Abstract
This paper reports loss of human factor VIII (hFVIII) inhibitory antibody in immunocompetent C57BL/6 mice. High-titer anti-hFVIII antibody developed in the mice within 7 to 14 days of intraportal administration of adeno-associated virus (AAV) carrying FVIII that coincided with a reduction in plasma hFVIII antigen. Bethesda titers (> 100 units) persisted relatively unchanged for 9 to 10 months. Unexpectedly, at 10 months after injection of the virus, hFVIII protein (up to 59 ng/mL) was detected in 3 mice at the same time as disappearance of hFVIII inhibitor. The level of hFVIII was similar to that found in immunodeficient mice receiving the same dose of recombinant AAV carrying hFVIII without hFVIII inhibitor. These results suggest that tolerance to hFVIII can be induced by sustained expression of hFVIII in a mouse model. Further elucidation of this observation may affect use of FVIII gene transfer in the treatment of inhibitor-positive patients with hemophilia A.
Introduction
Gene therapy for hemophilias has become an exciting prospect for long-term curative treatment.1Advances in gene transfer for hemophilia A and B have shown the feasibility of this curative procedure.2,3 However, the immune response against human factor VIII (hFVIII) may be a difficult barrier to successful gene therapy for hemophilia. Inhibitory anti-hFVIII antibodies (inhibitors) develop in up to 30% of patients with hemophilia A receiving infusions of factor VIII (FVIII) concentrates.4-6 The mechanism responsible for development of the anti-FVIII inhibitor remains unclear, although several factors may affect its formation, including the type of FVIII mutation, the FVIII product used, and patients' immune response.4-6Currently, patients with the inhibitor are treated with induction of immune tolerance7 or administration of porcine FVIII,8 activated factor complexes,9immunosuppressive agents,10 or activated factor VIIa.11,12 Successful induction of immune tolerance can be achieved by continuous infusion of a high-dose FVIII concentrate to “desensitize” patients.7 11 Because of the desirability of achieving long-term factor expression through gene transfer, coupled with the potential of inhibitor development, we performed experiments addressing inhibitor formation and tolerance in gene transfer.
We previously reported sustained expression of hFVIII in mice with use of recombinant adeno-associated virus (rAAV). When rAAV vector carrying hFVIII complementary DNA was administered by the intraportal route into immunocompetent C57BL/6 mice, high-titer anti-hFVIII inhibitor was detected.3 These data are consistent with findings of earlier studies in which inhibitor against hFVIII was detected in immunocompetent mice receiving either repeated administration of purified recombinant hFVIII protein or gene transfer of the hFVIII gene.13 14 We here report that the antibody response to hFVIII expressed from rAAV vector diminishes over time.
Study design
Vector constructs, cell cultures, rAAV production and purification, the enzyme-linked immunosorbent assay (ELISA) for hFVIII antigen, and the essays for activated partial thromboplastin time, Coatest FVIII activity, and Bethesda inhibitor for hFVIII were as described previously, as were the animal care and surgical procedures.3
Results and discussion
A total of 1010 to 1011 rAAV/DLZ6 virions expressing functional B-domain–deleted (BDD) hFVIII were injected into the portal vein of 4- to 6-week-old male C57BL/6 mice or nonobese diabetic/severe combined immunodeficiency disease (NOD/SCID) mice. Up to 27.5% (55 ng/mL) of the normal hFVIII antigen level was detected in the plasma of the NOD/SCID mice. However, maximum hFVIII levels in the C57BL/6 mice were only 6 to 10 ng/mL. High-titer hFVIII inhibitor was observed in plasma as early as 1 week after injection in all C57BL/6 mice given rAAV/DLZ6. Anti-hFVIII inhibitor titer increased to the maximum level 9 to 12 weeks after injection.3
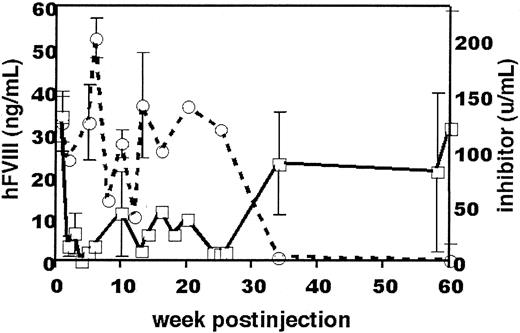
Six C57BL/6 mice were given an injection of rAAV/DLZ6. Three of the 6 mice were killed 9 months after administration of rAAV vectors for performance of histologic and molecular analyses. The 3 surviving mice were tested for hFVIII antigen at bimonthly intervals (it has now been 1.5 years since those mice received the rAAV/DLZ6 injection). At 10 months after injection, the hFVIII antigen level in 2 of the 3 mice increased from a range of 3% to 5% to 29% (58 ng/mL) of the normal level of hFVIII. Coincident with this rise in antigen levels was disappearance of the anti-hFVIII inhibitor in plasma in the 3 mice (Figure 1). These results are consistent with previous observations of development of anti-hFVIII inhibitor in either adult immunocompetent C57BL/6 mice or neonatal FVIII knockout mice (C57BL/6 background) after repeated infusions of hFVIII.13 14 The immature immune status of neonates or the relatively high FVIII levels may have accounted for the lower incidence of inhibitor formation in those investigations.
Level of hFVIII antigen and hFVIII inhibitor titer in C57BL mice (n = 3) after administration of rAAV and BDD-hFVIII.
Solid line indicates hFVIII antigen level; and broken line, anti-BDD-hFVIII inhibitor titer. The ELISA standard curve for hFVIII was obtained by using pooled normal human plasma diluted in either barbital-buffered saline or mouse plasma. Values (± SEM) represent the averages of three animals.
Level of hFVIII antigen and hFVIII inhibitor titer in C57BL mice (n = 3) after administration of rAAV and BDD-hFVIII.
Solid line indicates hFVIII antigen level; and broken line, anti-BDD-hFVIII inhibitor titer. The ELISA standard curve for hFVIII was obtained by using pooled normal human plasma diluted in either barbital-buffered saline or mouse plasma. Values (± SEM) represent the averages of three animals.
Our experimental data are consistent with observations of immune tolerance in hemophilia patients with inhibitor. A wide range of doses of FVIII are used clinically to induce tolerance; administration of either high- or low-dose FVIII regimens is effective.15Recombinant adenovirus, which is well-known to elicit cell-mediated immune response, did not provoke an inhibitor response to hFVIII in C57Bl/6 mice.16 Adenoviral production of supratherapeutic amounts of hFVIII (5- to 10-fold greater than physiologic levels) may facilitate immediate desensitization and immune tolerance that occurs with repeated administration of high-dose FVIII to patients with hemophilia A who have high-titer inhibitor.7 11
In another report,17 retroviral gene transfer and bone marrow transplantation induced immunologic unresponsiveness in 50% of FVIII knockout mice (C57BL/6 background). Although no hFVIII protein or activity was detected, it was postulated that low-level exposure of protein in antigen-presenting cells could induce tolerance. This induction of central tolerance produced by means of hematopoietic chimerism allows tolerization of developing lymphocytes in the bone marrow and thymus. In contrast, our results suggest that peripheral tolerance of mature lymphocytes was induced by constant exposure of hFVIII protein leading to B-cell and T-cell anergy.18 The reemergence of FVIII also confirms that rAAV-mediated transduced hepatocytes are stably maintained and not eliminated by cytotoxic immune effector cells.19 20
Gene therapy holds the promise of radically changing the way in which hemophilia is treated. Investigation of the mechanism responsible for development of anti-hFVIII inhibitor in animal models is important not only for preclinical evaluation of protocols for hemophilia A gene therapy but also because it will contribute to the elucidation of inhibitor formation and its treatment in patients with hemophilia. Our results demonstrate that persistent expression of hFVIII in immunocompetent mice mediates immune tolerance.
Funded by National Institutes of Health grant RO1 DK54419. C.E.W. is a recipient of the Lucille Markey Trust.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Christopher E. Walsh, UNC Gene Therapy Center, Rm 7101, Thurston Bldg, CB 7352, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599; e-mail:cwalsh@med.unc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal