It has been proposed that in the early stages of human immunodeficiency (HIV) infection, before the loss of CD4+ T cells, inhibition of IL-12 production from host antigen-presenting cells plays a critical role in the suppression of T-helper cell type 1 responses. Activation of the Gi-protein–coupled high-affinity N-formyl peptide receptor by f-met-leu-phe and HIV-derived peptide T-20–suppressed IL-12 p70 production from human monocytes in response to both T-cell–dependent and T-cell–independent stimulation are reported. Activation of the low-affinity N-formyl peptide receptor by the HIV-derived F-peptide suppressed IL-12 production more modestly. This suppression was pertussis toxin sensitive and was selective for IL-12; the production of IL-10, transforming growth factor-β, and tumor necrosis factor-α was unaltered. The production of IL-12 p70 by dendritic cells was unaffected by these peptides despite functional expression of the high-affinity fMLP receptor. These findings provide a potential direct mechanism for HIV-mediated suppression of IL-12 production and suggest a broader role for G-protein–coupled receptors in the regulation of innate immune responses.
Introduction
Suppression of interleukin-12 (IL-12) production during infection with the human immunodeficiency virus (HIV) may contribute significantly to host immunosuppression. This may be particularly relevant early in the course of infection, when patients still have substantial numbers of uninfected CD4+ T cells yet do not mount normal T-helper cell type 1 (Th1) responses.1-7 In support of this possibility, the in vitro production of IL-12 by both peripheral blood mononuclear cells and purified monocytes from HIV-infected patients is significantly and selectively reduced in response to stimulation.8-14 It has also been reported that the capacity to produce IL-12 p40 is increased after intensive antiretroviral therapy, though this finding has not been supported by all investigators.15-17 Furthermore, the addition of exogenous IL-12 to cultured cells from HIV-infected patients restores cell-mediated immune responses and augments cytotoxic T lymphocyte activity against HIV-specific antigens.18-21Finally the decreased capacity to produce IL-12 has also been proposed to enhance apoptosis of CD4+ Th1 cells in HIV-infected persons.22-25
Two synthetic peptides from HIV-1—T-20, which corresponds to the carboxy-terminal ectodomain sequence of gp41 of the HIV-1 LAI strain, and F-peptide, which corresponds to the V4-C4 region of gp120 of the HIV-1 Bru strain—bind to the high-affinity (FPR) and the low-affinity (FPRL-1) formyl-peptide receptors, respectively.26,27These peptides appear to be largely conserved in strains of HIV, and activation of these receptors by T-20 and F-peptide induce both calcium flux and chemotaxis in human monocytes and neutrophils.26,27 In addition, both peptides can affect HIV infectivity. F-peptide down-regulates the expression and the function of the HIV coreceptors CCR5 and CXCR4 on human monocytes, and T-20 peptide inhibits the formation of the gp41 hairpin structure that mediates fusion of the viral and cell membranes, preventing viral infection.27-31 On the basis of these observations, the administration of T-20 peptide to humans infected with HIV has entered clinical trials, where it has been shown to decrease viral burden.32
Both FPR and FPRL-1 are members of the 7-transmembrane domain family of receptors, and they signal through a Gi-coupled heterotrimeric G-protein complex. These receptors were initially characterized as receptors for the prototypic bacterial chemoattractant f-met-leu-phe (fMLP) and are expressed by human monocytes and by a variety of other human cells.33 Although no other naturally occurring ligands have been identified for FPR, 2 endogenous ligands for FPRL-1, the lipid metabolite lipoxin A4, and serum amyloid A, have been described.34,35 Studies in our laboratory and others36-39 have demonstrated that selective chemokines and chemoattractants, such as the monocyte chemoattractant protein-1 and the anaphylatoxin C5a (which also signal via Gi-coupled–receptors) suppress IL-12 production by human and murine antigen-presenting cells. This suggested that signaling through Gi-coupled FPR and FPRL-1 by T-20 and F-peptide may also suppress IL-12 p70 production. We now report that the activation of formyl-peptide receptors by HIV-derived peptides suppresses IL-12 p70 production from human monocytes in response to T-cell–dependent and T-cell–independent stimuli. This inhibition is pertussis toxin sensitive and independent of known autocrine inhibitors of IL-12 synthesis, and it likely occurs at the level of transcription. Interestingly, the suppressive effect was not seen with monocyte-derived dendritic cells, despite functional expression of the high-affinity FPR receptor. These findings provide a novel mechanism by which HIV antigens may directly inhibit IL-12 production by human monocytes and a novel function for formyl-peptide receptors.
Materials and methods
Isolation and stimulation of human monocytes
Human monocytes obtained from healthy donors (n = 16) by standard leukapheresis were purified by counterflow centrifugation (elutriation), which yielded cells of uniform forward/side scatter and were 95% to 99% CD14+ by flow cytometry. Monocytes were cultured at a density of 2 × 106 cells/mL at 37°C and 6% CO2 in 1 mL cRPMI: RPMI 1640 (Biofluids, Rockville, MD) supplemented with 10% fetal calf serum (Biofluids), 15 mM HEPES (Biofluids), 200 mM glutamine, 5% NCTC-109, 100 μg/mL penicillin and streptomycin, and 50 μg/mL gentamicin. Monocytes were preincubated for 1 hour with either media alone or with varying concentrations of either fMLP (Sigma Chemical, St Louis, MO), T-20 peptide (derived from gp41 HIV-1 LAI, AA 643-678): YSTLIHSLIEESQNQQEKNEQELLEL-DKWASLWNWF-NH2,26or F-peptide (derived from gp120 HIV-1 Bru, AA 414-434): EGSDTITLPCRIKQFINMWQE-NH2.27 Monocytes were stimulated with 100 ng/mL interferon (IFN)-γ (R&D Systems, Minneapolis, MN) and either 0.01% (wt/vol) Staphylococcus aureus, Cowan strain I (SAC; Calbiochem, San Diego, CA), 100 ng/mL lipopolysaccharide (LPS; E coli serotype O127:B8, Sigma), or 3 μg/mL recombinant trimerized human CD40L (kindly provided by Immunex, Seattle, WA). To evaluate pertussis toxin sensitivity, monocytes were cultured for 4 hours in the presence or absence of 250 ng/mL purified pertussis toxin (List Biological Laboratories, Campbell, CA) and then were stimulated with SAC (0.01%) and IFN-γ (100 ng/mL). Twenty-four hours after stimulation, cytokine production was assayed by enzyme-linked immunosorbent assay (ELISA). Antibody pairs (clone 2495.11 and polyclonal goat IgG) for human IL-12 p70 were obtained from R&D Systems, and antibody pairs for IL-10 (clones AHC8102 and AHC7109) and tumor necrosis factor (TNF)-α (clones AHC3712 and AHC3419) were obtained from Biosource International (Camarillo, CA) and used according to the manufacturer's suggestions. Assays for transforming growth factor (TGF)-β1 were performed using ELISA kits from R&D Systems and used according to the manufacturer's instructions. Statistical analysis was performed using paired Student t tests comparing the maximal cytokine induction in response to stimulation with SAC and IFN-γ alone to stimulated cytokine production in the presence of varying concentrations of fMLP, T-20, or F-peptide.
Quantitative polymerase chain reaction for IL-12 p35 and p40 mRNA expression
Elutriated human monocytes (107) were pretreated with fMLP, T-20 peptide, or F-peptide (all at 10−4 M) for 30 minutes and then were stimulated with SAC (0.01%) and IFN-γ (100 ng/mL) for 6 hours. Total RNA was extracted using STAT-60 (Tel-Test, Friendswood, TX), and cDNA was generated using 5.0 μg RNA as a template and Superscript II (Gibco BRL, Gaithersburg, MD) with random hexamer priming. Quantitative real-time polymerase chain reaction (PCR) was performed by TaqMan (PE Applied Biosystems, Foster City, CA) using sequence-specific probes and primers for IL-12 p35, IL-12 p40, and GAPDH obtained from the manufacturer. PCR was performed using 10-fold serial dilutions of templates to ensure linear-phase amplification, and gene expression was normalized for GAPDH expression.
Isolation and stimulation of dendritic cells
Immature dendritic cells were derived from elutriated monocytes as described by Bender et al.40 Briefly, monocytes were cultured for 7 to 9 days in cRPMI supplemented every other day with 100 ng/mL IL-4 (Prepro Tech, Rocky Hill, NJ) and 100 ng/mL granulocyte macrophage–colony-stimulating factor (GM-CSF; R&D Systems). Nonadherent cells were harvested by gentle washing, and most (70%-90%) were confirmed by flow cytometry to be CD1a (clone HI149; PharMingen, San Diego, CA) high and CD83 (clone HB15e; PharMingen) low. More than 95% of these cells excluded trypan blue and demonstrated characteristic dendrite formation by phase-contrast microscopy. Immature dendritic cells were resuspended in cRPMI at a density of 106 cells/mL, pretreated with either fMLP (10−5 M), T-20 peptide (10−5 M), or F-peptide (10−5 M) for 1 hour, and stimulated with 100 ng/mL IFN-γ and either 0.01% SAC or 3 μg/mL CD40L. Twenty-four hours after stimulation, IL-12 p70 production was assayed by ELISA as described above.
RT-PCR of stimulated human monocyte-derived dendritic cells
Immature monocyte-derived dendritic cells were generated as described previously. CD1a+ cells were isolated by flow cytometry sorting performed on a FACStar cell sorter (Becton Dickinson, San Jose, CA). The final cell population was more than 97% CD1a+. Total RNA was obtained using STAT-60, and cDNA was generated using 2.5 μg RNA as a template and Superscript II. PCR amplification was performed using 5-fold dilutions of cDNA template to ensure linear-phase amplification of PCR products and normalized for GADPH expression. PCR amplification of FPR and FPRL cDNA was performed under the following conditions: initial denaturation at 94°C for 2 minutes and then 40 cycles at 94°C for 45 seconds, 57.5°C for 60 seconds, and 70°C for 90 seconds, and a final elongation step at 70°C for 5 minutes. These primer pairs were used: GADPH, sense-TGAAGGTCGAGTCAACGGATTTGGT, antisense CATGTGGGCCATGAGGTCCACCAC; FPR, sense CAAGATGGAGACAAATTCCTCTC, antisense GAGCAGAGCCATCACCCAGGGCCCAA; and FPRL-1, sense CTGTACT-TTCAACTTTGCATCC, antisense ATTTCCCAACTCCACTTACC. PCR products were resolved by gel electrophoresis on a 1.5% agarose gel, and stained with ethidium bromide.
Calcium flux studies
Intracellular calcium flux studies using flow cytometry analysis was performed as described by Rabin et al.41 Briefly, monocyte-derived dendritic cells (2 × 107) were suspended in HBSS-HEPES (HBSS supplemented with 10 mM HEPES, Ca++, Mg++, and 1% fetal calf serum). Indo-1 and pleuronic detergent (Molecular Probes, Eugene, OR) were added at final concentrations of 5 μM and 300 μg/mL, respectively. The cell suspension was incubated at 30°C for 45 minutes with gentle agitation. Cells were then washed twice with the HBSS-HEPES, stained with anti-CD1a, and washed again. Calcium flux for CD1a+dendritic cells was performed using a FACSVantage flow cytometer (Becton Dickinson) equipped with an argon laser tuned to 488 nM and a krypton laser tuned to 360 nM. Indo-1 fluorescence was analyzed at 390/20 nM and 530/20 nM for bound and free calcium, respectively. Before stimulation, cell suspensions were warmed at 37°C for 3 minutes. The CD1a+ cell population was gated, and baseline fluorescent ratios were collected for 30 seconds. Cells were then stimulated with either fMLP (10−5 M), T-20 peptide (10−5 M), or F-peptide (10−5 M) followed by fMLP (10−8 M). Collections continued until calcium flux returned to basal levels. Changes in Indo-1 fluorescence were expressed as the ratio of bound to free intracellular calcium, and scattergrams represented the entire CD1a+ cell population at the time of stimulation. Data analysis was performed using Flowjo software (Tree Star, San Carlos, CA).
Results
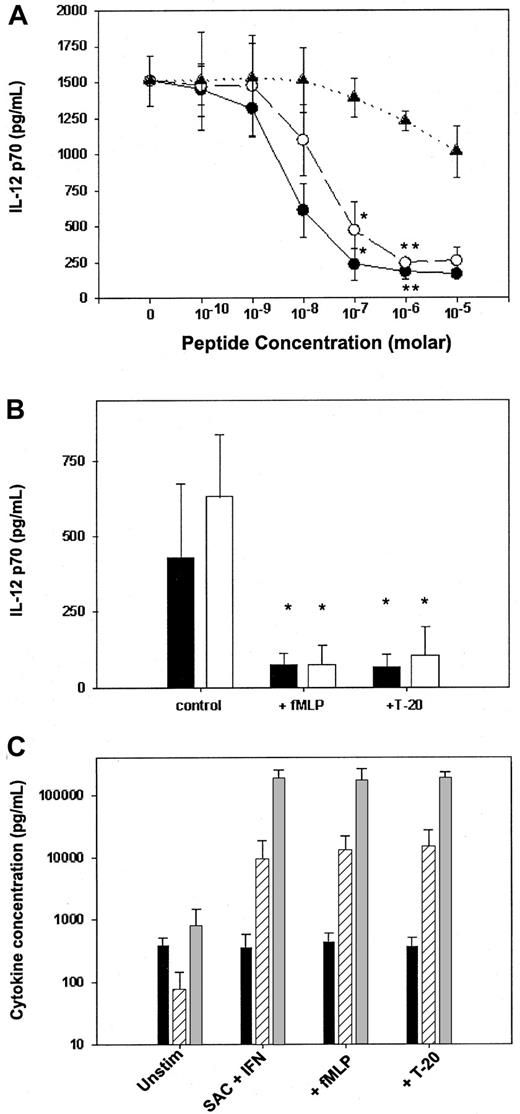
To explore the ability of FPR ligands to suppress IL-12 p70 production, we preexposed elutriated human peripheral blood monocytes to varying concentrations of T-20, F-peptide, or fMLP in vitro and subsequently stimulated the monocytes with 2 potent inducers of IL-12 p70 production—SAC and IFN-γ. Both T-20 and fMLP dramatically suppressed IL-12 p70 production in a dose-dependent manner (Figure1A), with maximal inhibition (more than 90%) occurring at concentrations below 10−5 M. F-peptide also suppressed IL-12 p70 production; however, the level of inhibition was modest (30%) despite high peptide concentrations (10−5 M), suggesting that signaling through FPRL-1 has a minimal effect on IL-12 production. The dose-response curve for fMLP was also consistent with a primary role for FPR signaling given that significant suppression of IL-12 occurred at the concentration range associated with high-affinity binding of fMLP to FPR on human monocytes (10−9 and 10−7 M).26 27 fMLP and T-20 each showed a similar potent inhibitory effect (Figure 1B) when monocytes were stimulated with 2 other known inducers of IL-12—LPS and IFN-γ—or with soluble trimerized CD40L and IFN-γ. These findings demonstrated that T-20 and fMLP can suppress monocyte production of IL-12 p70 in response to T-cell–dependent and T-cell–independent signals.
fMLP and T-20 peptide selectively suppress IL-12 p70 production by elutriated human monocytes.
(A) Monocytes were preincubated with varying concentrations of fMLP (●), T-20 peptide (○), or F-peptide (▴) and then were stimulated with SAC and IFN-γ. (B) fMLP and T-20 suppress both T-cell–dependent and T-cell–independent production of IL-12. Elutriated monocytes were pretreated with either fMLP (10−5 M) or T-20 peptide (10−5 M) and were stimulated with IFN-γ and either LPS (▪) or CD40L (■). (C) fMLP and T-20 selectively suppress IL-12 production. IL-10 (▨), TGF-β (▪), and TNF-α (░) production by human monocytes stimulated with IFN-γ and SAC after preincubation with either media alone, fMLP (10−5 M), or T-20 peptide (10−5 M). Cytokine concentrations were measured 24 hours after stimulation by ELISA. Data are mean ± 1 SD from monocytes obtained from 3 separate donors. (*P < .01; **P < .005).
fMLP and T-20 peptide selectively suppress IL-12 p70 production by elutriated human monocytes.
(A) Monocytes were preincubated with varying concentrations of fMLP (●), T-20 peptide (○), or F-peptide (▴) and then were stimulated with SAC and IFN-γ. (B) fMLP and T-20 suppress both T-cell–dependent and T-cell–independent production of IL-12. Elutriated monocytes were pretreated with either fMLP (10−5 M) or T-20 peptide (10−5 M) and were stimulated with IFN-γ and either LPS (▪) or CD40L (■). (C) fMLP and T-20 selectively suppress IL-12 production. IL-10 (▨), TGF-β (▪), and TNF-α (░) production by human monocytes stimulated with IFN-γ and SAC after preincubation with either media alone, fMLP (10−5 M), or T-20 peptide (10−5 M). Cytokine concentrations were measured 24 hours after stimulation by ELISA. Data are mean ± 1 SD from monocytes obtained from 3 separate donors. (*P < .01; **P < .005).
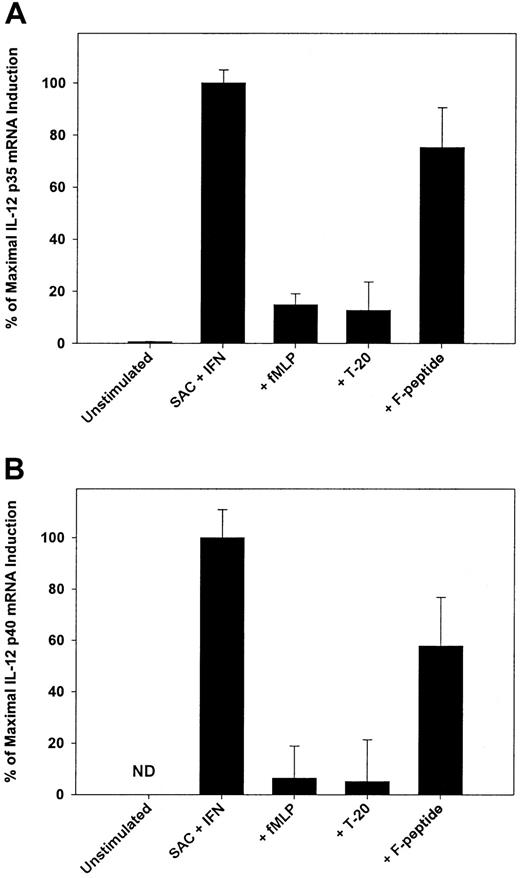
Suppression of IL-12 p70 production by T-20 and fMLP was selective (Figure 1C) in that the production of TNF-α, IL-10, and TGF-β by stimulated monocytes was unaltered by preexposure to the peptides. The addition of neutralizing antibodies to IL-10, TGF-β, or indomethacin to inhibit the production of PGE2 had minimal effect on reversing the suppression of IL-12 (data not shown), suggesting that T-20 and fMLP work independently of known autocrine inhibitors of IL-12 synthesis. We then determined whether T-20 and fMLP acted to suppress IL-12 mRNA accumulation and found that the pretreatment of monocytes with either T-20 or fMLP resulted in a marked reduction in the levels of mRNA for both IL-12 p40 and p35 (Figure2A-B). IL-12 p35 and p40 mRNA levels were suppressed by nearly 90% and 95%, respectively, by preexposure to T-20 or fMLP in comparison to levels induced by SAC and IFN-γ stimulation in the absence of pretreatment. Pretreatment with F-peptide resulted in a more modest reduction in IL-12 mRNA levels. These data imply that, as with other inhibitors of IL-12 production shown to regulate IL-12 at the level of transcription, the primary effect of FPR signaling is to inhibit gene transcription.42 43Alternatively, it is also possible that FPR signaling effects mRNA degradation; this possibility is currently being explored.
FPR activation suppresses mRNA.
Activation of FPR by fMLP or T-20 suppresses the level of mRNA for both IL-12 p35 (A) and IL-12 p40 (B). Human monocytes were stimulated for 6 hours with SAC and IFN-γ in the presence or absence of FPR and FPR-1 ligands (10−5 M). Taqman was used to isolate and quantitate RNA. Suppression of IL-12 mRNA was expressed as percentage maximal induction relative to peak expression with SAC and IFN-γ stimulation because there was no detectable constitutive expression of IL-12 p40 mRNA. Data are mean ± 1 SD from replicate samples from 3 separate donors. All samples were normalized for GADPH expression performed concurrently with IL-12 mRNA amplification. ND, not detected.
FPR activation suppresses mRNA.
Activation of FPR by fMLP or T-20 suppresses the level of mRNA for both IL-12 p35 (A) and IL-12 p40 (B). Human monocytes were stimulated for 6 hours with SAC and IFN-γ in the presence or absence of FPR and FPR-1 ligands (10−5 M). Taqman was used to isolate and quantitate RNA. Suppression of IL-12 mRNA was expressed as percentage maximal induction relative to peak expression with SAC and IFN-γ stimulation because there was no detectable constitutive expression of IL-12 p40 mRNA. Data are mean ± 1 SD from replicate samples from 3 separate donors. All samples were normalized for GADPH expression performed concurrently with IL-12 mRNA amplification. ND, not detected.
To confirm the role and specificity of GI signaling in the suppression of IL-12 production by FPR ligands, before the addition of either fMLP or T-20, monocytes were pretreated with pertussis toxin, which inhibits Gi signaling by adenosine phosphate ribosylation of a cysteine residue of the carboxy-terminus of the Giα subunit, and then were stimulated with SAC and IFN-γ. The addition of pertussis toxin completely reversed the suppression of IL-12 production by both peptides (Figure3). Significantly, pertussis toxin did not have the capacity to induce IL-12 production in the absence of additional stimuli. However, pertussis toxin pretreatment augmented the production of IL-12 in response to SAC and IFN-γ in the absence of fMLP and T-20 and reversed the ability of fMLP and T-20 to inhibit IL-12 production. These findings provide support to prior studies suggesting a critical role of Giα-mediated signaling pathways in the regulation of IL-12 production.44-48
Suppression of IL-12 p70 production by fMLP and T-20 peptide is reversed by pertussis toxin.
Monocytes were cultured for 4 hours with 250 ng/mL pertussis toxin (▩) and then stimulated with SAC and IFN-γ in the presence or absence of fMLP (10−5 M) or T-20 peptide (10−5 M). ■, control. IL-12 p70 concentrations were measured 24 hours after stimulation by ELISA. Data are mean ± 1 SD from the monocytes of 3 separate donors (*P < .05; **P < .01). ND, not detected.
Suppression of IL-12 p70 production by fMLP and T-20 peptide is reversed by pertussis toxin.
Monocytes were cultured for 4 hours with 250 ng/mL pertussis toxin (▩) and then stimulated with SAC and IFN-γ in the presence or absence of fMLP (10−5 M) or T-20 peptide (10−5 M). ■, control. IL-12 p70 concentrations were measured 24 hours after stimulation by ELISA. Data are mean ± 1 SD from the monocytes of 3 separate donors (*P < .05; **P < .01). ND, not detected.
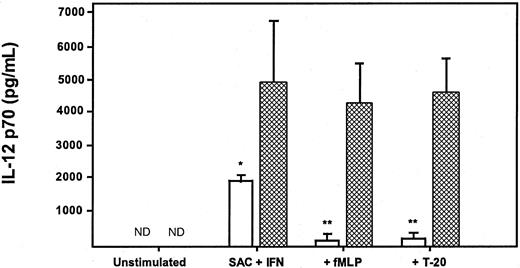
In a final series of experiments, we determined whether the effects seen on human monocytes could be applied to IL-12 p70 production by dendritic cells. Immature dendritic cells were derived from human monocytes cultured for 7 to 9 days in GM-CSF and IL-4.40These dendritic cells produced high levels of IL-12 p70 (Figure4) when stimulated with SAC and IFN-γ or CD40L and IFN-γ. However, the production of IL-12 p70 was not suppressed by preexposure to either fMLP or T-20 peptide, even at high concentrations (10−5 M). Interestingly, the preexposure of dendritic cells to pertussis toxin failed to augment the production of IL-12, as was seen in the elutriated monocytes (data not shown), suggesting that Giα signaling pathways may not play a critical role in the modulation of IL-12 production in dendritic cells.
Neither fMLP, T-20 peptide, nor F-peptide suppresses IL-12 p70 production by monocyte-derived dendritic cells.
Immature dendritic cells (CD1ahigh/CD83low) were generated from human monocytes cultured for 7 to 9 days in GM-CSF and IL-4 and then were stimulated with IFN-γ and either SAC (■) or CD40L (▨) in the presence or absence of fMLP (10−5 M), T-20 (10−5 M), or F-peptide (10−5 M). IL-12 p70 concentrations were measured 24 hours after stimulation by ELISA. Data are mean ± 1 SD from dendritic cells derived from 3 separate donors (all P > .05).
Neither fMLP, T-20 peptide, nor F-peptide suppresses IL-12 p70 production by monocyte-derived dendritic cells.
Immature dendritic cells (CD1ahigh/CD83low) were generated from human monocytes cultured for 7 to 9 days in GM-CSF and IL-4 and then were stimulated with IFN-γ and either SAC (■) or CD40L (▨) in the presence or absence of fMLP (10−5 M), T-20 (10−5 M), or F-peptide (10−5 M). IL-12 p70 concentrations were measured 24 hours after stimulation by ELISA. Data are mean ± 1 SD from dendritic cells derived from 3 separate donors (all P > .05).
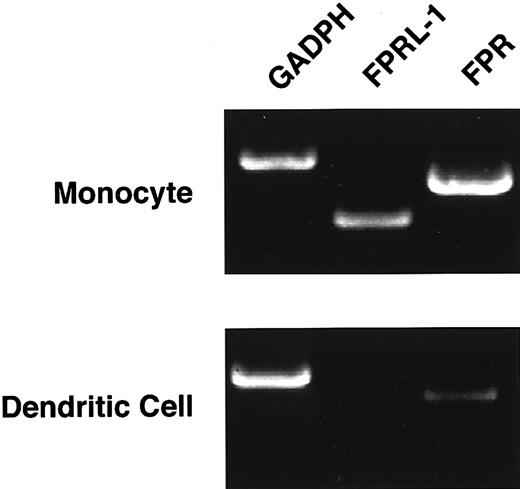
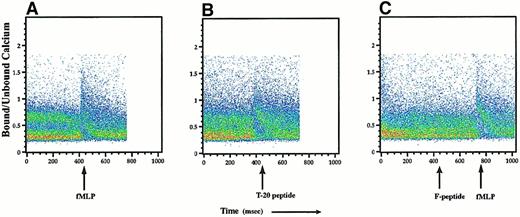
We explored the possibility that this lack of suppression resulted from low or absent expression of FPR and FPRL-1 by dendritic cells. Using semiquantitative reverse transcription (RT)-PCR of RNA isolated from flow cytometry-sorted monocyte-derived dendritic cells, FPR mRNA was modestly reduced in immature dendritic cells when compared to monocytes (Figure 5), whereas these cells expressed little, if any, FPRL-1 mRNA. Finally, we performed flow cytometry-based calcium flux assays on dendritic cells (gated on CD1a+cells) to determine whether the reduced levels of mRNA for FPR and FPRL-1 correlated with the functional expression of these receptors. Exposure to fMLP at concentrations of 10−5 M or 10−8 M readily induced a calcium flux in the CD1a+ cell population, as did exposure to the T-20 peptide (Figure 6A-B). In contrast, treatment with F-peptide, even at high concentrations (10−5 M), failed to induce a measurable calcium flux (Figure 6C). These data suggest that though CD1a+ dendritic cells exhibit functional expression of the high-affinity fMLP receptor, FPR, they lack expression of functional FPRL-1.
Expression of the fMLP receptors is reduced in immature dendritic cells.
Monocyte-derived dendritic cells were FACS sorted to obtain greater than 97% CD1a+ cells. mRNA was isolated, and semiquantitative RT-PCR was performed to determine the level of FPR and FPRL-1 mRNA expression by immature dendritic cells relative to primary monocytes. Data represent results of 3 similar, independent experiments.
Expression of the fMLP receptors is reduced in immature dendritic cells.
Monocyte-derived dendritic cells were FACS sorted to obtain greater than 97% CD1a+ cells. mRNA was isolated, and semiquantitative RT-PCR was performed to determine the level of FPR and FPRL-1 mRNA expression by immature dendritic cells relative to primary monocytes. Data represent results of 3 similar, independent experiments.
Immature dendritic cells have functional expression of FPR but not of FPRL-1.
Dendritic cells were loaded with the calcium fluorochrome Indo-1, and intracellular calcium flux by CD1a+ cells was assayed by FACS analysis. Dendritic cells were stimulated with 10−5 M fMLP, 10−5 M T-20 peptide, or 10−5 M F-peptide followed by 10−8 M fMLP. Calcium flux is expressed as the ratio of bound to free intracellular calcium, and scattergrams represent changes in ratios of bound to free calcium for the gated CD1a+ population. FACS data are representative of 2 independent experiments yielding similar results.
Immature dendritic cells have functional expression of FPR but not of FPRL-1.
Dendritic cells were loaded with the calcium fluorochrome Indo-1, and intracellular calcium flux by CD1a+ cells was assayed by FACS analysis. Dendritic cells were stimulated with 10−5 M fMLP, 10−5 M T-20 peptide, or 10−5 M F-peptide followed by 10−8 M fMLP. Calcium flux is expressed as the ratio of bound to free intracellular calcium, and scattergrams represent changes in ratios of bound to free calcium for the gated CD1a+ population. FACS data are representative of 2 independent experiments yielding similar results.
The reason for the lack of suppression of IL-12 production by immature dendritic cells after exposure to ligands for FPR is unclear. It is possible that the density of FPR expressed by dendritic cells is insufficient to generate the secondary signaling events necessary to suppress IL-12 yet is sufficient to induce a calcium flux. Alternatively, signaling pathways that are independent of those mediating calcium flux may be required for the suppression of IL-12 production, and such pathways are not active in dendritic cells. Given that supraphysiologic concentrations of fMLP failed to suppress IL-12 (up to 10−3 M, data not shown) and that pertussis toxin failed to augment IL-12 production, as occurred with purified monocytes, the latter mechanism is more likely than the former.
Discussion
The finding of selective regulation of IL-12 production in monocytes compared to dendritic cells has been previously reported in respect to infection with HIV and Leishmania as well as with other suppressors of IL-12.39 49-51 Our findings regarding the ability of fMLP to suppress IL-12 p70 production in monocytes, but not dendritic cells, suggests 2 possible roles for this signaling pathway in modulating the local immune microenvironment. The first role is the regulation of IFN-γ production by natural killer (NK) cells, which is dependent on IL-12 production by monocytes/macrophages. Because dendritic cells act to prime T-cell responses in draining lymphatic tissue, the effect of this pathway on T-cell responses may be indirect, possibly through the inhibition of IFN-γ production by NK cells, which subsequently acts to limit the production of IL-12 p70 by dendritic cells. The second role is the ability to regulate secondary T-cell responses because IL-12 production by macrophages is important for cell survival and for the augmentation of IFN-γ production by differentiated Th1 cells.
Our studies provide several novel and potentially important findings. First, they demonstrate that signals through N-formyl-peptide receptors can result in the suppression of early innate immunity by inhibiting the production of IL-12 from monocytes/macrophages, suggesting a novel function for a family of receptors thought only to have “pro-inflammatory” properties (eg, the attraction of neutrophils and monocytes and the induction of cytokines such as TNF-α). Second, the current findings provide a potential direct mechanism by which HIV infection may inhibit immune responses in vivo. In support of this possibility, antibodies to T-20 peptide have been detected in the serum of patients with HIV, indicating that these peptide domains may be exposed to the immune system during HIV infection in vivo.52 How and where this exposure occurs is unclear; gp41 has been identified in the brain tissue of patients with HIV encephalopathy, in macrophages of patients with HIV-associated myopathy, and in circulating immune complexes of patients with HIV.53,54 In addition, the normally cryptic component of gp41 containing T-20 is exposed during the fusion process and thus may directly interact with formyl-peptide receptors on target cells. Third, these findings also suggest that studies involving the treatment of HIV-infected patients with T-20 peptide should address the possibility that such treatment may result in the suppression of IL-12 production, which could have immunologic consequences. This may be particularly important because the concentrations of T-20 required to suppress IL-12 production in vitro are at or below the levels of T-20 required in humans to suppress viral burdens in vivo.32 Together with the current findings, these studies suggest that FPR signaling through Gi-linked signaling pathways may have broader effects on immune responses than previously thought.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Brian L. Kelsall, Immune Cell Interaction Unit, Mucosal Immunity Section, National Institutes of Health, Bldg 10, Rm 11N238, 10 Center Dr, Bethesda, MD 20892-1890; e-mail:kelsall@nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal