Abstract
Transfection of tumor cells with the gene encoding the costimulatory molecule B7-1 (CD80), the ligand for CD28 and cytotoxic T lymphocye antigen-4 on T cells, has been shown to result in potent T-cell–mediated antitumor immunity. As an alternative approach, this study analyzed the costimulatory capacity of a human B7-1 immunoglobulin G (IgG) fusion protein targeted to the cell membrane of human acute myeloid leukemia (AML) blasts. Flow cytometric analysis revealed a low constitutive expression of B7-1 on human AML blasts (on average, 3.0 ± 4.3%; n = 50). In contrast, the expression of B7-2 (CD86) was highly heterogeneous and higher in AML blasts of French-American-British classification types M4 and M5 (P < .0001). The B7-1 IgG fusion protein used in this study efficiently costimulated the proliferation of resting and preactivated T cells when immobilized on plastic. After preincubation with B7-1 IgG, specific binding of the fusion protein to the high-affinity Fcγreceptor I (CD64) on leukemic cells was demonstrated and was found to increase the proliferation of both allogeneic and autologous T cells in costimulation experiments. Furthermore, targeting of B7-1 IgG to the tumor membrane resulted in increased proliferation of autologous remission T cells and had the potential to generate an enhanced redirected cytotoxic T-cell response against autologous AML blasts. In summary, the targeting of B7-1 IgG fusion protein described in this study represents a strategy alternative to gene therapy to restore the expression of the costimulatory molecule B7-1 on human AML blasts, thereby enhancing their immunogenicity for autologous T cells. This new approach may have implications for T-cell–mediated immunotherapy in AML.
Introduction
During the late 1970s, a T-cell–mediated graft-versus-leukemia effect was recognized in patients with acute myeloid leukemia (AML) and other hematological malignancies who underwent allogeneic bone marrow transplantation.1,2Furthermore, recurrence of chronic myeloid leukemia, and to a lesser extent AML, after allogeneic stem cell transplantation can be successfully treated with donor-lymphocyte infusions.3These observations have led to an interest in immunotherapy of AML by the adoptive transfer of leukemia-reactive T cells generated in vitro. This adoptive T-cell therapy represents a potentially specific and efficacious treatment modality, as has been shown for the treatment of EBV (Epstein-Barr virus)–related lymphoproliferative disease with EBV-specific T-cell clones.4 One important factor for the success of adoptive T-cell therapies in AML might be the capacity of AML cells to function as antigen-presenting cells (APCs). It is well established that T cells depend on costimulatory signals from the APCs in addition to the antigen-specific and major histocompatibility complex (MHC)–restricted signal provided by ligation of the clonotypic T-cell receptor (TCR). The most important costimulatory pathway consists of the interaction of CD28 and cytotoxic T lymphocye antigen-4 (CTLA-4) on T cells with their ligands B7-1 (CD80) and B7-2 (CD86) on the APCs.5,6 Recent evidence suggests that the final outcome of T-cell activation is modulated by activating and inhibitory signals induced by ligation of CD28 and CTLA-4, respectively. Therefore, one way to improve the APC function of AML cells is by manipulation of this costimulatory pathway. Indeed, studies using murine models of AML have shown that transfection with the costimulatory molecule B7-1 results in increased immunogenicity of the leukemic blasts, leading to the generation of cytotoxic T lymphocytes with antileukemic reactivity. Importantly, these leukemia-reactive cytotoxic T cells are capable of eradicating the disease at a low tumor burden,7-9 which resembles minimal residual disease in human AML.
This study evaluated an alternative approach to gene therapy aiming at enhancing the costimulatory capacity of AML blasts. To this end, a human B7-1 immunoglobulin G (IgG) fusion protein, consisting of the extracellular domain of B7-1 fused to the Fc portion of IgG1, was used. The rationale behind this approach is as follows: the Fc portion of B7-1 IgG should permit binding of the fusion protein to the cell membrane of AML blasts via their high-affinity Fcγreceptor I (FcγRI) (CD64). Thereby, AML blasts should acquire a B7-1+ phenotype resulting in enhanced costimulation of host T cells via CD28/CTLA-4. In particular, the aims of this study were to determine (1) to what extent human AML blasts express the costimulatory molecules B7-1 and B7-2, (2) whether targeting of the B7-1 IgG fusion protein to the AML membrane is feasible, and (3) whether this targeting enhances the costimulatory activity of AML blasts for the proliferation of autologous remission T cells.
Materials and methods
Flow cytometric analysis of AML blasts
Mononuclear cells were isolated from bone marrow or peripheral blood of 50 patients with AML by Ficoll-Hypaque gradient centrifugation. The mean percentage of leukemic blasts as determined morphologically was 74% ± 19% (range, 28%-99%) before Ficoll-Hypaque gradient centrifugation. AML blasts were either subjected directly to flow cytometric analysis or cryopreserved for later analysis. We incubated 2.5 to 5.0 × 105AML blasts with pretitrated amounts of monoclonal antibody (mAb) 104/CD80 (Immunotech, Marseilles, France) and IT2.2/CD86 (Pharmingen, San Diego, CA), and used irrelevant mAbs of the same isotype IgG1 and IgG2a (Becton Dickinson, Mountain View, CA), respectively, as negative controls. The leukemic blasts were counterstained with mAbs directed against 8G12/CD34 (Becton Dickinson), P67.6/CD33 (Becton Dickinson), or SJ1D1/CD13 (Immunotech) to determine the percentage of B7+ blasts as accurately as possible by excluding contaminating lymphoid cells from the analysis. After 30 minutes of incubation at 4°C, cells were washed and resuspended in fluorescence-activated cell sorting (FACS) buffer containing 0.5% bovine serum albumin (Behring, Marburg, Germany) and 0.01% NaN3 (Merck, Darmstadt, Germany) in phosphate-buffered saline (PBS) (Biochrom, Berlin, Germany). To exclude dead cells from the analysis, cell suspensions were incubated with propidium iodide (Molecular Probes, Leiden, The Netherlands) at 2 μg/mL. Finally, stained cells were washed and analyzed on a FACScan cytofluorometer by means of the Cell Quest software package (Becton Dickinson). To block nonspecific binding via FcRs, human IgG (Behring) and rabbit serum (Sigma, Deisenhofen, Germany) were present throughout all incubation steps at concentrations of 12 mg/mL and 50 mg/mL, respectively.
Cell lines
U937, a human leukemic cell line with monoblastic features expressing high-affinity FcγRI, was maintained in suspension culture in B medium containing RPMI 1640 (Gibco, Grands Islands, NY) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Biochrom), 2 mM L-glutamine (Biochrom), and 1 mM sodium pyruvate (Biochrom) at 37°C in 5% CO2. For the culture of adherent COS7m6 cells, an SV40-transformed monkey kidney epithelial cell line, COS medium consisting of Dulbecco modified Eagle medium (DMEM) (Gibco) supplemented with 10% heat-inactivated FCS, 2 mM L-glutamine, and 1 mM sodium pyruvate was used. Cells were cultured at 37°C in 5.5% CO2. Furthermore, cells were tested for the presence of mycoplasma by a DNA staining method using bisbenzimide (Biochrom) every 4 weeks. Contaminated cultures were discarded.
Transient expression of B7-1 IgG in COS7m6 cells
The vector pCDM8–B7-1 IgG (kindly provided by B. Seed, Boston, MA) encoding the human B7-1 IgG fusion protein was transiently expressed in COS7m6 cells with the diethylaminoethyl-dextran (DEAE)-dextran method as previously described.10Briefly, COS7m6 cells at 50% confluence in 10 cm dishes (Costar, Cambridge, MA) were transfected with 1 μg/mL purified B7-1 IgG plasmid in medium containing DMEM supplemented with 3% heat-inactivated FCS, 2 mM L-glutamine, 1 mM sodium pyruvate, 4 μg/mL DEAE-dextran, and 1 μM chloroquine diphosphate (Sigma). After approximately 4 hours at 37°C in 5.5% CO2, the transfection medium was removed. Then, the cells were incubated in a 10% dimethyl sulfoxide solution (Serva, Heidelberg, Germany) for 2 minutes and washed with COS medium. After 7 more days of culture in COS medium, the cell supernatant was harvested and tested for the presence of B7-1 IgG. In analogous fashion, a human CD8 IgG fusion protein (vector kindly provided by B. Seed, Boston, MA), consisting of the extracellular domain of CD8 fused to the Fc portion of IgG, was produced by transfection of COS7m6 cells.
Detection of B7-1 IgG in the supernatant of COS7m6 cells
A sandwich enzyme-linked immunosorbent assay (ELISA) specific for the Fc portion of human IgG molecules was used to detect B7-1 IgG in the harvested supernatant. Serial dilutions of the supernatant (triplicates) were incubated in 96-well flat-bottomed Maxi-sorp plates (Nunc, Roskilde, Denmark) coated with a rat antihuman IgG/Fc capture antibody (Dako, Glostrup, Denmark) used at 1 μg/mL. After thorough washing with 0.1% Tween 20 (Sigma) in PBS, the alkaline phosphatase–conjugated second antihuman IgG/Fc antibody (Dako) was added at a concentration of 0.25 μg/mL. Then, the plates were washed again; the substrate p-nitrophenylphosphate was added; and the extinction was measured photometrically at 405 nm. Human immunoglobulin (Behring) at 0.5 μg/mL served as a standard to determine the concentration of B7-1 IgG in the supernatant. To detect the B7-1 portion of the fusion protein, U937 cells were preincubated with 200 μL supernatant/5 × 105 cells and washed extensively with B medium to remove unbound fusion protein. After the cells were stained with the mAb 104/CD80, they were subjected to FACS analysis as described above. Preincubation with COS7m6 cell supernatant containing a human CD8 IgG fusion protein served as a negative control.
Isolation of responder T cells
Peripheral blood mononuclear cells (PBMCs) were isolated from healthy donors or from AML patients while in complete remission by Ficoll-Hypaque gradient centrifugation. Contaminating erythrocytes were removed by hypotonic lysis. For the isolation of resting T cells, these PBMCs were subjected to further purification steps by means of nylon-wool filtration and depletion by adherence to plastic. In the case of remission PBMCs of AML patients, the total mononuclear cell fraction was used as responder population. To generate preactivated T cells, PBMCs were stimulated with 10 μg/mL phytohemagglutinin (PHA) (Biochrom) in B medium at 37°C in 5% CO2 for 5 days. Thereafter, the cells were rested in B medium without PHA for 24 hours before being used for subsequent experiments. The immunophenotype of both resting and preactivated T cells was characterized by flow cytometry with the following mAbs: MY31/CD56 (Becton Dickinson) and J4.119/CD19, RMO52/CD14, B8.12.2/HLA-DR, and B1.49.9/CD25 (all Immunotech). Cells were counterstained with SK7/CD3 (Becton Dickinson). Irrelevant mAbs of the appropriate isotype were used as negative controls. The percentage of CD3+ cells of resting and preactivated T-cell populations was 84.1% ± 5.4% and 95.9 ± 5.5%, respectively (n = 7). The expression of the activation markers HLA-DR and CD25 (interleukin-2αR [IL-2αR]) was determined as 3.6% ± 2.2% and 3.4% ± 4.1% (n = 7) for resting and as 61.7% ± 8.4% and 92.0 % ± 2.8% (n = 7) for preactivated T cells.
Binding of B7-1 IgG to the tumor membrane
U937 cells were preincubated with various amounts of B7-1 IgG or CD8 IgG in the absence or presence of the CD64-blocking mAb 197 (Medarex, Annandale, NJ) at B7-1 IgG–to–mAb 197 ratios ranging from 1:10 to 2:1 at 4°C. After careful washing, the cells were stained with the mAb 104/CD80 and analyzed by flow cytometry as described above.
T-cell costimulation by plastic-immobilized B7-1 IgG
For the stimulation of resting and preactivated T cells with plastic-immobilized B7-1 IgG, 96-well flat-bottomed plates (Corning, New York, NY) were coated with 10 μg/mL goat antimouse IgG/Fc and goat antihuman IgG/Fc antisera (both Sigma) at 4°C in coating buffer consisting of 50 mM Tris-HCl (Serva)/pH 9.5 for 2 to 4 hours. Plates were washed with RPMI 1640, and blocking buffer with 0.1% bovine serum albumin in PBS was added to reduce nonspecific binding. After a further 16 to 18 hours at 4°C and extensive washing steps with RPMI 1640, 50 μL per well of various concentrations of OKT3/mouse antihuman CD3 mAb (Ortho, Raritan, NJ) and 50 to 100 ng CD8 IgG per well or B7-1 IgG per well were added. The plates were incubated at 37°C in 5% CO2 for 2 hours and washed thoroughly again with RPMI 1640. Finally, 5 × 104 T cells per well were added to the plates and cultured in B medium in a final volume of 200 μL for 48 to 72 hours before they were pulsed with 1 μCi3H-thymidine per well (Amersham, Little Chalfont, United Kingdom) for a further 16 to 18 hours. We determined3H-thymidine-uptake 3H-TdR by liquid scintillation counting.
Allogeneic and autologous mixed lymphocyte tumor cultures
Allogeneic and autologous mixed lymphocyte tumor cultures (AMLTCs) were performed with the use of U937 cells and T cells from 2 healthy donors, and with AML blasts cryopreserved at the time of the initial diagnosis and remission T cells of AML patient no. 39, respectively. T cells were isolated from patient no. 39 (a 64-year-old woman with AML-M5a) after 2 cycles of induction and 1 cycle of consolidation chemotherapy when the patient was in complete remission. Date of first diagnosis in patient number 39 was August 23, 1995. Blast cells were isolated from bone marrow and frozen down in multiple aliquots of 1.5 × 107 cells per 2 mL FCS per vial. Following aspiration and Ficoll-Hypaque gradient centrifugation, 1 mL bone marrow contained 6 × 107 mononuclear cells. The preparation contained 1% contaminating T and B cells, respectively, as determined by staining for CD7+and CD19+ cells. The purity of blast cells as assessed by morphology was greater than 95%; therefore, no further purification steps were performed. The immunophenotypes were as follows: CD33, 90%; CD64, 88%; CDw65, 33%; CD13, 59%; CD15, 15%; CD14, 40%; CD4, 81%; HLA-DR, 73%; CD34, 7%; CD10, 1%; CD117, 2%; and CD54, 41%. Remission T cells that were used as responding cells for cytotoxicity testing were obtained in February 2000 and were used directly following venipuncture. Both stimulator cell populations (U937 and AML blasts) were inactivated by incubating 5 × 106 cells per 2 mL B medium in 1 mg/mL mitomycin C (Kyowa Hakko Kogyo, Tokyo, Japan) at 37°C in 5% CO2 for 30 minutes. Soluble mitomycin C was removed by extensive washing with PBS. Next, stimulator cells were preincubated with saturating amounts of CD8 IgG or B7-1 IgG (U937 cells: 5 to 7 μg/106 cells; AML blasts: 5 to 10 μg/106 cells) at 4°C for 30 to 60 minutes. Unbound fusion proteins were removed by 3 washing steps with B medium. Binding of CD8 IgG and B7-1 IgG was checked by FACS analysis with mAb B9.11/CD8 and mAb 104/CD80 (both Immunotech), respectively. In addition, FcγRI expression was studied by means of 32.2/CD64 mAb (Medarex). To measure the proliferation of the responder T cells in AMLTC, 5 × 104 T cells per well were cultured in 96-well flat-bottomed plates coated with OKT3 as described above. At the initiation of culture, we added 1 × 105 stimulator cells (U937 cells or AML blasts) per well, resulting in an R-to-S ratio of 1:2. Cells were incubated in a final volume of 200 μL per well for 48 to 72 hours before 3H-thymidine uptake was determined as described earlier.
AMLTC and cell-mediated cytotoxicity assay
Cytotoxic effector cells were generated as follows: 106/mL remission PBMCs of patient no. 39 with AML-M5a were activated with 10 μg/mL PHA for 3 days in B medium followed by washing and culture in B medium alone at 105/mL for 24 hours. We then transferred 5 × 104/100 μL PHA blasts to microwells of 96-flat bottom plates (Costar 3599). Plates had been pretreated with 10 μg/mL goat antimouse IgG/Fc (Sigma) in 100 mM Tris HCl (pH 9.0) for 2 hours at 37°C, followed by blocking with 0.1% bovine serum albumin in PBS at 4°C overnight. Various concentrations of OKT3 or B medium alone were added in 50 μL per well for 2 hours at 37°C prior to 3 extensive washing steps with B medium (200 μL per well) to remove unbound antibody. Mitomycin C–inactivated AML-M5 blasts of patient no. 39 were coated with CD8 IgG or B7-1 IgG as described above and were used as stimulator cells. Binding of IgG fusion proteins was verified by flow cytometry as described. We added 105/100 μL stimulator cells to each well containing 5 × 104/100 μL responding T cells. At day 7, microcultures were restimulated by replacing 100 μL supernatant per well with 100 μL B medium containing freshly prepared CD8 IgG–coated or B7-1 IgG–coated AML-M5 stimulator cells. At day 14, cytotoxic activity of microcultures was determined by standard 4-hour51chromium (51Cr)–release assay. To this end, cryopreserved AML blasts of patient no. 39 (AML-M5a) were thawed 1 day before assay and were cultured overnight at 106 cells per milliliter in B medium. Target cells (1 to 2 × 106cells) in 100 μL FCS were labeled with 300 μCi51Na2CrO4 (Amersham) for 2 hours at 37°C and 5.5% CO2. Cells were washed once with B medium followed by a second incubation in 5 mL B medium for 30 minutes. Cells were washed twice with B medium. Target cells (5 × 103 cells) in 100 μL B medium were added to 100 μL B medium containing effector cells of the AMLTC described above. OKT3 at a final concentration of 80 ng/mL was added, and plates were centrifuged at 150g for 5 minutes prior to being incubated for 4 hours at 37°C and 5.5% CO2. Determinations were made in triplicate. Supernatants were then harvested by means of a Skatron system (Skatron, Lkier, Norway) and counted in a gamma counter (LKB Wallac, Bromma, Sweden) The percentage of specific51Cr-release was calculated by means of the following formula: percentage of specific release = (ER − SR) × 100/(MR − SR), where ER was experimental 51Cr-release, SR the spontaneous51Cr-release as measured in the supernatant of 5 × 103 target cells cultured in 200 μL B medium alone, and MR the maximum release after the addition of 100 μL 1% TritonX100 (Sigma). Spontaneous 51Cr-release had to be lower than 25% for results to be included into the final analysis.
Statistics
To compare the expression of B7-1 and B7-2 on AML blasts of different French-American-British (FAB) types, Kruskal-Wallis analysis was used. For comparison of the T-cell proliferation or T-cell cytotoxicity induced by CD8 IgG and B7-1 IgG, one-sided Wilcoxon tests for unpaired rank sums were calculated. A value of P = .05 was considered statistically significant.
Results
Expression of the costimulatory molecules B7-1 and B7-2 on AML blasts
The expression of the costimulatory molecules B7-1 and B7-2 on human AML blasts isolated from bone marrow or peripheral blood was investigated by flow cytometry with monoclonal antibodies in 50 patients. As shown in Figure 1, the percentage of leukemic blasts expressing B7-1 on their cell membrane was generally low (average expression: 3.0% ± 4.3%) regardless of FAB type. In contrast, the expression of B7-2 was found to be substantial, with an average expression of 23.9% ± 26.1%. Interestingly, the expression of B7-2 was highly heterogeneous and significantly higher in AML blasts of FAB types M4 and M5 with monocytic/monoblastic morphology (42.1% ± 20.9% and 42.8%± 32.5%) than in AML blasts belonging to the FAB types M0, M1, M2, and M3 (P < .0001 by Kruskal-Wallis analysis).
B7-expression on human AML blasts according to FAB type.
The expression of B7-1 (A) and B7-2 (B) on human AML blasts was studied by flow cytometry as described in “Materials and methods.” The average percentages of B7-1+ or B7-2+ blasts are shown as horizontal lines for the different FAB types.P = .736 for B7-1, P < .0001 for B7-2, by Kruskal-Wallis analysis.
B7-expression on human AML blasts according to FAB type.
The expression of B7-1 (A) and B7-2 (B) on human AML blasts was studied by flow cytometry as described in “Materials and methods.” The average percentages of B7-1+ or B7-2+ blasts are shown as horizontal lines for the different FAB types.P = .736 for B7-1, P < .0001 for B7-2, by Kruskal-Wallis analysis.
Plastic-immobilized B7-1 IgG costimulates the proliferation of T cells
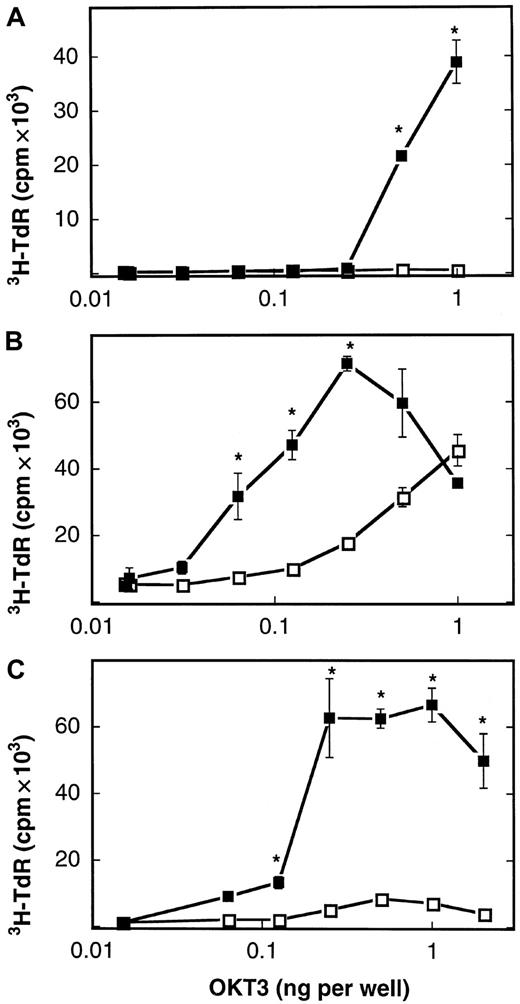
The described lack of B7-1 molecules expressed on the cell membrane of AML blasts constitutes a potential mechanism whereby leukemic cells escape the immune surveillance of autologous T lymphocytes. We therefore asked if a human B7-1 IgG fusion protein consisting of the extracellular domain of B7-1 and the Fc portion of IgG1 could compensate for the absent expression of B7-1 on the tumor membrane of human AML blasts. An expression vector (referred to as pCDM8–B7-1 IgG) containing the complementary DNA encoding the extracellular domain of human B7-1 fused to the hinge CH2-CH3 domains (Fc portion) of human IgG1 was kindly provided by B. Seed (Boston, MA). After transient expression of the pCDM8–B7-1 IgG plasmid in COS7m6 cells, the presence of an intact fusion protein consisting of both the Fc and the B7-1 portion could be demonstrated in the harvested cell supernatant. First, the concentration of B7-1 IgG in the supernatant was determined to be 14.8 ± 3.0 μg/mL (n = 3) by an ELISA specific for the Fc portion of human immunoglobulins. Second, FACS analysis revealed that the B7-1 portion was recognized by a monoclonal antibody reacting with B7-1 after preincubation of U937 cells with the B7-1 IgG COS cell supernatant as described in “Materials and methods.” In the next set of experiments, the biologic activity of the described B7-1 IgG fusion protein was tested. To this aim, the costimulatory capacity of B7-1 IgG was studied when coimmobilized on plastic with OKT3 as a surrogate signal 1 (imitating the interaction of antigen-MHC complexes with the TCR). We used 3 responder T-cell populations: resting T cells isolated from healthy donors, PHA-preactivated T cells isolated from healthy donors, and PHA-preactivated T cells isolated from AML patients in complete remission. As shown in Figure 2, the B7-1 IgG fusion protein used in this study efficiently costimulated the proliferation of all 3 responder T-cell populations at a concentration of 50 to 100 ng per well in the presence of OKT3 (P = .004 by Mann-Whitney test). A human CD8 IgG fusion protein, in contrast, failed to enhance the proliferation of these T cells. Similar results were obtained with the use of T cells from 4 healthy donors; B7-1 IgG enhanced proliferation 5- to 65-fold. We conclude that the B7-1 IgG fusion protein provides efficient costimulation for the proliferation of normal resting and preactivated T cells, including remission T cells of AML patients.
Costimulation of T cells by plastic-immobilized B7-1 IgG.
Resting T cells isolated from a healthy donor (A), T cells from a healthy donor preactivated with PHA (B), and PHA-preactivated remission T cells from patient no. 39 (AML-M5a) (C) were cultured in 96-well plates (5 × 104 cells per well) coated with CD8 IgG (■) or B7-1 IgG (▪, 75 to 100 ng per well) and the indicated concentrations of OKT3. 3H-thymidine-uptake was determined after 72 hours of culture as described in “Materials and methods.” Data are expressed as mean ± SD of triplicate wells. T cells alone: 0.3 ± 0.1 cpm × 103 (A); 2.9 ± 0.8 cpm × 103 (B); 0.3 ± 0.03 cpm × 103(C). Asterisks indicate P = .05 values by one-sided Wilcoxon tests for unpaired rank sums compared with CD8 IgG–induced proliferation.
Costimulation of T cells by plastic-immobilized B7-1 IgG.
Resting T cells isolated from a healthy donor (A), T cells from a healthy donor preactivated with PHA (B), and PHA-preactivated remission T cells from patient no. 39 (AML-M5a) (C) were cultured in 96-well plates (5 × 104 cells per well) coated with CD8 IgG (■) or B7-1 IgG (▪, 75 to 100 ng per well) and the indicated concentrations of OKT3. 3H-thymidine-uptake was determined after 72 hours of culture as described in “Materials and methods.” Data are expressed as mean ± SD of triplicate wells. T cells alone: 0.3 ± 0.1 cpm × 103 (A); 2.9 ± 0.8 cpm × 103 (B); 0.3 ± 0.03 cpm × 103(C). Asterisks indicate P = .05 values by one-sided Wilcoxon tests for unpaired rank sums compared with CD8 IgG–induced proliferation.
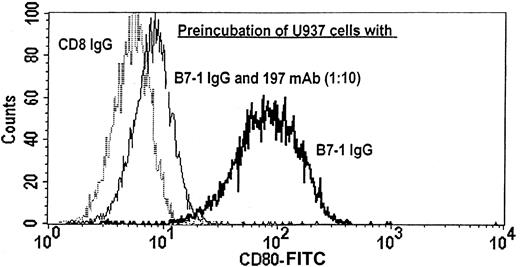
Specific binding of B7-1 IgG to the tumor membrane via high-affinity FcγRI
To test whether the B7-1 IgG fusion protein is capable of binding to the tumor membrane, the monoblastic cell line U937 was used as a model system. This leukemic cell line is particularly suited for this kind of experiment because it has a high-level expression of high-affinity FcγRI and does not express B7-1 (data not shown). U937 cells were analyzed for binding of B7-1 IgG to their cell membrane by means of FACS analysis with a monoclonal antibody directed against B7-1 before and after preincubation with B7-1 IgG. Whereas fewer than 1% of U937 cells expressed B7-1 on their cell surface without preincubation, or after preincubation with the control fusion protein CD8 IgG, more than 99% of these cells were rendered B7-1+ after preincubation with 0.5 μg or more B7-1 IgG per 106 cells associated with a greater than 14-fold increase in the mean fluorescence intensity of the CD80 staining (data not shown). To further delineate the binding properties of B7-1 IgG, U937 cells were preincubated with B7-1 IgG in the absence or presence of the 197 mAb, which interferes with the binding of immunoglobulins (and fusion proteins consisting of Fc portions) to high-affinity FcγRI by blocking the IgG-binding region of these receptors. Figure3 shows a greater than 90% reduction in the binding of B7-1 IgG to U937 cells by a concentration of the blocking 197 mAb exceeding the concentration of B7-1 IgG by 1 order of magnitude. In conclusion, B7-1 IgG is capable of binding to the tumor membrane and this binding is dependent on, and specifically mediated by, high-affinity FcγRI expressed on the tumor cell surface.
Binding of B7-1 IgG to the tumor membrane by means of high-affinity FcγRI.
U937 cells were preincubated with B7-1 IgG in the absence or presence of the CD64-blocking mAb 197. After extensive washing of the cells, membrane-bound B7-1 IgG was detected by FACS analysis with mAb 104/CD80. CD8 IgG was used as a negative control. The figure shows a representative FACS histogram of this experiment with a B7-1 IgG–to–197 mAb ratio of 1:10.
Binding of B7-1 IgG to the tumor membrane by means of high-affinity FcγRI.
U937 cells were preincubated with B7-1 IgG in the absence or presence of the CD64-blocking mAb 197. After extensive washing of the cells, membrane-bound B7-1 IgG was detected by FACS analysis with mAb 104/CD80. CD8 IgG was used as a negative control. The figure shows a representative FACS histogram of this experiment with a B7-1 IgG–to–197 mAb ratio of 1:10.
Costimulatory activity of membrane-bound B7-1 IgG in an allogeneic tumor model
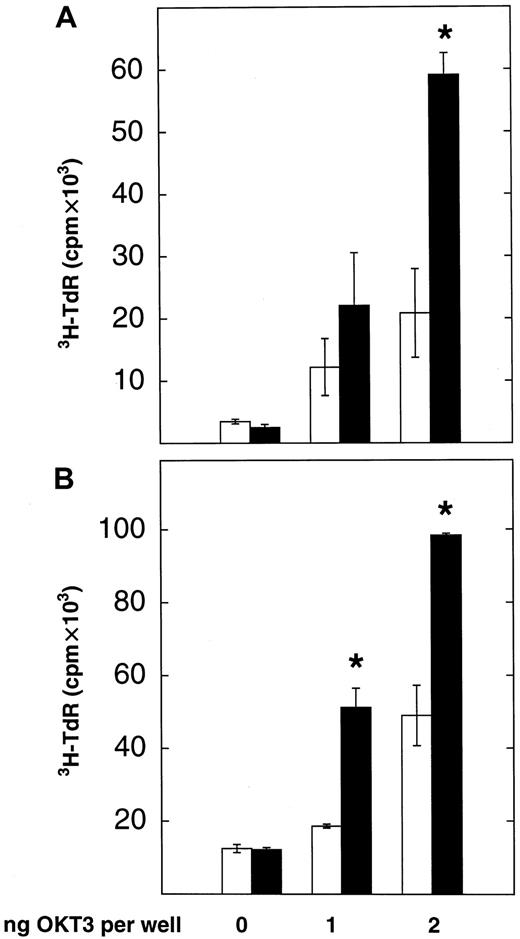
After the demonstration that both the B7-1 and the Fc portion of the B7-1 IgG fusion protein retained biological activity, the costimulatory properties of the fusion protein in an allogeneic leukemia model were studied. This consisted of U937 cells as stimulator cells and T cells isolated from healthy donors as responder cells, either resting or preactivated with PHA. As described earlier, plastic-immobilized OKT3 was used as a surrogate signal 1. The proliferation of the responder T cells was measured in the presence of U937 cells, which were inactivated with mitomycin C and preincubated with saturating amounts of either CD8 IgG or B7-1 IgG.3H-thymidine-uptake assays (Figure4 shows 1 of 2 experiments with identical results) revealed that U937 cells preincubated with B7-1 IgG significantly enhanced the proliferation of both resting and PHA-preactivated allogeneic T cells in the presence of OKT3 compared with U937 cells preincubated with CD8 IgG (P = .006 by Mann-Whitney test). In the absence of OKT3, no significant T-cell proliferation could be detected. Thus, the B7-1 IgG fusion protein exerts costimulatory activity for the proliferation of T cells in an allogeneic leukemia model.
B7-1 IgG costimulates allogeneic T cells after binding to the tumor membrane.
Resting (A) or PHA-preactivated (B) T cells isolated from a healthy donor (5 × 104 per well) were cocultured with U937 cells (1 × 105 per well), which were inactivated with mitomycin C and preincubated with CD8 IgG (■) or B7-1 IgG (▪) (5 to 7 μg/106 cells) in 96-well plates coated with OKT3.3H-thymidine-uptake was measured after 48 to 72 hours of culture as described in “Materials and methods.” Data are expressed as mean ± SD of triplicate wells. T cells (resting/preactivated) alone: 0.3 ± 0.02 cpm × 103/2.9 ± 0.8 cpm × 103. U937 cells (CD8 IgG/B7-1 IgG) alone: 5.5 ± 2.2 cpm × 103/3.9 ± 0.8 cpm × 103. Asterisks indicate P = .05 values by one-sided Wilcoxon tests for unpaired rank sums as compared with CD8 IgG-induced proliferation.
B7-1 IgG costimulates allogeneic T cells after binding to the tumor membrane.
Resting (A) or PHA-preactivated (B) T cells isolated from a healthy donor (5 × 104 per well) were cocultured with U937 cells (1 × 105 per well), which were inactivated with mitomycin C and preincubated with CD8 IgG (■) or B7-1 IgG (▪) (5 to 7 μg/106 cells) in 96-well plates coated with OKT3.3H-thymidine-uptake was measured after 48 to 72 hours of culture as described in “Materials and methods.” Data are expressed as mean ± SD of triplicate wells. T cells (resting/preactivated) alone: 0.3 ± 0.02 cpm × 103/2.9 ± 0.8 cpm × 103. U937 cells (CD8 IgG/B7-1 IgG) alone: 5.5 ± 2.2 cpm × 103/3.9 ± 0.8 cpm × 103. Asterisks indicate P = .05 values by one-sided Wilcoxon tests for unpaired rank sums as compared with CD8 IgG-induced proliferation.
Costimulation of remission T cells by autologous AML blasts after targeting of B7-1 IgG to the tumor membrane
Finally, the costimulatory activity of B7-1 IgG was investigated in an autologous AML system. Analogous with U937 cells, preincubation with B7-1 IgG induced a 20-fold increase in the mean fluorescence intensity of the CD80 staining and therefore in the expression of B7-1 on the cell surface of the leukemic blast cells of patient no. 39 (AML-M5a) (Figure 5A). This corresponded to the high level of high-affinity FcγRI expressed by these AML blasts, leading to a percentage of greater than 95% B7-1+blasts after preincubation with B7-1 IgG. Next, AMLTC experiments measuring the T-cell proliferation by 3H-thymidine uptake were performed. To this aim, PBMCs of AML patient no. 39 were isolated while she was in complete remission and either left untreated (resting) or preactivated with PHA. We stimulated these responder T cells with plastic-immobilized OKT3 and mitomycin C–inactivated AML blasts (cryopreserved at the time of the initial diagnosis), which had been preincubated with either B7-1 IgG or CD8 IgG as described above. Figure5B shows a representative experiment demonstrating that the AML blasts of patient no. 39 were not capable of costimulating the proliferation of autologous resting remission T cells despite the fact that 59% of these leukemic blast cells expressed B7-2 (data not shown). However, when PHA-preactivated T cells were used as responder cells, AML blasts preincubated with B7-1 IgG showed a 5.5-fold higher costimulatory activity than AML blasts preincubated with CD8 IgG in the presence of OKT3 (P = .006 by Mann-Whitney test). In summary, the targeting of a human B7-1 IgG fusion protein to the tumor membrane of AML blasts expressing high-affinity FcγRI is feasible, leading to a significant increase in their costimulatory activity for the proliferation of autologous remission T cells. To address the question of whether B7-1 IgG targeting also increased differentiation into cytotoxic effector cells, AMLTCs were set up that used AML-M5 blasts of patient no. 39 as stimulator cells and autologous remission T cells as responders. In addition, submitogenic concentrations of plastic-bound OKT3 were incorporated in the stimulation protocol as a substitute for T-cell receptor activation. Redirected cytotoxicity against AML-M5 blasts in the presence of soluble OKT3 was measured on day 14 of AMLTC by standard 4-hour 51Cr-release assay. As is evident from Table 1, stimulation of autologous T cells with AML-M5 blasts precoated with control CD8 IgG twice on day 0 and 7 induced moderate T-cell cytotoxicity against autologous AML cells. There was a small but significant increase in antileukemic cytotoxic activity when autologous AML stimulator cells carried B7-1 IgG on the cell surface. CD3 cross-linking by OKT3 was required during the induction phase, arguing against effective recognition of an intrinsic unknown tumor antigen by T cells. In conclusion, there is indication that B7-1 IgG targeting to AML blasts has the potential to increase the differentiation of autologous T cells into cytotoxic effector cells.
Costimulation of remission T cells by autologous AML blasts after targeting of B7-1 IgG to the tumor membrane.
(A) Expression of B7-1 on the leukemic blasts of patient no. 39 (AML-M5a) after preincubation with CD8 IgG or B7-1 IgG (5 to 10 μg/106 cells). The expression of high-affinity FcγRI was determined at the same time. (B) PBMCs of the same patient were isolated while she was in complete remission and either left untreated or preactivated with PHA as described in “Materials and methods.” AMLTC with mitomycin C–inactivated and CD8 IgG–pretreated (■) or B7-1 IgG–pretreated (▪) AML blasts was performed at an R-to-S ratio of 1:2 in the absence or presence of OKT3.3H-thymidine-uptake was measured after 48 to 72 hours of culture as described earlier. Results from triplicate cultures are given as mean ± SD. T cells (resting/preactivated) alone: 0.5 (0.08 cpm × 103/0.3 ± 0.03 cpm × 103. AML blasts (CD8 IgG/B7-1 IgG) alone: 0.3 ± 0.07 cpm × 103/0.3 ± 0.09 cpm × 103.
Costimulation of remission T cells by autologous AML blasts after targeting of B7-1 IgG to the tumor membrane.
(A) Expression of B7-1 on the leukemic blasts of patient no. 39 (AML-M5a) after preincubation with CD8 IgG or B7-1 IgG (5 to 10 μg/106 cells). The expression of high-affinity FcγRI was determined at the same time. (B) PBMCs of the same patient were isolated while she was in complete remission and either left untreated or preactivated with PHA as described in “Materials and methods.” AMLTC with mitomycin C–inactivated and CD8 IgG–pretreated (■) or B7-1 IgG–pretreated (▪) AML blasts was performed at an R-to-S ratio of 1:2 in the absence or presence of OKT3.3H-thymidine-uptake was measured after 48 to 72 hours of culture as described earlier. Results from triplicate cultures are given as mean ± SD. T cells (resting/preactivated) alone: 0.5 (0.08 cpm × 103/0.3 ± 0.03 cpm × 103. AML blasts (CD8 IgG/B7-1 IgG) alone: 0.3 ± 0.07 cpm × 103/0.3 ± 0.09 cpm × 103.
Induction of redirected antileukemic T-cell cytotoxicity following stimulation with B7-1 immunoglobulin G-coated acute myeloid leukemia-M5 blasts in autologous mixed-lymphocyte tumor culture
| Plastic-bound OKT3 (ng per well) . | Specific lysis from AML-M5 blasts by remission T cells (%) stimulated with AML-M5 blasts according to pretreatment of blasts . | ||
|---|---|---|---|
| CD8 IgG . | B7-1(CD80) IgG . | P value* . | |
| 0 | 7.8 ± 3.1 | 5.0 ± 0.5 | — |
| 1 | 23.7 ± 1.0 | 26.3 ± 1.8 | 0.05 |
| 2 | 26.0 ± 0.2 | 30.0 ± 3.6 | 0.05 |
| 4 | 24.4 ± 1.6 | 28.5 ± 2.0 | 0.05 |
| Plastic-bound OKT3 (ng per well) . | Specific lysis from AML-M5 blasts by remission T cells (%) stimulated with AML-M5 blasts according to pretreatment of blasts . | ||
|---|---|---|---|
| CD8 IgG . | B7-1(CD80) IgG . | P value* . | |
| 0 | 7.8 ± 3.1 | 5.0 ± 0.5 | — |
| 1 | 23.7 ± 1.0 | 26.3 ± 1.8 | 0.05 |
| 2 | 26.0 ± 0.2 | 30.0 ± 3.6 | 0.05 |
| 4 | 24.4 ± 1.6 | 28.5 ± 2.0 | 0.05 |
Remission PHA blasts, 5 × 104 per well (activated for 3 days), of patient no. 39 with AML-M5a were stimulated with submitogenic concentrations of plastic-bound OKT3 as indicated, and with autologous mitomycin C-inactivated AML blasts (105per well) precoated with saturating concentrations of CD8 IgG and B7-1 IgG on day 0 and 7. Fusion cytokine binding was verified as stated in legend to Figure 3. On day 14 of mixed-lymphocyte tumor culture, cytotoxicity of responding T cells against autologous AML-M5 blasts (5 × 103 per well) in the presence of soluble OKT3 (80 ng/mL) was measured by standard 51Cr-release assay. Mean and SD of triplicate measurements are shown. Spontaneous51Cr-release was 18.2%.
AML indicates acute myloid leukemia; IgG, immunoglobulin G; PHA, phytohemagglutinin; 51Cr, 51chromium.
CD8 IgG-pretreated versus B7-1 IgG-pretreated stimulator cells compared by Wilcoxon test for unpaired rank sums.
Discussion
Our results indicate that human AML blasts generally do not express the costimulatory molecule B7-1 on their cell membrane. This might represent an important mechanism of immune escape in AML. Consistent with this, Cardoso et al11 reported that human B7 (pre-B) acute lymphoblastic leukemia cells can induce T-cell anergy to alloantigen, which can be prevented by up-regulation of B7 expression through stimulation via the CD40 pathway. The expression of B7-2 was found to be substantially higher than that of B7-1, confirming results of a previous study.12 Furthermore, it is notable that AML blasts of FAB types M4 and M5 expressed significantly more B7-2 than blasts of other FAB types. We suggest that this reflects the biology of their nonmalignant cellular counterparts, ie, monocytes, which express B7-2 constitutively.13 However, several lines of evidence suggest that B7-2 does not play a major role in costimulating antileukemic T-cell immunity in AML: (1) In our study, the B7-1−B7-2+ blasts of patient no. 39 (AML-M5a) were capable only of costimulating autologous T cells after targeting of B7-1 IgG to their cell membrane (Figure 5B). (2) One recent study even reported a worse clinical outcome for AML patients whose leukemic blasts were B7-2+.14 (3) Finally, in most murine tumor models including AML, B7-1 was more effective than B7-2 in costimulating antitumor T-cell responses.9
The underlying mechanism for the superiority of B7-1 over B7-2, as far as costimulated tumor immunity is concerned, might be related to the more efficient generation of cytotoxic T lymphocytes by B7-1.15 In addition, costimulation of T cells by B7-1 has been shown to result in a higher activity of factors important for transcriptional regulation of the IL-2 gene, such as CD28RE, AP-1, and nuclear factor κB, thereby leading to a more sustained IL-2 production than B7-2 costimulation.16 An interesting possibility might be that B7-2 expressed on tumor cells preferentially triggers an inhibitory signal through CTLA-4, as has been demonstrated in a murine model of T-cell lymphoma.17
On the basis of these data, we used a human B7-1 IgG fusion protein to enhance the costimulatory activity of AML blasts. Our experiments show that an intact B7-1 IgG molecule with both a biologically active Fc and a biologically active B7-1 portion is obtained after transient expression in mammalian cells. The Fc portion of the fusion protein was capable of binding to a plastic surface, as demonstrated by ELISA. The B7-1 portion revealed potent costimulatory activity for the proliferation of both resting and preactivated T cells obtained from different donors. This costimulatory capacity of B7-1 IgG in plastic-bound form is similar to that reported in another study.18 As a new approach, we were able to show that B7-1 IgG as an example of a human fusion protein consisting of the Fc portion of IgG can be targeted to the cell membrane of leukemic cells. The binding of B7-1 IgG was dose-dependent, correlated with the expression of CD64, and was inhibited by a mAb that blocks the IgG-binding region of CD64. Hence, these data provide evidence that the targeting is mediated by the interaction of B7-1 IgG with high-affinity FcγRI expressed on the cell membrane of leukemic cells. We reasoned that CD64 as a target molecule on human AML blasts has several potential advantages: (1) FcγRI is the only Fc receptor capable of binding human monomeric IgG with high-affinity (ka = 108 to 109M−1).19 This allows efficient and specific binding of B7-1 IgG to CD64+ AML blasts. (2) As a specific myeloid antigen, CD64 is expressed on all AML cases at levels sufficient for recognition by cytotoxic T cells, as we previously demonstrated in anti-CD3–redirected cytotoxicity assays with AML blasts as target cells.20 (3) In nonmalignant cells, the expression of CD64 is restricted to commited myeloid progenitor cells and monocytes/macrophages.21
Thus, the B7-1 IgG fusion protein should selectively target CD64+ AML blasts while sparing nonmalignant CD34+ hematopoietic stem cells with long-term repopulating potential. In support of this hypothesis, we have shown in a previous study that AML blasts targeted with a CD3 monoclonal antibody (which binds to CD64 like B7-1 IgG) are preferentially lysed by autologous cytotoxic T cells compared with nonmalignant hematopoietic stem cells.20 In summary, CD64 as a target molecule in AML combines the advantage of being expressed in all AML cases at levels sufficient for recognition by cytotoxic T cells with a very restricted pattern of expression in normal cells.
Importantly, the binding of B7-1 IgG to CD64 resulted in an improved costimulatory activity of the leukemic cells for the proliferation of T cells. We could provide this proof of concept using the leukemic cell line U937 and freshly isolated human AML blasts in an allogeneic and autologous sytem, respectively. Therefore, targeting of B7-1 IgG to the cell membrane of AML blasts restores their costimulatory activity for autologous remission T cells by correcting their deficient expression of B7-1. The challenge in the development of immunotherapy for AML is to design strategies that induce both proliferative and cytotoxic autologous T-cell responses that eliminate residual tumor cells, thereby preventing relapse of the disease. So far, this could not be accomplished by stimulating T cells with leukemia-specific antigens. We provide evidence that costimulation mediated by B7-1 IgG targeted to AML blasts, apart from causing T-cell proliferation, increases differentiation of autologous T cells into cytotoxic effector cells. However, we could only demonstrate this effect of B7-1 IgG when the remission T cells were preactivated with PHA. Several possible explanations could account for this observation. First, AML cells may produce immunosuppressive factors, such as IL-10 or transforming growth factor-β, that interfere with T-cell activation and T-cell effector function. Second, the pool of CD4+CD45RA+ T cells in tumor patients is depleted after previous chemotherapy and can take up to 2 years to recover.22 Finally, T cells of patients with AML, even while in complete remission, show abnormalities of their TCR/CD3 signal transduction pathway, eg, reduced expression of the ζ-chain of the CD3 complex.23 It is known that preactivated T cells have less stringent requirements for full activation compared with naive T cells, especially as far as the costimulatory signal 2 is concerned.24 This may explain our finding that autologous remission T cells are capable of responding to costimulation by B7-1–targeted AML blasts only after preactivation with PHA.
In conclusion, B7-1 IgG fusion protein targeting of tumor cells is a new approach with the following features: (1) correction of a molecular deficit (ie, B7-1[CD80] expression and costimulatory activity) possibly contributing to the immune escape of AML cells by a recombinant fusion protein (ie, B7-1[CD80] IgG) targeted to the tumor membrane; (2) use of CD64 as a target molecule on AML blasts for immunoglobulin fusion proteins containing an Fc portion; and (3) local enrichment of molecules important for T-cell activation close to the tumor cell by a membrane-bound recombinant fusion protein (ie, B7-1[CD80] IgG) instead of systemic delivery of the soluble form of the same protein. Other approaches involving manipulation of the costimulatory B7-CD28 pathway to increase the immunogenicity of AML blasts include gene transfection25,26 and induction of B7-molecules by cytokines.27 The B7-1 targeting described here, in contrast, is technically simple without requiring extensive in vitro manipulation of the leukemic cells. This could be of value in future immunotherapeutic interventions in AML.
We thank Alexandra Bittroff-Leben and Gudrun Bochert for technical assistance.
Supported by the Deutsche Krebshilfe (W65/94/No2) and the Sonderforschungsbereich 506 (C4/R5).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael Notter, Department of Hematology, Oncology and Transfusion Medicine, Freie Universität Berlin, Universitätsklinikum Benjamin Franklin, Hindenburgdamm 30, 12200 Berlin, Germany.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal