Abstract
Antibody-targeted chemotherapy is a promising therapy in patients with acute myeloid leukemia (AML). In a phase II study of Mylotarg (CMA-676, gemtuzumab ozogamicin), which consists of a CD33 antibody linked to calicheamicin, saturation and internalization by leukemic and normal myeloid cells were analyzed in 122 patients with relapsed AML. Peripheral blood samples were obtained just before and 3 and 6 hours after the start of the first and second Mylotarg treatment cycles. Within 3 to 6 hours after infusion, near complete saturation of CD33 antigenic sites by Mylotarg was reached for AML blasts, monocytes, and granulocytes, whereas Mylotarg did not bind to lymphocytes. Saturation levels prior to the start of the second Mylotarg treatment cycle were significantly increased compared with background levels before the start of the first cycle. This apparently was caused by remaining circulating Mylotarg from the first treatment cycle (∼2 weeks earlier). On binding of Mylotarg to the CD33 antigen, Mylotarg was rapidly internalized, as determined by the decrease in maximal surface membrane Mylotarg binding. Internalization of Mylotarg was also demonstrated in myeloid cells in vitro and was confirmed by confocal laser microscopy. In vitro studies using pulse labeling with Mylotarg showed a continuous renewed membrane expression of CD33 antigens, which can significantly increase the internalization process and thereby the intracellular accumulation of the drug. Finally, Mylotarg induced dose-dependent apoptosis in myeloid cells in vitro. These data indicate that Mylotarg is rapidly and specifically targeted to CD33+ cells, followed by internalization and subsequent induction of cell death.
Introduction
Although 70% to 80% of adult patients with acute myeloid leukemia (AML) can be successfully induced into first remission by chemotherapy, most of these patients ultimately have a relapse within 2 years.1 In addition, chemotherapy is accompanied by substantial side effects because the cytotoxic drugs not only kill the leukemic cells but also dividing cells of other hematopoietic and nonhematopoietic lineages. Therefore, it is clear that new or additional therapy approaches are required. A promising therapy is antibody-targeted chemotherapy, in which the cytotoxic agent is linked to an antibody recognizing the leukemic cells.2 Such an approach allows the specific delivery of the cytotoxic drug to the leukemic cells and is likely to result in less toxic side effects.
In AML, the CD33 antigen is an appropriate target because AML blast cells express the CD33 antigen in more than 90% of patients, whereas hematopoietic stem cells, lymphoid cells, and nonhematopoietic cells do not express the CD33 antigen.3-5 The CD33 antigen, a member of the immunoglobulin superfamily, is a membrane-bound glycoprotein of 67 kd. Although it has been shown that the CD33 antigen is a sialic acid-binding receptor,6 7 its function on myeloid cells remains to be established.
Mylotarg (gemtuzumab ozogamicin, previously known as CMA-676) is an antibody-targeted chemotherapy agent consisting of the humanized murine CD33 antibody (clone P67.6) to which the calicheamicin γ1derivative is attached via a hydrolyzable bifunctional linker.8 It is assumed that binding of Mylotarg to the CD33 antigen results in internalization followed by the release of the potent antitumor antibiotic calicheamicin. Calicheamicin is a member of the enediyne family of molecules and binds to the minor groove of DNA, causing double-strand breaks and apoptosis.9 10 However, there is no direct proof thus far that the antitumor effect of Mylotarg therapy is indeed mediated by this mechanism.
Recently, Mylotarg was tested for the treatment of relapsed or refractory AML in a phase I dose-escalation study.11 In 8 of 40 patients, complete disappearance of leukemic cells was observed, and Mylotarg was reasonably well tolerated, even at the highest dose of 9 mg/m2. In the phase II studies on Mylotarg, we analyzed the saturation of CD33 antigenic sites by Mylotarg on CD33+ AML blast cells as well as on normal leukocytes. In addition, we investigated whether and to what extent Mylotarg is internalized by CD33+ cells. The processes of saturation and internalization are both crucial for determining the amount of calicheamicin delivered into the leukemic cells, and thus probably also for the killing of these cells.
In this report, we present data on the in vivo and in vitro binding of Mylotarg to leukemic and normal CD33+ myeloid cells. In addition, we provide evidence that on binding of Mylotarg to the CD33 antigen, the complex is rapidly internalized and that this internalization process continues because of renewed expression of CD33 antigens on the cellular surface. Finally, we show that internalization of Mylotarg results in the dose-dependent induction of apoptosis.
Patients, materials, and methods
Patients
European patients (n = 122) with AML in first relapse, enrolled in the European phase II Mylotarg protocols 0903B1-202-EU (n = 76) and 0903B1-203-EU (n = 46) between August 1998 and March 2000, were included in this study. Patients included in the 202-EU study were more than 18 years old and had a first remission duration of at least 6 months. Patients included in the 203-EU study were more than 60 years old and had a first remission duration of at least 3 months. All patients were required to have CD33+ AML as determined by immunophenotyping (see below) of a bone marrow (BM) aspirate obtained within 1 week before the start of the Mylotarg therapy. The AML was considered to be CD33+ if at least 80% of the myeloid blast cells expressed the CD33 antigen in sufficient amounts; that is, the fluorescence intensity of the CD33 labeling was at least 4 times as high as the fluorescence intensity of an isotype-matched control antibody. Informed consent was obtained from all patients in accordance with the institutional review boards of the participating clinical sites.
Protocol
In these open, single-arm multicenter phase II studies, Mylotarg (Wyeth-Ayerst Laboratories, St Davids, PA) was administered as a single 2-hour intravenous infusion at a dose of 9 mg/m2. In general, each patient received 2 Mylotarg doses with at least 14 days between the doses. A delay of the second dose beyond 14 days was allowed for acute treatment of infections and other medical problems. Before the start of each Mylotarg treatment cycle and 3 and 6 hours after the start of each Mylotarg treatment cycle, peripheral blood (PB) samples were obtained, immediately placed on ice (4°C) and shipped overnight at 4°C to Immunology Rotterdam by express courier (World Courier, Hoofddorp, The Netherlands). Before the start of the first and, in a limited number of patients, before the start of the second Mylotarg treatment cycle, serum samples were obtained and shipped overnight on dry ice to Immunology Rotterdam by express courier.
Immunophenotyping
The BM samples of all 122 AML patients were immunophenotyped as described previously.11 12 Briefly, 100 μL whole BM was incubated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), and peridin chlorophyll protein (PerCP)-conjugated monoclonal antibodies (BD Biosciences, San Jose, CA) for 20 minutes at room temperature. After lysing red blood cells (RBCs) by ammonium chloride (10 minutes at room temperature) and washing the cells with phosphate-buffered saline pH 7.8 (PBS)/0.2% bovine serum albumin (BSA), at least 10 000 events were acquired on a FACScan flow cytometer (BD Biosciences). Data were analyzed by using CellQuest software (BD Biosciences).
Cell lines, mononuclear cells, and monocytes
The myeloid cell lines HL-6013 and MEG-0114 were cultured in RPMI (Bio-Whittaker, Verviers, Belgium) supplemented with 10% fetal calf serum (FCS; Bio-Whittaker), penicillin G sodium (100 U/mL; Gist-Brocades, Delft, The Netherlands) and streptomycin sulfate (0.1 mg/mL; Biochrom, Berlin, Germany). Mononuclear cells (MNCs) were obtained by Ficoll-Paque (Pharmacia, Uppsala, Sweden) density centrifugation of PB. Monocytes were obtained after Ficoll-Paque and Percoll (Pharmacia) density centrifugation of buffy coats as described previously.15
In vitro incubation of cells with Mylotarg
For continuous-labeling experiments, whole PB or BM (100 μL) was incubated with 5 μg/mL Mylotarg (unless indicated otherwise; doses and concentrations of Mylotarg are reported with respect to its protein portion, which comprises 97% of the molecule) at 37°C for different periods of time, after which RBCs were lysed by ammonium chloride (10 minutes, 4°C). After washing the leukocytes with PBS/2% FCS, the cell suspension was used for analysis of saturation and modulation (see below). MNCs, monocytes, and cell lines were suspended in RPMI/10% FCS at a concentration of 2 × 106 cells/mL and incubated with 5 μg/mL Mylotarg (unless indicated otherwise) at 37°C for different periods of time. After washing the cells with PBS/2% FCS, the cell suspension was used for analysis of saturation and modulation.
For pulse-labeling experiments, cells were incubated with Mylotarg (5 μg/mL) at 37°C for 15 minutes, after which nonbound Mylotarg was removed by washing the cells with RPMI. The cell pellet was suspended in RPMI/10% FCS (2 × 106 cells/mL) and incubated at 37°C for different periods of time, after which the cells were used for analysis of saturation and modulation.
To analyze whether Mylotarg present in patient serum could still bind to myeloid cells, monocytes (0.25 × 106) were incubated with 500 μL serum obtained from patients just before the start of the first or second Mylotarg treatment cycle. Serum obtained from healthy controls was used as a negative control, whereas serum obtained just after start of the Mylotarg treatment was used as a positive control. After incubation at 4°C for 1 hour, the monocytes were washed twice with PBS/2% FCS, after which they were used for analysis of saturation.
Saturation and modulation analysis
After in vivo or in vitro exposure to Mylotarg or in vitro incubation with patient serum, cell suspensions (RBC-lysed whole PB, RBC-lysed whole BM, MNCs, monocytes, or cell lines) were diluted in PBS/2% FCS to 2 × 106 cells/mL. To detect Mylotarg bound to the cells, cells were incubated with biotin-conjugated mouse antihuman IgG4 antibodies (Caltag Laboratories, Burlingame, CA), followed by streptavidin-FITC (Biosource, Nivelles, Belgium) as a second-step reagent. To detect maximal Mylotarg binding, cells were first incubated with excess Mylotarg (final concentration: 10 μg/mL), followed by successive incubation with biotin-conjugated mouse antihuman IgG4 antibodies and streptavidin-FITC. As a negative control, cells were incubated with streptavidin-FITC alone. To detect CD33 antigenic sites not occupied by Mylotarg, cells were incubated with CD33-PE (clone P67.6; BD Biosciences) or, as a negative control, IgG1-PE (BD Biosciences). To improve separate analysis of different leukocyte subsets, CD45-PerCP (clone 2D1; BD Biosciences) was added to each patient sample from April 1999 onward. All incubations were done for 20 minutes at 4°C (to prevent internalization during immunostaining) and were followed by washing the cells twice with PBS/2% FCS. Data on at least 10 000 events were acquired on a FACScan flow cytometer and analyzed by using CellQuest software.
Saturation was defined as (specific fluorescence intensity of bound Mylotarg)/(specific fluorescence intensity maximal Mylotarg binding) × 100%. Modulation was defined as 1−(specific fluorescence intensity maximal Mylotarg binding at time pointx)/(specific fluorescence intensity maximal Mylotarg binding at time point 0) × 100%, in which time point 0 is the time point just before infusion of Mylotarg in patients or just before addition of Mylotarg to cell or blood suspensions. Analysis was performed on each individual leukocyte population, as defined by scatter characteristics or CD45 expression levels or both.12 16 For analysis of saturation and modulation by purified monocytes, cells were gated based on staining with CD14-PE or CD14-FITC.
The percentage of AML blast cells present in PB samples obtained just before the start of the first and second Mylotarg treatment cycle was determined by flow cytometric analysis.12 16 If less than 5% blast cells were present in the PB sample, generally no reliable saturation data could be obtained.
Confocal laser microscopy
The myeloid cell line MEG-01 (0.4 × 106 cells) was incubated with the FITC-conjugated CD33 antibody P67.6 (BD Biosciences). After incubation at 37°C for various periods of time, the cells were washed twice with PBS/BSA and suspended in PBS/BSA. The cells were then incubated (4°C, 20 minutes) with CD13 (BD Biosciences), washed twice, and incubated (4°C, 20 minutes) with tetramethyl rhodamine-conjugated goat antimouse antibodies (GαM-TRITC; Central Laboratory of the Blood Transfusion Service, Amsterdam, The Netherlands). After washing the cells twice, suspension preparations were made in Vectashield (Vector Laboratories, Burlingame, CA). The suspension preparations were analyzed on a Zeiss LSM 410 laser-scanning confocal microscope (Zeiss, Oberkochen, Germany).17 The 488-nm line of an argon laser and the 543-nm line of a helium-neon laser were used for the excitation of the FITC and TRITC labels, respectively. Emission spectra of both labels were separated with a 560-nm beam splitter and passed through a 510- to 540-nm band pass filter (FITC) or a more than 570 nm long pass filter (TRITC). Images were taken at optimal lateral resolution (pixel size 104 nm). Cross sections (perpendicular to the cover slip) were imaged with an axial (ζ) pixel size of 104 nm.
Detection of apoptosis
Apoptosis was analyzed by labeling the cells with annexin V-FITC and propidium iodide (PI)18 using the IQP apoptosis kit (Immunoquality Products, Groningen, The Netherlands) according to the manufacturer's instructions. Annexin V stains phosphatidylserine residues that normally are present in the inner cell membrane only, whereas apoptotic cells express them on the outer cell membrane as well. Therefore, the combination of annexin V and DNA staining by PI allows the discrimination between nonpermeable apoptotic cells (annexin V+, PI−) and permeable late-apoptotic/necrotic cells (annexin V+, PI+).
Statistical analysis
Data were analyzed as of March 31, 2000. Differences in maximal Mylotarg binding, saturation, and modulation between different leukocyte subsets were determined by using the unpaired Studentt test, whereas differences in maximal Mylotarg binding, saturation, and internalization between the first and second Mylotarg treatment cycle were determined by the paired Student t test (unless indicated otherwise). The Mann-Whitney U test was used to determine differences in the in vitro saturation and modulation processes. In all tests, a P value of less than .05 was considered significant.
Results
Maximal Mylotarg binding to leukocytes
We first investigated the maximal binding of Mylotarg to different leukocyte subsets. Blood samples from patients were obtained just before the start of the Mylotarg infusion (t = 0) and maximal Mylotarg binding was analyzed by incubating the cells with an excess of Mylotarg in vitro. Mylotarg bound to myeloid blast cells (range: 16.6-86.8 arbitrary units) and monocytes (range: 26.0-113.0), whereas the binding to granulocytes was significantly less (range: 8.3-41.9; Figure 1A). Barely detectable amounts of Mylotarg bound to lymphocytes. The maximal Mylotarg binding to PB blast cells was related to the level of CD33 expression by BM blast cell as was analyzed for inclusion of the AML patients into the Mylotarg protocol (r = 0.64; P < .05).
Maximal Mylotarg binding to leukocyte subsets.
Maximal Mylotarg binding to different leukocyte subsets was analyzed prior to start of the first (hatched bars) and second (open bars) Mylotarg treatment cycle by incubating PB with an excess of Mylotarg in vitro, followed by detection with biotin-conjugated antihuman IgG4 and streptavidin-FITC. (A) Maximal Mylotarg binding to AML blast cells (cycle 1: n = 86; cycle 2: n = 35), monocytes (n = 33; n = 45), granulocytes (n = 55; n = 32), and lymphocytes (n = 61; n = 43). Patient numbers differ between leukocyte subsets and between cycle 1 and cycle 2 because not all patients received a second treatment cycle, in some patients the PB sample was not available or of too low quality, or too few events were available for a reliable analysis of all leukocyte subsets. (B) Maximal Mylotarg binding to AML blast cells from patients showing less than 5% blast cells in their PB just prior to the start of the second treatment cycle (blast cell reducers; n = 27) or 5% or more blasts in PB (blast cell nonreducers; n = 31 and n = 26 for cycle 1 and cycle 2, respectively). Maximal Mylotarg binding data for cycle 2 could not be obtained in the blast cell reducers, because the number of blast cells was too low to perform a reliable analysis. ND indicates no data. Data are expressed as mean ± SD. Significant differences (P < .05; indicated by the asterisks [unpairedt test]) were observed in maximal Mylotarg binding to blast cells between cycle 1 and cycle 2 and between cycle 1 data of the blast cell reducers and blast cell nonreducers.
Maximal Mylotarg binding to leukocyte subsets.
Maximal Mylotarg binding to different leukocyte subsets was analyzed prior to start of the first (hatched bars) and second (open bars) Mylotarg treatment cycle by incubating PB with an excess of Mylotarg in vitro, followed by detection with biotin-conjugated antihuman IgG4 and streptavidin-FITC. (A) Maximal Mylotarg binding to AML blast cells (cycle 1: n = 86; cycle 2: n = 35), monocytes (n = 33; n = 45), granulocytes (n = 55; n = 32), and lymphocytes (n = 61; n = 43). Patient numbers differ between leukocyte subsets and between cycle 1 and cycle 2 because not all patients received a second treatment cycle, in some patients the PB sample was not available or of too low quality, or too few events were available for a reliable analysis of all leukocyte subsets. (B) Maximal Mylotarg binding to AML blast cells from patients showing less than 5% blast cells in their PB just prior to the start of the second treatment cycle (blast cell reducers; n = 27) or 5% or more blasts in PB (blast cell nonreducers; n = 31 and n = 26 for cycle 1 and cycle 2, respectively). Maximal Mylotarg binding data for cycle 2 could not be obtained in the blast cell reducers, because the number of blast cells was too low to perform a reliable analysis. ND indicates no data. Data are expressed as mean ± SD. Significant differences (P < .05; indicated by the asterisks [unpairedt test]) were observed in maximal Mylotarg binding to blast cells between cycle 1 and cycle 2 and between cycle 1 data of the blast cell reducers and blast cell nonreducers.
For monocytes and granulocytes, the maximal Mylotarg binding before the start of the second Mylotarg treatment cycle was similar to the maximal Mylotarg binding before the start of the first Mylotarg treatment cycle (Figure 1A). In contrast, the mean maximal Mylotarg binding to myeloid blast cells before the start of the second Mylotarg treatment cycle was significantly reduced as compared to the mean maximal Mylotarg binding before the start of the first cycle.
Because reliable Mylotarg binding data could, in general, not be obtained if the percentage of blast cells in the PB was less than 5%, the mean maximal Mylotarg binding at cycle 2 was obtained from only those patients with still more than 5% blasts in their PB. We therefore hypothesized that the observed decrease in mean maximal Mylotarg binding at the start of the second Mylotarg treatment cycle was related to disappearance of PB blast cells (to < 5%) during the first Mylotarg treatment cycle in those patients with blast cells expressing high levels of CD33 antigenic sites (ie, high maximal Mylotarg binding). We defined these patients as “blast cell reducers,” whereas patients with 5% or more blast cells in their PB at the start of the second Mylotarg treatment cycle were defined as “blast cell nonreducers.” Indeed, before the first Mylotarg treatment cycle, blast cells from the blast cell reducers bound significantly more Mylotarg than blast cells from the blast cell nonreducers (Figure 1B). Thus, patients with high maximal Mylotarg binding on their blast cells appeared to show a more profound disappearance of blast cells from their PB. As a consequence, the mean maximal Mylotarg binding at cycle 1, obtained from all patients, was higher than the mean maximal Mylotarg binding at cycle 2, which could only be obtained from blast cell nonreducers (Figure 1A). In the latter group, the mean maximal Mylotarg binding before the start of the first and second Mylotarg treatment cycle was similar (Figure 1B).
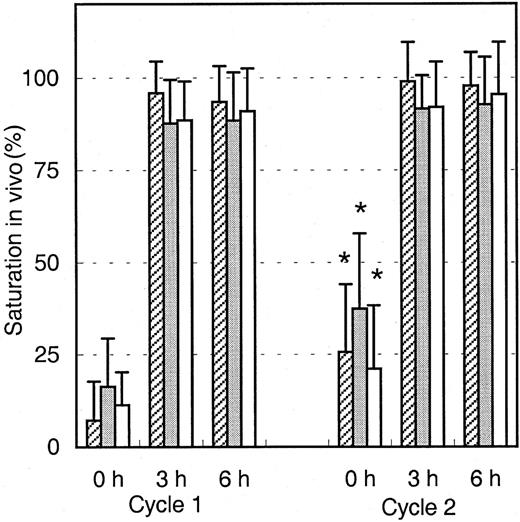
Saturation of Mylotarg in vivo
To analyze the binding of infused Mylotarg to leukemic and normal myeloid cells, PB samples were obtained just before infusion of Mylotarg and 3 and 6 hours after the start of each Mylotarg treatment cycle. Before the start of the first Mylotarg treatment cycle, only background saturation levels were observed (Figure2). However, 3 and 6 hours after start of the first Mylotarg infusion, near-complete saturation of CD33 antigenic sites on the 3 investigated types of CD33+ myeloid cells was observed (Figure 2). Before the start of the second Mylotarg treatment cycle, significantly increased saturation levels were observed on blast cells, monocytes, and granulocytes as compared to the background saturation levels before the start of the first Mylotarg treatment cycle. Three and 6 hours after the start of the second Mylotarg treatment cycle, near-complete saturation of CD33 antigenic sites was observed again. Overall, these data indicate that the Mylotarg dosage used (9 mg/m2) is sufficient to saturate CD33 antigenic sites on leukemic and normal CD33+ cells in PB.
Saturation of Mylotarg to myeloid cells in vivo.
Saturation of Mylotarg just before and 3 and 6 hours after the start of each Mylotarg treatment cycle was analyzed by comparing Mylotarg bound in vivo with the maximal Mylotarg binding. The different myeloid cells were determined by their scatter and CD45-binding characteristics: AML blast cells (hatched bars; cycle 1: n = 86; cycle 2: n = 35), monocytes (gray bars; n = 33; n = 45), and granulocytes (white bars; n = 55; n = 32). Significant differences (P < .05; indicated by the asterisks) were observed between cycle 1 and cycle 2 at the 0-hour time point.
Saturation of Mylotarg to myeloid cells in vivo.
Saturation of Mylotarg just before and 3 and 6 hours after the start of each Mylotarg treatment cycle was analyzed by comparing Mylotarg bound in vivo with the maximal Mylotarg binding. The different myeloid cells were determined by their scatter and CD45-binding characteristics: AML blast cells (hatched bars; cycle 1: n = 86; cycle 2: n = 35), monocytes (gray bars; n = 33; n = 45), and granulocytes (white bars; n = 55; n = 32). Significant differences (P < .05; indicated by the asterisks) were observed between cycle 1 and cycle 2 at the 0-hour time point.
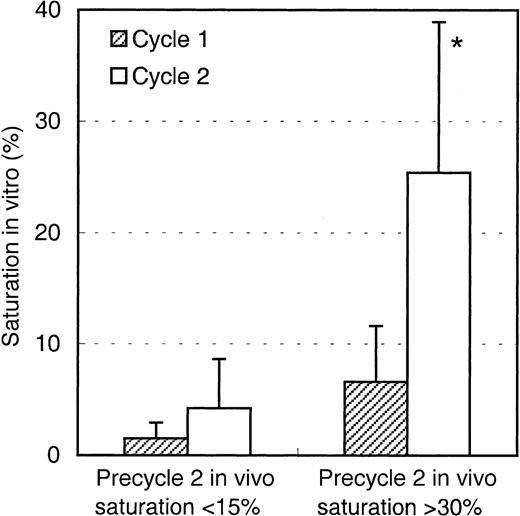
The saturation levels detected before the start of the second Mylotarg treatment cycle suggest that Mylotarg remaining from the first Mylotarg treatment cycle is still circulating and able to bind to the CD33-expressing cells. To investigate this hypothesis, monocytes obtained from healthy controls were incubated with serum obtained from patients just before start of the first or second Mylotarg treatment cycle, after which saturation of CD33 antigenic sites was analyzed. As shown in Figure 3, serum obtained prior to cycle 1 did not result in significant saturation levels. In contrast, incubation with serum obtained just prior to the second Mylotarg treatment cycle resulted in significantly increased saturation levels. These levels of in vitro saturation were related to the saturation observed in the in vivo PB samples obtained before the second Mylotarg treatment cycle; that is, serum from patients with high in vivo saturation levels (> 30%) before the start of the second Mylotarg treatment cycle also showed a high in vitro saturation, whereas patients with low in vivo saturation levels (< 15%) before the start of the second Mylotarg treatment cycle also showed a low in vitro saturation (Figure 3). Incubation of monocytes with serum obtained just after the start of Mylotarg treatment resulted in complete saturation, whereas incubation with serum obtained from healthy volunteers resulted in background saturation levels (< 10%). These results indicate that the increased saturation observed before the second Mylotarg treatment cycle is indeed caused by circulating Mylotarg remaining from the first treatment cycle, generally given 2 weeks earlier.
Saturation of Mylotarg present in patient serum to monocytes in vitro.
Purified monocytes from healthy controls were incubated with patient serum obtained just before the start of the first (hatched bars) or second (open bars) Mylotarg treatment cycle, after which saturation was analyzed by comparing Mylotarg bound in vitro with the maximal Mylotarg binding. Patients showing more than 30% saturation in vivo at the start of the second treatment cycle (n = 7) were compared with patients showing less than 15% saturation in vivo at the start of the second treatment cycle (n = 3). Data are expressed as mean ± SD. A significant difference (P < .05; indicated by the asterisk) was observed between in vitro saturation with precycle 1 serum and in vitro saturation with precycle 2 serum in the patients with more than 30% saturation in vivo. Saturation levels less than 10% should be regarded as baseline levels.
Saturation of Mylotarg present in patient serum to monocytes in vitro.
Purified monocytes from healthy controls were incubated with patient serum obtained just before the start of the first (hatched bars) or second (open bars) Mylotarg treatment cycle, after which saturation was analyzed by comparing Mylotarg bound in vitro with the maximal Mylotarg binding. Patients showing more than 30% saturation in vivo at the start of the second treatment cycle (n = 7) were compared with patients showing less than 15% saturation in vivo at the start of the second treatment cycle (n = 3). Data are expressed as mean ± SD. A significant difference (P < .05; indicated by the asterisk) was observed between in vitro saturation with precycle 1 serum and in vitro saturation with precycle 2 serum in the patients with more than 30% saturation in vivo. Saturation levels less than 10% should be regarded as baseline levels.
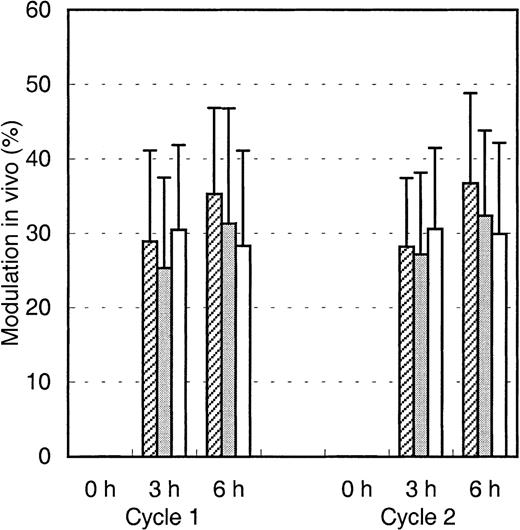
Modulation of Mylotarg in vivo
We then hypothesized that Mylotarg, after binding to the myeloid cells, is internalized into the cells and thus would cause a decrease in CD33 expression and in maximal Mylotarg binding. We therefore evaluated this modulation, which we defined as the decrease in maximal Mylotarg binding over time, relative to the maximal Mylotarg binding just prior to exposure of the cells to Mylotarg. Three hours after starting the first Mylotarg treatment cycle, rapid modulation of Mylotarg (25%-30%) was observed (Figure4). Six hours after the start of the Mylotarg infusion, modulation by myeloid blast cells and monocytes showed a limited (but statistically significant) increase (to 30%-40%), whereas no further modulation by granulocytes was observed (Figure 4). Also after the second Mylotarg treatment cycle, rapid modulation was observed (Figure 4). The levels and kinetics of modulation were comparable between both Mylotarg treatment cycles. These data support the assumption that after binding of Mylotarg to the CD33 antigen present on leukemic and normal myeloid cells, the antibody-antigen complex is down-modulated and thus may become internalized.
Modulation of Mylotarg by myeloid cells in vivo.
Modulation of Mylotarg was analyzed 3 and 6 hours after the start of each Mylotarg treatment cycle by comparing the maximal Mylotarg binding at each time point with the maximal Mylotarg binding just before the start of the corresponding treatment cycle. The different myeloid cells were analyzed separately, based on their scatter or CD45-binding characteristics: AML blast cells (hatched bars; cycle 1: n = 86; cycle 2: n = 34), monocytes (gray bars; n = 33; n = 45), and granulocytes (white bars; n = 54; n = 32).
Modulation of Mylotarg by myeloid cells in vivo.
Modulation of Mylotarg was analyzed 3 and 6 hours after the start of each Mylotarg treatment cycle by comparing the maximal Mylotarg binding at each time point with the maximal Mylotarg binding just before the start of the corresponding treatment cycle. The different myeloid cells were analyzed separately, based on their scatter or CD45-binding characteristics: AML blast cells (hatched bars; cycle 1: n = 86; cycle 2: n = 34), monocytes (gray bars; n = 33; n = 45), and granulocytes (white bars; n = 54; n = 32).
Internalization of Mylotarg in vitro
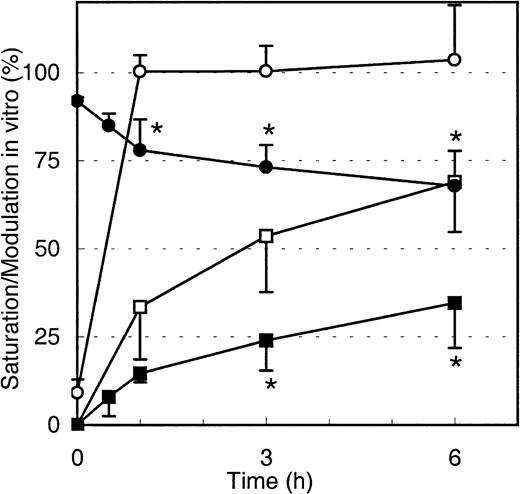
To test if the decrease in maximal Mylotarg binding over time is due to internalization of Mylotarg and not the result of other biologic mechanisms (eg, shedding of antigens or trapping of cells with high Mylotarg binding in the spleen), we analyzed down-modulation in vitro. Purified monocytes, whole blood (both from healthy controls), whole BM from an AML patient, and the myeloid cell lines MEG-01 and HL-60 were incubated with Mylotarg. The results showed a rapid saturation of CD33 antigenic sites. In addition, we observed a clear decrease in the maximal Mylotarg binding over time, implying that down-modulation occurred. Representative results for monocytes are shown in Figure5. No decrease in maximal Mylotarg binding over time was observed when monocytes were incubated at 4°C (data not shown).
Modulation of Mylotarg by monocytes in vitro.
Monocytes were continuously incubated (open symbols; n = 5) or pulse labeled for 15 minutes (black symbols; n = 4) with Mylotarg in vitro. Saturation (circles) and modulation (squares) were analyzed at different time points during the continuous incubation (from start onward) or from the end of the 15-minute pulse labeling onward. Data are expressed as mean ± SD. Significant differences (P < .05; indicated by the asterisks) were observed for modulation or saturation between continuously incubated and pulse-labeled monocytes.
Modulation of Mylotarg by monocytes in vitro.
Monocytes were continuously incubated (open symbols; n = 5) or pulse labeled for 15 minutes (black symbols; n = 4) with Mylotarg in vitro. Saturation (circles) and modulation (squares) were analyzed at different time points during the continuous incubation (from start onward) or from the end of the 15-minute pulse labeling onward. Data are expressed as mean ± SD. Significant differences (P < .05; indicated by the asterisks) were observed for modulation or saturation between continuously incubated and pulse-labeled monocytes.
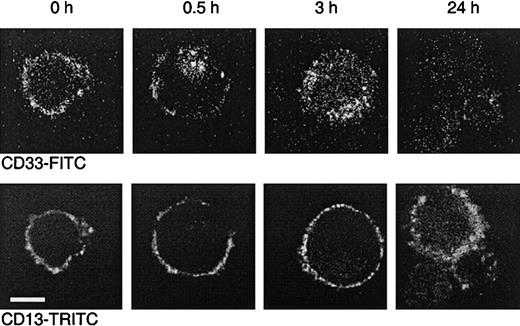
To further demonstrate that internalization occurs, MEG-01 cells were incubated with the FITC-labeled CD33 antibody P67.6. This antibody is the murine version of the humanized antibody used in Mylotarg and behaves comparably in in vitro saturation/modulation analysis. MEG-01 cells were chosen because they show similar saturation and modulation behavior as monocytes, but are larger, thereby making them more suitable for analysis by confocal laser microscopy. A CD13 antibody was used as a positive control for the visualization of the cell membrane. As shown in Figure 6, incubation of MEG-01 cells with P67.6-FITC resulted in a clear membrane-bound staining pattern. However, after incubation at 37°C, a clear shift in the distribution of P67.6 occurred; after 0.5 hours the antibody was detected in the majority of the cells both on the cell membrane and in intracellular locations, whereas after 3 hours the majority of the antibody had become internalized. After incubation for 24 hours at 37°C, only low levels of intracellular antibody were detected, which may be due to degradation or to quenching of the fluorochrome by the low pH in lysosomes that are assumed to fuse with the endosomes containing internalized antibody. These in vitro experiments demonstrate that Mylotarg can indeed efficiently be internalized by cells expressing CD33.
Dual color laser microscopic images showing the internalization of the CD33 antibody P67.6.
The CD33+ myeloid cell line MEG-01 was incubated at 37°C with P67.6-FITC for different periods as indicated, followed by labeling with CD13 and GαM-TRITC. The upper panel shows the P67.6-FITC staining (green channel), and the bottom panel shows the red channel of exactly the same image displaying the membrane staining with CD13/GαM-TRITC (bar = 10 μm).
Dual color laser microscopic images showing the internalization of the CD33 antibody P67.6.
The CD33+ myeloid cell line MEG-01 was incubated at 37°C with P67.6-FITC for different periods as indicated, followed by labeling with CD13 and GαM-TRITC. The upper panel shows the P67.6-FITC staining (green channel), and the bottom panel shows the red channel of exactly the same image displaying the membrane staining with CD13/GαM-TRITC (bar = 10 μm).
Continuous renewed expression of CD33 antigenic sites promotes internalization of Mylotarg
Binding of Mylotarg to CD33 antigenic sites on leukemic and normal myeloid cells and subsequent internalization of the antibody-antigen complex are dynamic processes. We hypothesized that after internalization of CD33 antigenic sites, new CD33 antigenic sites might be expressed on the cell membrane, which may again bind Mylotarg and subsequently be internalized. To test this hypothesis, monocytes, AML blast cells, and myeloid cell lines (HL-60 and MEG-01) were pulse labeled with Mylotarg. After 15 minutes of labeling, resulting in a virtually complete saturation of CD33 antigenic sites, nonbound Mylotarg was washed away and the cells were incubated at 37°C for several periods of time. As shown in Figure 5 for monocytes, saturation levels gradually decreased, indicating the appearance of empty CD33 antigenic sites. Furthermore, modulation observed after pulse labeling was significantly decreased compared to modulation observed after continuous exposure of monocytes to Mylotarg. Comparable data were found for the other myeloid cells analyzed. These data suggest that new CD33 antigenic sites are expressed on the cell membrane after binding and subsequent internalization of Mylotarg and that this renewed expression of CD33 antigenic sites contributes significantly to the amount of internalized Mylotarg.
Mylotarg induces apoptosis in myeloid cells in vitro
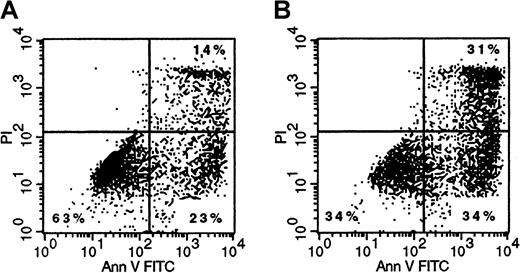
Finally, we analyzed whether binding and subsequent internalization of Mylotarg resulted in apoptosis of the myeloid cells. Three and 6 hours after the start of each Mylotarg treatment cycle, we did not detect apoptotic cells in PB samples of AML patients. In addition, Mylotarg treatment did not appear to activate myeloid cells, as assessed by the stable expression of several markers that are related to activation of myeloid cells (CD16, CD32, CD64, CD66, HLA-DR). In accordance with these in vivo data, we also did not observe increased numbers of apoptotic cells in monocytes and the myeloid cell line HL-60 after culturing these cells with Mylotarg for 24 or 48 hours. However, a dose-dependent increase in the percentage of early apoptotic (annexin V+/PI−) and late apoptotic/necrotic (annexin V+/PI+) cells was observed after culturing monocytes (Figure7), AML blast cells (data not shown), or HL-60 cells (Table 1) with Mylotarg for 72 and 96 hours. In contrast, no increased apoptosis was detected in lymphocytes cultured in the presence of Mylotarg for up to 96 hours. In vivo samples were not available from comparable time points. These data indicate that Mylotarg is able to induce cell death, in part by apoptosis, selectively in CD33-expressing cells.
Mylotarg induces apoptosis in monocytes.
Monocytes were incubated with (1 μg/mL; right plot) or without (left plot) Mylotarg for 72 hours at 37°C, after which cells were labeled with annexin V-FITC and PI, and analyzed on a flow cytometer. The annexin V−/PI− cells are nonapoptotic cells, the annexin V+/PI− cells are early apoptotic cells, and the annexin V+/PI+ cells are late apoptotic/necrotic cells. One representative experiment of 3 is shown.
Mylotarg induces apoptosis in monocytes.
Monocytes were incubated with (1 μg/mL; right plot) or without (left plot) Mylotarg for 72 hours at 37°C, after which cells were labeled with annexin V-FITC and PI, and analyzed on a flow cytometer. The annexin V−/PI− cells are nonapoptotic cells, the annexin V+/PI− cells are early apoptotic cells, and the annexin V+/PI+ cells are late apoptotic/necrotic cells. One representative experiment of 3 is shown.
Mylotarg induces apoptosis in HL-60 cells in a dose-dependent manner*
| . | Concentration Mylotarg . | |||||
|---|---|---|---|---|---|---|
| 0 pg/ mL . | 1 pg/ mL . | 10 pg/ mL . | 100 pg/ mL . | 1 μg/ mL . | 10 μg/ mL . | |
| Annexin V+/PI−(%) | ||||||
| At 72 h | 12.8 | 11.4 | 12.8 | 17.1 | 19.0 | 21.6 |
| At 96 h | 7.5 | 10.8 | 13.8 | 17.2 | 17.2 | 21.0 |
| Annexin V+/PI+(%) | ||||||
| At 72 h | 18.9 | 23.1 | 27.5 | 38.4 | 39.8 | 38.7 |
| At 96 h | 20.6 | 22.9 | 31.3 | 44.1 | 51.2 | 50.9 |
| Total annexin V+(%) | ||||||
| At 72 h | 31.7 | 34.5 | 40.3 | 55.5 | 58.8 | 60.3 |
| At 96 h | 28.1 | 33.6 | 45.1 | 61.3 | 68.4 | 71.9 |
| . | Concentration Mylotarg . | |||||
|---|---|---|---|---|---|---|
| 0 pg/ mL . | 1 pg/ mL . | 10 pg/ mL . | 100 pg/ mL . | 1 μg/ mL . | 10 μg/ mL . | |
| Annexin V+/PI−(%) | ||||||
| At 72 h | 12.8 | 11.4 | 12.8 | 17.1 | 19.0 | 21.6 |
| At 96 h | 7.5 | 10.8 | 13.8 | 17.2 | 17.2 | 21.0 |
| Annexin V+/PI+(%) | ||||||
| At 72 h | 18.9 | 23.1 | 27.5 | 38.4 | 39.8 | 38.7 |
| At 96 h | 20.6 | 22.9 | 31.3 | 44.1 | 51.2 | 50.9 |
| Total annexin V+(%) | ||||||
| At 72 h | 31.7 | 34.5 | 40.3 | 55.5 | 58.8 | 60.3 |
| At 96 h | 28.1 | 33.6 | 45.1 | 61.3 | 68.4 | 71.9 |
Data from one representative experiment of 3 are shown.
Discussion
This study demonstrates that Mylotarg, given as a 2-hour intravenous infusion, rapidly binds to CD33 antigenic sites expressed on leukemic and normal myeloid cells in blood, but not to lymphocytes. The applied Mylotarg dose of 9 mg/m2 results in near-complete saturation of CD33 antigenic sites within 3 hours after the start of the 2-hour infusion. Furthermore, we show that Mylotarg is rapidly internalized after binding to CD33 antigenic sites, both in vivo and in vitro. Continuous renewed expression of CD33 antigens on the cellular surface of myeloid cells was observed, which significantly increased the amount of internalized Mylotarg. Finally, we demonstrate that Mylotarg binding and internalization results in the induction of cell death in CD33+ myeloid cells.
The mean maximal binding of Mylotarg to myeloid blast cells was comparable to the maximal binding of Mylotarg to monocytes, whereas the maximal binding of Mylotarg to granulocytes was significantly lower and no significant binding of Mylotarg to lymphocytes was observed. These data are in accordance with the known expression levels of the CD33 antigen on these cells.3-5 The 122 relapsed AML patients included in this study all had CD33+ blast cells, although the level of CD33 expression varied (from 5.9 to 210.9 times over background). Interestingly, it appeared that patients with less than 5% blasts cells in their PB just prior to the start of the second Mylotarg treatment cycle showed significantly higher maximal Mylotarg binding levels prior to start of the first Mylotarg treatment cycle as compared with patients with 5% or more blasts in their PB just prior to the start of the second cycle (Figure 1B). These data suggest that high expression levels of CD33, and consequently high maximal Mylotarg binding and large amounts of internalized Mylotarg, are related to a more efficient clearance of myeloid blast cells from the PB. It will be of interest to analyze whether CD33 expression levels are also related to the clearance of myeloid blasts from the BM, but these data are not yet fully available. However, preliminary multivariate analysis shows that CD33 expression levels are not correlated with response or overall survival (unpublished results).
As suggested in the limited number of patients in the phase I study, a near-complete saturation of CD33 antigenic sites was reached with the 9 mg/m2 dose, both at the first and the second Mylotarg treatment cycle.11 In agreement with these results, near-complete saturation of CD33 antigenic sites was observed in a clinical study with the CD33 antibody M195 administered in a comparable dose (3 mg/m2).19 In our study, saturation levels just before start of the second cycle were still relatively high. This appeared to be caused by circulating Mylotarg, apparently remaining from the first treatment cycle. Pharmacokinetic data have shown that the mean half-life of Mylotarg is 72 hours.20This would theoretically mean that Mylotarg will still be present in the blood at the start of the second treatment cycle (in general 14 days after the start of the first cycle) at a concentration comparable with a dose of approximately 0.3 mg/m2. As shown in the Mylotarg phase I study, patients treated with 0.25 mg/m2Mylotarg have 20% to 50% saturation of CD33 antigenic sites on their PB blast cells.11 These percentages are comparable to the saturation levels we have found in the in vivo saturation study before cycle 2 and the in vitro saturation study using serum obtained from patients just before the start of the second Mylotarg treatment cycle (Figures 2 and 3).
After binding of Mylotarg to CD33 antigenic sites in vivo, the complex is rapidly modulated (∼40% at 6 hours). We defined this in vivo modulation as the decrease in maximal Mylotarg binding over time, and thereby did not directly measure intracellular Mylotarg. One may argue that this decrease in maximal Mylotarg binding is not due to internalization, but to other immunobiologic mechanisms. However, several experiments support our assumption that the decrease in maximal Mylotarg binding is due to internalization. First, the number of myeloid blast cells did not significantly change during the first 6 hours after the start of the infusion, suggesting that there is no removal of cells from the leukemic blast cell population (data not shown). Second, modulation was also observed in several myeloid cell types in vitro. Third, no decrease in maximal Mylotarg binding was observed when cells were kept at 4°C (eg, during overnight transportation of the samples from the clinical site to the central laboratory) in contrast to the decrease in maximal Mylotarg binding observed with incubation at 37°C. Finally, in the CD33+ myeloid cell line MEG-01, confocal laser microscopy demonstrated internalization of the CD33 antibody P67.6, which has exactly the same antigen specificity of Mylotarg (Figure 6).
Modulation of CD33 antibodies has also been demonstrated in previous in vitro studies using radiolabeled CD33 antibodies (M195, P67.6).21-23 In agreement with our results, approximately 40% modulation was observed after 3 to 6 hours,21,23 and it was suggested that after endocytosis the antibody is routed to lysosomes, where it is proteolytically degraded.22 In a phase I trial of 131I-labeled M195, modulation was also observed in vivo as determined by comparing the counts per minute before and after washing with acid to remove membrane-bound M195.19,24 However, it should be noted that radionuclides (especially 131I) may be rapidly excreted from the cells after lysosomal catabolism of antibodies.24
Modulation was comparable between myeloid blast cells and monocytes, but granulocytes seemed to behave somewhat differently, because there was no further increase in the level of modulation between 3 and 6 hours after the start of the Mylotarg infusion. This is probably due to the recruitment of granulocytes from the BM into the PB, because the percentage of (mainly immature) granulocytes significantly increased between 3 and 6 hours after the start of the infusion (data not shown). It may be that penetration of Mylotarg into the BM is slightly delayed as compared to the presence of Mylotarg in the PB. As a result, the newly recruited granulocytes may have had a delayed binding of Mylotarg and thereby a delayed modulation.
The results of our pulse-labeling experiments suggest that, after internalization of Mylotarg bound to the CD33 antigen, renewed expression of CD33 antigens on the surface of the cells occurs. Previous studies19 24 as well as our present data show that “empty” CD33 antigens (not bound to Mylotarg) can be detected on leukemic blast cells 3 to 14 days after infusion of a (radionuclide- or drug-conjugated) CD33 antibody. However, these expression data mainly reflect the disappearance of the CD33 antibody from the circulation and thus the decrease in saturation levels, but these studies do not demonstrate ongoing (new) expression of CD33 antigens during CD33 antibody therapy. Our data strongly suggest that new CD33 antigenic sites are continuously being expressed on the surface of the cells, although pulse-labeling studies with 35S-methionine may be necessary to strengthen this conclusion.
Our combined results suggest that the binding of Mylotarg to the CD33 antigen, the subsequent internalization, and the expression of new CD33 molecules form an ongoing process, which probably contributes to a progressive accumulation of the drug. Therefore, we hypothesize that infusion of higher doses of Mylotarg might result in prolonged saturation and subsequent internalization levels, thereby resulting in higher levels of drug targeted to the leukemic cells and possibly better treatment results. It has been shown that supersaturating doses of the CD33 antibody M195 (36 mg/m2 daily for 4 days) are well tolerated and show antileukemic activity.25Alternatively it may be possible to use more frequent Mylotarg cycles to obtain prolonged saturating serum levels and thereby exploit the renewed CD33 antigen expression for more efficient drug targeting.
Calicheamicin binds to DNA, resulting in double-strand breaks and the induction of apoptosis.9,10 We now demonstrate that calicheamicin, conjugated to a CD33 antibody, is able to induce apoptosis in vitro in CD33-expressing cells but is unable to induce apoptosis in CD33− cells (ie, lymphocytes). These data are in accordance with a recent report showing the cytocidal effect of Mylotarg on CD33+ leukemic cell lines.26In our study, the induction of apoptosis was apparent after 72 to 96 hours of culturing the cells in the presence of Mylotarg. In accordance with these in vitro data, no apoptosis was observed in blood samples obtained within the first 6 hours after the start of Mylotarg treatment. Although several mechanisms may contribute to the removal or killing of leukemic cells (eg, complement-dependent cytotoxicity, antibody-dependent cell-mediated cytotoxicity),26 27 our in vitro data show that the antileukemic effect of Mylotarg is at least partly based on the induction of apoptosis in CD33-expressing cells.
Data from the Mylotarg phase II studies indicate that Mylotarg induces remission in 31% of the patients.28 It is not clear why only a subset of patients can be induced into complete remission. Data from the phase I study show that drug efflux may play a role.11 It may, however, be expected that the clinical response is in part dependent on the level of saturation or internalization or both. In our results for the 122 relapsed AML patients, the minimal level of saturation on blast cells was 72%, whereas internalization varied between 15% and 69% (6-hour time point). Nevertheless, we did not find a difference in the percentages saturation and internalization between patients who showed less than 5% blast cells in the PB prior to the start of the second treatment cycle and patients with still 5% or more blasts in their PB. Clearly, it will be of interest to correlate our saturation and internalization data with clinical outcome. These data are not yet fully available.
In conclusion, Mylotarg rapidly binds to CD33-expressing leukemic and normal cells, after which internalization occurs and apoptosis is induced. These data provide insight into the mechanism of action of Mylotarg and support the use of antibody-targeted chemotherapy in patients with AML. Current protocols are investigating the applicability of Mylotarg therapy in patients with myelodysplastic syndrome and the applicability of combined Mylotarg therapy and “classical” chemotherapy in AML patients.
We gratefully acknowledge Dr B. de Vos, Mrs C. Lejeune, Mrs C. Eten, and Dr L. Grassnickel for organizational support. We thank the Universitair Ziekenhuis Gasthuisberg Leuven (Department of Hematology; Prof M. Boogaerts), Hôpital Saint-Joseph (Department of Hemato-oncology; Dr P. Mineur), Academisch Ziekenhuis VU Amsterdam (Department of Internal Medicine/Hematology; Prof P. C. Huijgens), University Hospital Rotterdam (Department of Hematology; Prof B. Löwenberg), Hospital Edouard Herriot Lyon (Department of Hematology; Prof Fiere), C. H. U. Nantes-Hotel Dieu (Deparment of Hematology; Prof J.-L. Harousseau), Hôpital Saint-Louis (Services de Maladies du Sang; Prof H. Dombret), Hospital A. Mignot Le Chesnay (Department of Hematology; Prof S. Castaigne), Universitaire de Lille (Services de Maladies du Sang; Dr N. Cambier), University Clinic Mainz (Department of Hematology; Dr M. Theobald), Medizinische Hoschule Hannover (Department of Hematology/Oncology; Dr G. Heil), University Hospital Erlangen-Nuremberg (Department of Hematology/Oncolcogy; Prof M. Gramatzki), University Hospital Cologne (Department of Oncology; Dr D. Voliotis), University Hospital Dresden (Department of Hematology/Oncology; Prof Ehninger), Azienda Policlinico Universitario Roma (Department of Hematology; Prof F. Mandelli), Ospedale Mauriziano Umberto Torino (Department of Hematology; Prof M. Aglietta), Policlinico S. Matteo Pavia (Department of Hematology; Prof C. Bernasconi), Universita deglui Studi di Genova (Department of Internal Medicine; Prof M. Gobbi), Ospedali Riuniti di Reggio Calabria (Department of Hematology; Prof F. Nobile), Hospital Puerta de Hierro Madrid (Department of Hematology; Dr M.-N. Fernandez), Hospital de la Santa Creu I San Pau Barcelona (Department of Hematology; Dr J. Sierra), Hospital Clinico San Carlos Madrid (Department of Hematology; Dr D. Mediavilla), Hospital Duran I Reynals Barcelona (Department of Hematology; Dr Granena), Hospital Clinic I Provencial (Department of Hematology; Dr J. Esteve Reynar), Instituto Portugues de Oncologica Lisboa (Department of Hematology; Dr A. B. S. Parreira), Universitetssjh Linköping (Department of Hematology; Dr G. Juliusson), Huddinge sjh (Department of Hematology; Dr C. Paul), Karolinska Hospital (Department of Hematology; Dr L. Stenke), Akademiska Hospital Uppsala (Department of Hematology; Dr B. Simonsson), Norrlands University Hospital Umeå (Department of Hematology; Dr A. Wahlin), Cantonal Hospital Aarau (Department of Hematology; Dr M. Wernli), Cantonal Hospital St. Gallen (Department of Hematology; Dr U. Hess), University of Wales College of Medicine Cardiff (Department of Hematology; Prof A. Burnett), and University of College Hospital London (Department of Clinical Hematology; Dr A. Goldstone) for sending the patient samples. Dr D. Flowers and Mrs W. Hugens are acknowledged for their technical assistance, and Mrs A. Boon for secretarial assistance.
Departments of Immunology and Pathology, Erasmus University Rotterdam and University Hospital Rotterdam, Rotterdam, The Netherlands; Fred Hutchinson Cancer Research Center, Seattle, Washington; and Wyeth-Ayerst Research, Radnor, Pennsylvania.
M.S.B. is employed by Wyeth-Ayerst Research. This study was supported in part by research funding from Wyeth-Ayerst Research to J.J.M.v.D.
Submitted September 20, 2000; accepted January 5, 2001.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jacques J. M. van Dongen, Department of Immunology, Erasmus University Rotterdam, PO Box 1738, 3000 DR Rotterdam, The Netherlands; e-mail: vandongen@immu.fgg.eur.nl.

![Fig. 1. Maximal Mylotarg binding to leukocyte subsets. / Maximal Mylotarg binding to different leukocyte subsets was analyzed prior to start of the first (hatched bars) and second (open bars) Mylotarg treatment cycle by incubating PB with an excess of Mylotarg in vitro, followed by detection with biotin-conjugated antihuman IgG4 and streptavidin-FITC. (A) Maximal Mylotarg binding to AML blast cells (cycle 1: n = 86; cycle 2: n = 35), monocytes (n = 33; n = 45), granulocytes (n = 55; n = 32), and lymphocytes (n = 61; n = 43). Patient numbers differ between leukocyte subsets and between cycle 1 and cycle 2 because not all patients received a second treatment cycle, in some patients the PB sample was not available or of too low quality, or too few events were available for a reliable analysis of all leukocyte subsets. (B) Maximal Mylotarg binding to AML blast cells from patients showing less than 5% blast cells in their PB just prior to the start of the second treatment cycle (blast cell reducers; n = 27) or 5% or more blasts in PB (blast cell nonreducers; n = 31 and n = 26 for cycle 1 and cycle 2, respectively). Maximal Mylotarg binding data for cycle 2 could not be obtained in the blast cell reducers, because the number of blast cells was too low to perform a reliable analysis. ND indicates no data. Data are expressed as mean ± SD. Significant differences (P < .05; indicated by the asterisks [unpairedt test]) were observed in maximal Mylotarg binding to blast cells between cycle 1 and cycle 2 and between cycle 1 data of the blast cell reducers and blast cell nonreducers.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/10/10.1182_blood.v97.10.3197/6/m_h81011049001.jpeg?Expires=1769089382&Signature=aDbwVT7oy4~tQ82pTMyEsmcVBz3JXMN~qyfPvuvXvdFoXcmFHXadTWBd3q6rpL6wYQSW7N71zF3meHfxUdJirYroD7NPgwF5XSmFW8tTYNKjKYzmgMJxsoAXByB~5py581g2OtYeNo-hTZI6wdy6ZZ-lfWa10rBQ5AYLwZke~Q~QLF6AIyPZGiAK71lGDg0R-RIkwfsjyUnARqMK5YR7IUlL~YGtBNUJBhkARFY4n-yPjBWMgnqs2L6h~bDREMTfGeSvnAnjW16PG3HW14vgrRnju5eNWnlYf9-CwWCKeyXFtyaBFJQ-uhmCtsHOjH7SV47oUHq3fOTdGyfVIQ0VDg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal