the increased procoagulant activity in AMI and measured RNA expression and surface levels of TF and TFPI-1. To assess the functional consequences, we also measured circulating prothrombin fragments F1 + 2 and in vitro generation of FXa in the presence and absence of TFPI-1 antibodies. We sought to determine the role of surface-bound TFPI-1 in the regulation of TF proteolytic activity on circulating monocytes in AMI.
Patients, materials, and methods
Patient selection
The study group comprised 40 patients with AMI without preceding recurrent episodes of unstable angina. AMI diagnosis was based on a history of prolonged (more than 15 minutes) ischemic chest pain and significant ST-segment elevations. Twenty patients with stable angina undergoing elective stenting were included in the control group. Patients with interfering noncardiac diseases were excluded. The study was approved by the institutional ethics committee for human subjects. Informed consent was obtained from all patients.
In both groups, peri-interventional antithrombotic therapy consisted of abciximab (0.25 mg/kg bolus followed by continuous infusion, 10 μg/min for 12 hours) plus boluses of 70 U/kg heparin and 500 mg aspirin, intravenously. Postinterventional antithrombotic therapy consisted of 250 mg ticlopidine bid and 100 mg aspirin bid throughout the study. Serial peripheral venous blood samples were obtained immediately after stenting and at 24, 48, and 96 hours after stenting. For TFPI-1 surface expression, samples were also obtained before stenting in the control group. All blood samples were put on ice and processed immediately, as indicated below.
Immunoassays
Concentrations of TFPI-1 and F1 + 2 were determined by sandwich-type immunoassay (total TFPI-1, American Diagnostica, Pfungstadt, Germany; Enzygnost F1 + 2 micri, Behring Diagnostica, Marburg, Germany) using plasma samples from citrate-anticoagulated blood specimens. Detection limits were 0.36 ng/mL for TFPI-1 and 0.04 nmol/L for F1 + 2, and respective intra-assay variabilities for the lower assay ranges were 6.2% and 5%.
Flow cytometry
For flow cytometry, Cyfix (A. Ruf, Karlsruhe, Germany) fixed-blood samples were stained with fluorescein isothiocyanate (FITC)-conjugated anti-TF (American Diagnostica), anti–TFPI-1 (clone 4904; American Diagnostica), FITC-conjugated goat anti–mouse immunoglobulin (Dako, Hamburg, Germany) for the patient study, and FITC-labeled anti–TFPI-1 for the in vitro studies. Labeling was done as described previously.9 To identify monocytes, phycoerythrin (PE)–conjugated anti-CD14 monoclonal antibodies (clone Tük4; Coulter Electronics, Krefeld, Germany) were used. Flow cytometric analysis was performed using a FACScalibur (Becton Dickinson, Mountain View, CA). Fluorescence intensity of 5000 monocytes was recorded and analyzed using Cellquest software (Becton Dickinson). Flow cytometric measurement and analysis were performed as described.1
RNA preparation and reverse transcription–polymerase chain reaction
Total RNA was extracted from EDTA-anticoagulated blood samples using whole blood RNA extraction assays (RNeasy Blood Mini Kit; Quiagen, Crawley, United Kingdom) according to the manufacturer's instructions. Reverse transcription–polymerase chain reaction (RT-PCR) was performed as described7 with denaturing at 95°C for 1 minute, annealing at 60°C for 1 minute, and extending at 72°C for 1 minute repeated in 30 cycles. Primer sequences are summarized in Table 1. Serial dilutions of cDNA were examined to ensure that any effects on mRNA induction were not obscured due to a plateau effect in the PCR reactions. The density of the electrophoresed TF or TFPI-1 PCR product was related to the glyceraldehyde-3-phosphate dehydrogenase intensity of the same sample, and this ratio served as a measure of specific RNA content (NIH Image).
Sequences of oligonucleotide primers used for polymerase chain reaction
| Molecule . | Primer sequence . | PCR product . |
|---|---|---|
| TF | 5′ CTTGTGTAGAGATATAGCCAGG 3′ | 376 bp |
| 5′ GGGAACAAAAGTGAATGTGACC 3′ | ||
| TFPI-1 | 5′ CAACTCTGATACAAACGTGTTGA 3′ | 374 bp |
| 5′ CTACTACAATTCAGTCATTGGGA 3′ | ||
| GAPDH | 5′ GTTGTCATGGATGACCTTGGCC 3′ | 350 bp |
| 5′ CCACCCATGGCAAATTCCATGG 3′ | ||
| E-selectin | 5′ CTCTGACAGAAGAAGCCAAG 3′ | 235 bp |
| 5′ ACTTGAGTCCACTGAAGGCA 3′ |
| Molecule . | Primer sequence . | PCR product . |
|---|---|---|
| TF | 5′ CTTGTGTAGAGATATAGCCAGG 3′ | 376 bp |
| 5′ GGGAACAAAAGTGAATGTGACC 3′ | ||
| TFPI-1 | 5′ CAACTCTGATACAAACGTGTTGA 3′ | 374 bp |
| 5′ CTACTACAATTCAGTCATTGGGA 3′ | ||
| GAPDH | 5′ GTTGTCATGGATGACCTTGGCC 3′ | 350 bp |
| 5′ CCACCCATGGCAAATTCCATGG 3′ | ||
| E-selectin | 5′ CTCTGACAGAAGAAGCCAAG 3′ | 235 bp |
| 5′ ACTTGAGTCCACTGAAGGCA 3′ |
PCR indicates polymerase chain reaction; TF, tissue factor; TFPI-1, tissue factor pathway inhibitor-1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; bp, base pair.
Isolation of mononuclear cells and factor Xa generation
Mononuclear cells were isolated from citrate phospate dextrose acid-anticoagulated blood samples using Ficoll (Pharmacia Biotech, Freiburg, Germany) gradient separation as described.4 To measure Xa generation, cells (5 × 106) were resuspended in 100 μL cell buffer (21 mM HEPES, 137 mM NaCl, 5 mM KCl, 0.75 mM Na2HPO4, 5.5 mM glucose, 2 mM CaCl2, pH 7.4) and incubated with 100 μg/mL polyclonal anti–TFPI-1 IgG (American Diagnostica, Greenwich, CT), rabbit IgG (Sigma), or recombinant TFPI (American Diagnostica) at 37°C for 15 minutes, followed by the addition of factor VIIa (5 nM) and X (500 nM, American Diagnostica). Aliquots of 50 μL were taken after 30 minutes and quenched in 150 μL 0.1 M EDTA.8 The chromogenic substrate Spectozyme FXa (American Diagnostica) was added to 0.05 μg/mL, and FXa generation was determined after 30 minutes of reading the absorbances at 405 nm. Purified factor Xa (American Diagnostica) was used as a standard to calculate the amount of generated FXa. Regulated TF was defined as the ratio of [Xa generation with anti–TFPI-1 antibodies − Xa generation without anti–TFPI-1 antibodies/Xa generation with anti–TFPI-1 antibodies]. Interassay variability was 10%.
Triton X-114 fractionation to isolate membrane-associated TFPI-1
Mononuclear cells were adjusted in cell buffer to 6 × 106 cells/mL. To release GPI-anchored proteins, this suspension was incubated with phosphatidylinositol-specific phospholipase C (Boehringer Mannheim Biochemica, Indianapolis, IN) at 1 U/mL for 60 minutes at 37°C. Cells were washed once, and parts of the cells were stained for flow cytometry as described above. For Triton X-114 fractionation, the remaining cell pellets were lysed in 1% Triton X-114 in 0.1 M Tris, 10 mM EDTA, 2000 U/mL aprotinin, and 100 μM phenylmethylsulfonyl fluoride by incubating on ice for 15 minutes. Cell debris was removed by centrifugation at 14 000g at 4°C. Phase separation was induced by incubation of the cleared detergent lysate for 5 minutes at 37°C, followed by brief centrifugation at 14 000g to separate detergent and aqueous phase. The detergent pellicle was extracted with acetone, and the membrane proteins were resuspended in nonreducing sodium dodecyl sulfate (SDS)-sample buffer. Equivalents of 5 × 105cells were loaded per lane for separation by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting using polyclonal anti–TFPI-1 antibody (American Diagnostica) as described.8
Other methods
Serum creatine kinase concentrations were determined in the clinical chemistry laboratory. No endotoxin contamination of leukocyte suspensions or of buffers was detected (E-toxate; Sigma, Deisenhofen, Germany).
Statistical analysis
The Kolmogorov-Smirnov test showed that coagulation variables were not normally distributed. Differences between more than 2 matched samples were tested by the Friedman test, followed by the Wilcoxon matched-pair, signed-rank test, and differences between the study group and the control group were tested by the Mann-Whitney-Wilcoxon rank sum test or by the Fisher exact test, as appropriate. Correlations of parameters were calculated using linear regression analysis.P < .05 in the 2-tailed test was regarded as significant.
Results
Clinical and angiographic data
In the study group, time from onset of symptoms to start of intervention ranged from 0.5 to 8 hours, and mean plasma creatine kinase was 1325 U/L (range, 230-5962 U/L). None of the patients suffered reinfarction or death during the hospital stay. The study group did not differ significantly from the control group with respect to age, gender distribution, risk factor profile, medication, and target vessel (Table 2). Stenting was successful in all patients; it restored TIMI grade 3 flow and reduced the percentage diameter stenosis to less than 15%.
Baseline characteristics of study patients
| . | Study group (n = 40) . | Elective stenting (n = 20) . |
|---|---|---|
| Gender (M/F) | 29/11 | 15/5 |
| Age, y (range) | 61.8 (36-87) | 63.7 (31-79) |
| Active smokers, n (%) | 23 (56) | 10 (50) |
| Hypercholesterolemia, n (%) | 30 (75) | 15 (75) |
| Systemic hypertension, n (%) | 33 (83) | 14 (70) |
| Diabetes mellitus, n (%) | 10 (25) | 5 (25) |
| Prior myocardial infarction, n (%) | 4 (10) | 10 (50) |
| Prior PTCA, n (%) | 3 (8) | 1 (5) |
| Single-vessel disease, n (%) | 18 (45) | 5 (25) |
| Double-vessel disease, n (%) | 14 (35) | 8 (40) |
| Triple-vessel disease, n (%) | 8 (20) | 6 (30) |
| Target vessel, n (%) | ||
| LAD | 16 (40) | 8 (40) |
| LCx | 17 (43) | 7 (35) |
| RCA | 7 (18) | 5 (30) |
| Peak CK, U/L (range) | 1325 (230-5962) | < 80 |
| . | Study group (n = 40) . | Elective stenting (n = 20) . |
|---|---|---|
| Gender (M/F) | 29/11 | 15/5 |
| Age, y (range) | 61.8 (36-87) | 63.7 (31-79) |
| Active smokers, n (%) | 23 (56) | 10 (50) |
| Hypercholesterolemia, n (%) | 30 (75) | 15 (75) |
| Systemic hypertension, n (%) | 33 (83) | 14 (70) |
| Diabetes mellitus, n (%) | 10 (25) | 5 (25) |
| Prior myocardial infarction, n (%) | 4 (10) | 10 (50) |
| Prior PTCA, n (%) | 3 (8) | 1 (5) |
| Single-vessel disease, n (%) | 18 (45) | 5 (25) |
| Double-vessel disease, n (%) | 14 (35) | 8 (40) |
| Triple-vessel disease, n (%) | 8 (20) | 6 (30) |
| Target vessel, n (%) | ||
| LAD | 16 (40) | 8 (40) |
| LCx | 17 (43) | 7 (35) |
| RCA | 7 (18) | 5 (30) |
| Peak CK, U/L (range) | 1325 (230-5962) | < 80 |
Except for peak CK, none of the differences between the two groups were statistically significant.
PTCA indicates percutaneous transluminal coronary angioplasty; CK, creatine kinase; LAD, left anterior descending coronary artery; LCx, left circumflex coronary artery; RCA, right coronary artery.
Monocyte counts
Within the first 24 hours of stenting, monocyte counts increased to a similar extent in the control group (709 ± 43 × 103/μL vs 853 ± 60 × 103/μL; P = .01) and the study group (815 ± 44 × 103/μL vs 1044 ± 64 × 103/μL; P = .01), and they declined thereafter.
TF and TFPI-1 surface expression and RNA levels after direct stenting in AMI
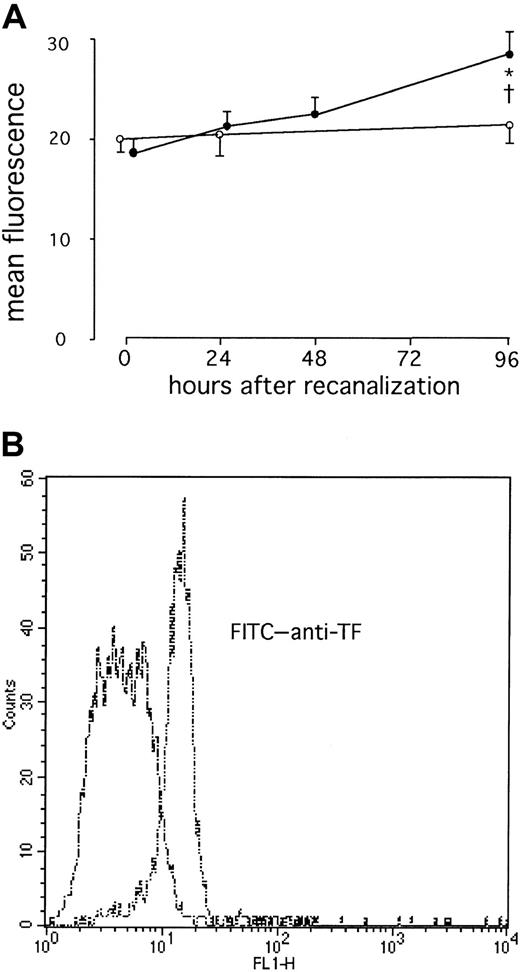
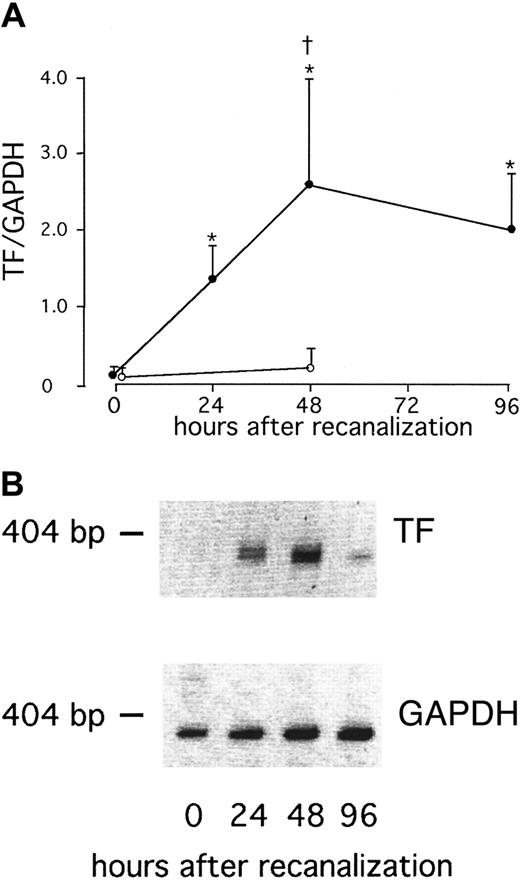
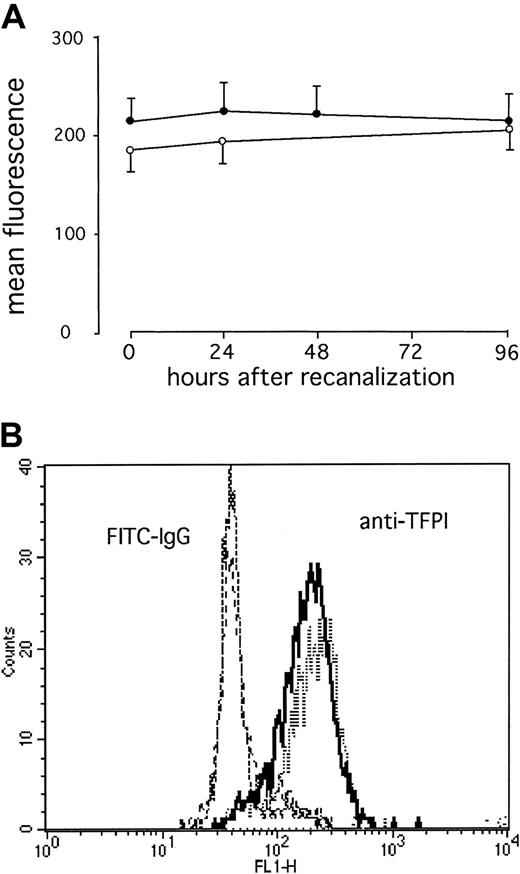
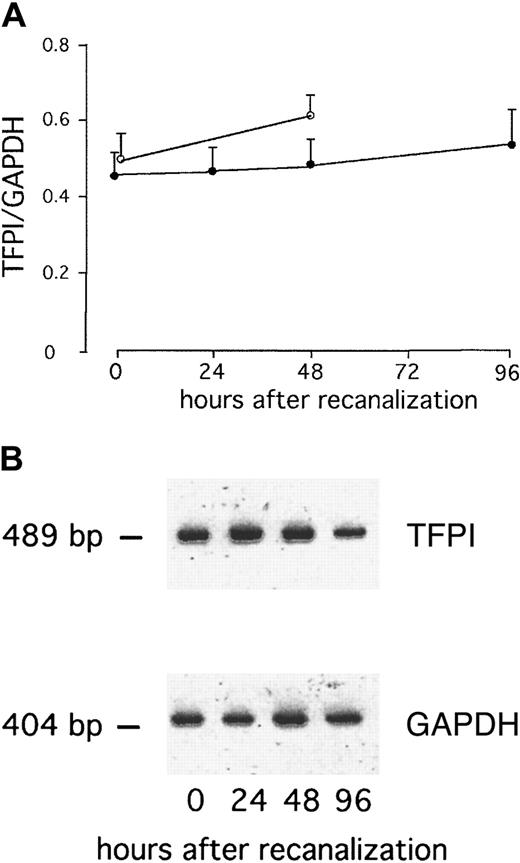
We found a significant increase in surface TF expression at 96 hours after direct stenting in AMI (P = .02; Figure1). This was preceded by a significant increase in TF RNA in whole blood at 48 hours (P = .009), whereas no TF RNA was detectable immediately after stenting in AMI (Figure 2). After elective stenting, no surface TF protein expression or TF RNA expression was found in whole blood (Figure 1). In contrast, surface expression of TFPI-1 on circulating monocytes of patients with AMI remained essentially unchanged during the study and was similar to TFPI-1 surface expression after elective stenting (Figure 3). In the control group, we were able to compare blood samples before and 30 minutes after the administration of 5000 IU heparin and did not find significant differences in TFPI-1 surface fluorescence (189 ± 9 and 193 ± 10 fluorescence; n = 20). We were unable to detect any significant changes in TFPI-1 RNA expression with time or between the groups (Figure 4). Plasma concentrations of total TFPI-1 were 192.8 ± 22.7 ng/mL immediately after intervention and decreased to 27.6 ± 3.5 ng/mL at 96 hours in patients with AMI (P < .001). Similar results were obtained in the control group. There was no RNA of E-selectin in circulating cells 96 hours after direct stenting during AMI (data not shown).
Increased TF surface expression on circulating monocytes after AMI.
(A) Serial changes in monocytic TF in patients with AMI (●) or in patients with stable angina (○). Values are expressed as mean ± SEM. *P = .02 compared to the values immediately after therapy. †P = .02 compared to the patients with stable angina. (B) Representative histograms from one patient with AMI at 96 hours (double dashes) and at 0 hours (dashes) are shown.
Increased TF surface expression on circulating monocytes after AMI.
(A) Serial changes in monocytic TF in patients with AMI (●) or in patients with stable angina (○). Values are expressed as mean ± SEM. *P = .02 compared to the values immediately after therapy. †P = .02 compared to the patients with stable angina. (B) Representative histograms from one patient with AMI at 96 hours (double dashes) and at 0 hours (dashes) are shown.
Increased TF RNA expression in whole blood after AMI.
(A) Serial changes in TF RNA expression of whole blood in patients with AMI after direct stenting (●) or in patients with stable angina after elective stenting (○). Values are expressed as mean ± SEM. *P < .01 compared to the values immediately after therapy. †P = .001 compared to the patients with stable angina. (B) RT-PCR products of one representative patient with AMI.
Increased TF RNA expression in whole blood after AMI.
(A) Serial changes in TF RNA expression of whole blood in patients with AMI after direct stenting (●) or in patients with stable angina after elective stenting (○). Values are expressed as mean ± SEM. *P < .01 compared to the values immediately after therapy. †P = .001 compared to the patients with stable angina. (B) RT-PCR products of one representative patient with AMI.
TFPI surface expression on circulating monocytes after AMI remains unchanged.
(A) Serial changes in monocytic TFPI-1 in patients with AMI after direct stenting (●) or in patients with stable angina after elective stenting (○). Values are expressed as mean ± SEM. (B) Representative histograms from one patient with acute AMI at 96 hours (dashes) and immediately after stenting (solid line) are shown (B).
TFPI surface expression on circulating monocytes after AMI remains unchanged.
(A) Serial changes in monocytic TFPI-1 in patients with AMI after direct stenting (●) or in patients with stable angina after elective stenting (○). Values are expressed as mean ± SEM. (B) Representative histograms from one patient with acute AMI at 96 hours (dashes) and immediately after stenting (solid line) are shown (B).
TFPI RNA expression in whole blood after AMI remain unchanged.
(A) Serial changes in TFPI-1 RNA expression of whole blood in patients with AMI after direct stenting (●) or in patients with stable angina after elective stenting (○). Values are expressed as mean ± SEM. (B) RT-PCR products of one representative patient with AMI (B).
TFPI RNA expression in whole blood after AMI remain unchanged.
(A) Serial changes in TFPI-1 RNA expression of whole blood in patients with AMI after direct stenting (●) or in patients with stable angina after elective stenting (○). Values are expressed as mean ± SEM. (B) RT-PCR products of one representative patient with AMI (B).
Functional consequences of TF and TFPI-1 expression
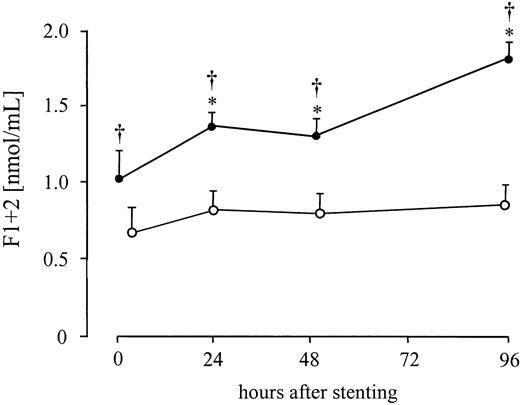
Purified mononuclear cells, obtained from patients with AMI, generated significant amounts of FXa after the addition of FVIIa and FX, whereas in the control group only minimal amounts of FXa were measured (Figure 5). As a functional correlate in vivo, a significant increase in the generation of prothrombin fragments F1 + 2 by 61% ± 13% was found at 24 hours and increased further by 103% ± 17% at 96 hours after stenting in AMI. Prothrombin fragments F1 + 2concentration did not show any significant changes after elective PTCA (Figure 6) but remained within the normal range.
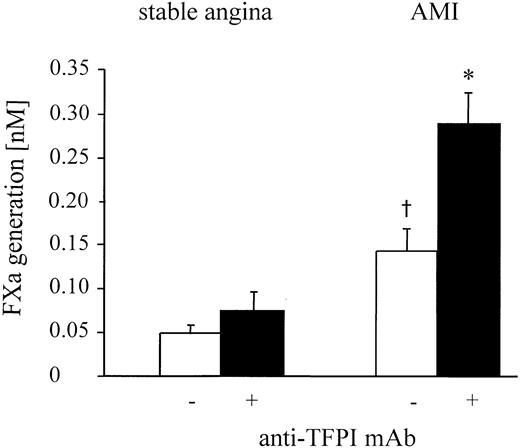
Surface-bound TFPI-1 inhibits TF activity on mononuclear cells after AMI.
TFPI-1–dependent inhibition of TF function 96 hours after stenting in patients with AMI or stable angina. FXa generation of mononuclear cells was measured in the presence and absence of anti–TFPI-1 antibody after the addition of factors VIIa and X. Values are expressed as mean ± SEM. *P = .001 compared to the values without anti-TFPI. †P = .04 compared to the patients with stable angina.
Surface-bound TFPI-1 inhibits TF activity on mononuclear cells after AMI.
TFPI-1–dependent inhibition of TF function 96 hours after stenting in patients with AMI or stable angina. FXa generation of mononuclear cells was measured in the presence and absence of anti–TFPI-1 antibody after the addition of factors VIIa and X. Values are expressed as mean ± SEM. *P = .001 compared to the values without anti-TFPI. †P = .04 compared to the patients with stable angina.
Systemic thrombin generation after AMI.
Serial changes in plasma levels of F1 + 2 in patients with AMI after direct stenting (●) or in patients with stable angina after elective stenting (○). Values are expressed as mean ± SEM. *P ≤ .03 compared to the values immediately after therapy. †P ≤ .04 compared to the patients with stable angina.
Systemic thrombin generation after AMI.
Serial changes in plasma levels of F1 + 2 in patients with AMI after direct stenting (●) or in patients with stable angina after elective stenting (○). Values are expressed as mean ± SEM. *P ≤ .03 compared to the values immediately after therapy. †P ≤ .04 compared to the patients with stable angina.
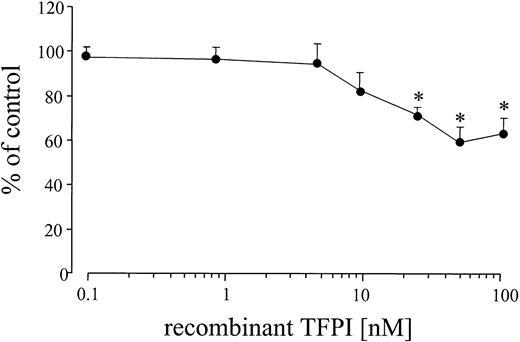
In the presence of an inhibitory TFPI-1 antibody, TF activity in the patients with AMI increased even further (Figure 5). This was specific for the TFPI-1 antibody because it was not observed after the addition of rabbit IgG (data not shown). By comparing cell surface FXa generation in the presence and absence of TFPI-1 antibodies, we determined that the percentage of TF activity inhibited by TFPI-1 after AMI was 53.6% ± 9%. After elective stenting no significant changes occurred compared to the values without TFPI-1 antibodies. There were no significant changes in TF activity after the addition of recombinant TFPI, up to concentrations of 10 nM. Only at concentrations of 25 nmol/L or greater was a significant decrease in Xa generation found (Figure 7).
Effect of recombinant TFPI-1 on TF activity of mononuclear cells after AMI.
Effect of increasing concentrations of recombinant TFPI-1 on TF activity 96 hours after stenting in patients with AMI (n = 12). FXa generation of mononuclear cells was measured in the presence and absence of recombinant TFPI-1 and addition of factors VIIa and X. Values are expressed as mean ± SEM. *P ≤ .008 compared to the control values.
Effect of recombinant TFPI-1 on TF activity of mononuclear cells after AMI.
Effect of increasing concentrations of recombinant TFPI-1 on TF activity 96 hours after stenting in patients with AMI (n = 12). FXa generation of mononuclear cells was measured in the presence and absence of recombinant TFPI-1 and addition of factors VIIa and X. Values are expressed as mean ± SEM. *P ≤ .008 compared to the control values.
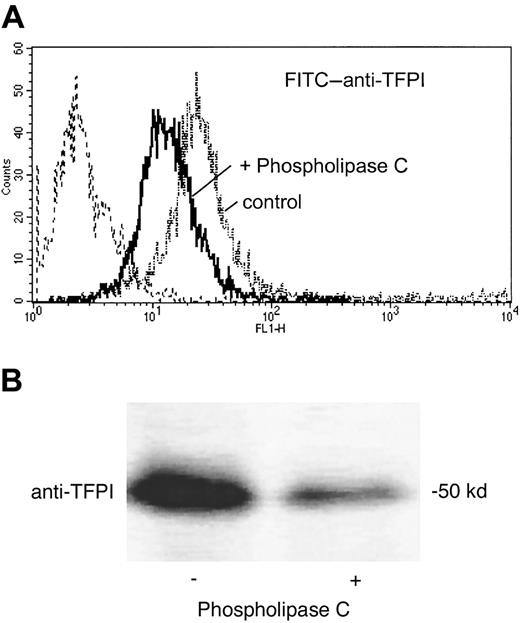
Binding sites for TFPI-1 on monocytes
Phospholipase C treatment was performed to distinguish between GPI-anchored and non–GPI-anchored binding sites for TFPI-1. Phospholipase C efficiently released endogenous, surface-bound TFPI-1, as shown by flow cytometry and Western blotting (Figure8). Surface immunofluorescence for TFPI-1 significantly (P = .03; n = 4) decreased from 186 ± 39 to 111 ± 26 mean fluorescence (mean fluorescence of irrelevant control antibody 10 ± 2 mean fluorescence).
TFPI-1 on mononuclear cells is GPI-anchored.
Effect of phospholipase C treatment on the association of TFPI-1 with mononuclear cells. Cells were incubated in the absence and in the presence of phospholipase C. TFPI-1 surface expression was analyzed by flow cytometry (A) and TX-114-soluble, membrane-associated TFPI-1 by Western blotting with anti–FPI-1 antibody (B).
TFPI-1 on mononuclear cells is GPI-anchored.
Effect of phospholipase C treatment on the association of TFPI-1 with mononuclear cells. Cells were incubated in the absence and in the presence of phospholipase C. TFPI-1 surface expression was analyzed by flow cytometry (A) and TX-114-soluble, membrane-associated TFPI-1 by Western blotting with anti–FPI-1 antibody (B).
Discussion
Major findings of our study are as follows: (1) in patients with AMI, monocytic TF expression is increased and functionally active; (2) surface-bound TFPI-1 is an important regulator of TF activity in monocytes; (3) inhibition of TF activity is mediated by surface-bound, GPI-anchored TFPI-1 but not by exogenously added recombinant TFPI-1 at concentrations comparable to those of circulating TFPI-1; and (4) inhibition of TF activity by surface-bound TFPI-1 limits the procoagulant effects of increased monocytic TF-expression in AMI.
As a cause for the increased procoagulant activity of mononuclear cells in AMI, we show increased expression of TF RNA and surface protein. Functional relevance in vitro is verified by increased FVIIa-dependent FXa formation. These in vitro findings correspond to an increased thrombin formation in patients with AMI, which we demonstrated by increased plasma concentrations of prothrombin fragments F1 + 2. This is consistent with TF-dependent thrombin generation, though we cannot directly prove a cause-and-effect relation because other pathways of thrombin generation may also play a role. We cannot attribute the increased TF expression to the stenting procedure itself, because it was not found in patients undergoing stenting for chronic stable angina. Previous studies suggest that the various inflammatory mediators released in AMI constitute a strong stimulus for TF expression in monocytes.4,10 11
Surface-bound TFPI-1 has been identified as potent inhibitor of TF activity in cultured endothelial cells.7,8 15 We show for the first time, to our knowledge, that this mechanism is also effective in monocytes. We also demonstrate the relevance of this mechanism in patients with AMI by showing that blockade of TFPI-1–dependent inhibition of TF by antibodies increased TF activity by approximately 50%.
In endothelial cells, we recently described quaternary complex formation of GPI-anchored TFPI-1 with the TF-FVIIa-FXa assembly and subsequent translocation in caveolae as a major mechanism of TF inhibition by TFPI-1. The regulation of TF by TFPI-1 in mononuclear cells found in this study exhibited central characteristics of this mechanism. Phospholipase C treatment removed TFPI-1 from the cell surfaces, indicating that the large amounts of surface-bound TFPI-1 were GPI-anchored. The importance of GPI-anchored TFPI-1 is corroborated by the inability of exogenously added recombinant TFPI-1 to further inhibit TF activity. This was observed at concentrations comparable to those of circulating TFPI-1 in the patients after AMI. In addition, it has to be considered that most plasma TFPI-1 is bound to lipoproteins and lacks anticoagulant function.12 However, we cannot exclude other potential pathways for TF inhibition by TFPI-1, such as scavenging of TF-FVIIa-FXa complexes by lipoprotein-related protein-bound TFPI.
In addition, binding of TFPI-1 to heparan sulfate proteoglycans13 must be considered. Therapeutic administration of heparin increases the circulating levels of TFPI-1 in plasma by releasing TFPI-1 from heparan sulfate or other glycosaminoglycans on the endothelium.14 All our patients received peri-interventional heparin, and, accordingly, we found increased plasma TFPI-1 concentrations. Because we did not find significant changes in surface TFPI-1 expression before and after heparin in the control group and we could not release TFPI-1 after incubation with unfractionated heparin in vitro (data not shown), the contribution of monocytic glycosaminoglycan-bound TFPI-1 to the increase in plasma TFPI-1 levels after heparin administration may be considered negligible.
Based on these findings, we suggest that quaternary complex formation of GPI-anchored TFPI-1 with the TF-FVIIa-FXa-assembly as the central mechanism for TFPI-1–dependent TF inhibition on monocytes of patients with AMI. In endothelial cells, the underlying mechanism for this inhibition is translocation to caveolae, representing detergent-insoluble microdomains.7,8,15 The GPI anchor of TFPI-1 is sufficient for this translocation8,15 and for the reconstitution of TF inhibition.7 In monocytes, surface-bound TFPI-1 also localizes to detergent-insoluble microdomains (W. Ruf, personal communication). Because monocytes do not express caveolin,6 these detergent-insoluble microdomains may be rafts.16 This is concurrent with our finding that in monocytes part of the endogenous TFPI-1 is GPI-anchored because GPI-anchored proteins partition into rafts.16 Thus, we suggest that the inhibition of TF by TFPI-1 in monocytes is mediated through GPI-anchored TFPI-1 in rafts.
Monocytes constitutively express TFPI-1, but TFPI-1 regulation by inflammatory mediators is still controversial. Although serum and growth factors have been shown to induce TFPI-1 in smooth muscle and endothelial cells,17-19 conflicting results have been obtained for monocytes.5 20 In our study, the regulation of monocytic TFPI-1 by inflammatory mediators did not appear to play a major role. Contrary to our findings on TF, we did not observe the up-regulation of monocytic TFPI-1 during reperfusion in AMI. Nevertheless, the constitutive expression of TFPI-1 on the monocytic surface was sufficient to down-regulate induced TF activity after AMI.
In studies of monocyte function there is always concern about changes occurring during and after blood sampling. We, therefore, ensured that the time between sampling and processing was identical in all specimens. We also routinely checked for endotoxin contamination that might have induced functional changes ex vivo.
To investigate monocytic RNA expression, we used semiquantitative RT-PCR of whole blood RNA because the number of cells available from the patients was limited. We ensured a linear relation between RNA concentration and cDNA product assessed by densitometric analysis. Despite these efforts, we cannot disregard that we might have missed minute changes in TFPI-1 RNA. Nevertheless, our semiquantitative approach was able to demonstrate changes in TF RNA robustly. In circulating leukocytes, monocytes have been shown to express significant amounts of tissue factor. Because the generation of TF in neutrophils is minimal, if at all present, and TF is not expressed in lymphocytes, the pronounced changes in TF RNA reflect monocyte activation.21-23
To ensure that the increase in TF mRNA level was not caused by detached activated endothelial cells, we analyzed E-selectin RNA expression in circulating cells. There was no mRNA expression for E-selectin in circulating cells 96 hours after direct stenting during AMI. Therefore, the increase in TF mRNA level reflects monocyte activation.
Systemic procoagulant changes in AMI corrupt the success of thrombolytic treatment and contribute to the risk for reinfarction and thrombo-embolic events. Our study identifies molecular mechanisms of increased procoagulant activity and its regulation in AMI. It demonstrates increased TF activity of mononuclear cells, which is only partially inhibited by GPI-anchored TFPI-1. Specific inhibition of TF-activity may thus be particularly effective in AMI. This may be achieved by recombinant inactive FVIIa or by the administration of specific antibodies, an approach that has been successfully adopted in experimental septic shock.24 25 Another therapeutic concept suggested by our study is the strengthening of inhibition by TFPI-1. Based on our findings, we suggest that exogenously added TFPI-1 has to be engineered to enable GPI anchoring to achieve this effect.
We thank Dr L. V. M. Rao for the polyclonal anti–TFPI-1 antibody. We also thank Dr W. Koch, C. Huber, T. Nordte, V. Malouvier, and R. Vukovich for invaluable technical assistance.
Supported in part by grants from the Deutsche Forschungsgemeinschaft (Ne 540/1-2) and the Bayrische Wissenschaftsministerium (Bayrischer Habilitationsförderpreis) (I.O.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ilka Ott, Deutsches Herzzentrum der Technischen Universität München, Lazarettstrasse 36, 80636 Munich, Germany; e-mail: ott@dhm.mhn.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal