Abstract
Pericellular proteolysis plays an important role in cell migration and the formation of new capillary structures. The plasminogen activator/plasmin and matrix degrading metalloproteinase (MMP) cascades act together in the remodeling of matrix and cell-matrix contacts. Previously we have shown that the formation of capillary structures by human foreskin microvascular endothelial cells (hMVECs) in a 3-dimensional fibrin matrix requires a functional urokinase-type plasminogen activator receptor (u-PAR). Here we report on the unexpected finding that inhibition of hMVEC-derived MMP activity by BB94 (batimastat) increased the outgrowth of capillary structures in a fibrin matrix. BB94 prevented the release of the u-PA binding domain D1 of u-PAR and thereby increased the number of functional u-PARs on hMVECs without affecting the u-PAR messenger RNA levels. Comparison of various types of protease inhibitors pointed to the prime involvement of MMP activity. Using recombinant MMPs it was shown that MMP-12 activity was able to release the D1 domain of cellularly expressed u-PAR. In addition, the expression of MMP-12 in control and basic fibroblast growth factor/tumor necrosis factor-α–stimulated hMVECs was shown by reverse transcriptase–polymerase chain reaction, suggesting that endothelial cell–derived MMP-12 may be involved in angiogenesis-related u-PAR shedding. This new mechanism of u-PAR cleavage provides new insights into the mutual interactions between the MMP and u-PA/plasmin systems. Moreover, it may be helpful in the interpretation of recent data on the use of specific MMP inhibitors in the treatment of several types of cancer.
Introduction
The urokinase-type plasminogen activator (u-PA) and its cellular receptor (u-PAR, CD87) are involved in cell migration such as occurs during tumor invasion and angiogenesis.1 The u-PAR regulates the u-PA activity on the cell surface not only by mediating the localization of u-PA and the internalization of u-PA/inhibitor complexes but also by an increase in the efficiency of plasminogen activation by u-PAR–bound u-PA.2 Pro–u-PA bound to the receptor can be converted into the protease u-PA and subsequently be inhibited by plasminogen activator inhibitor type 1 (PAI-1). The u-PA:PAI-1 complex is subsequently internalized and degraded in the lysosomes, while the u-PAR is recycled to the cell surface. Besides a role in controlling u-PA activity, u-PAR is involved in activating cell signaling pathways, including diacylglycerol formation, activation of a serine kinase, focal adhesion kinase, tyrosine kinase(s), and the activation of the Janus kinase/signal transducer and activator of transcription pathway. Furthermore, u-PAR enforces cellular interaction with the extracellular matrix, in particular by binding to the matrix protein vitronectin and to integrins. Recently, u-PAR has been shown to possess chemokine-like activity mediating chemotaxis of monocytes, neutrophils, and smooth muscle cells.3 4
The u-PAR is a glycosyl phosphatidylinositol (GPI)-anchored receptor that contains 3 homologous domains. The amino-terminal domain (D1) is involved in both the binding of u-PA5,6 and the interaction with vitronectin,7,8 but the other 2 domains of u-PAR are also indispensable for high-affinity interactions.9,10 The GPI anchor of u-PAR can be cleaved by GPI-specific phospholipases, resulting in a soluble form of u-PAR (su-PAR). Recently, Wilhelm et al showed that a cellular phospholipase D might be involved in the release of su-PAR.11 In addition to the shedding of the full-size u-PAR, the finding of truncated D2D3 fragments of u-PAR on the cell membrane implies that D1 domains can also be cleaved directly from u-PAR. Cleavage of u-PAR between domains D1 and D2 can be mediated by proteolytic activity of u-PA, directly or indirectly via the plasminogen activation/plasmin12,13 or by other proteases such as chymotrypsin and elastase.5,14 A similar region has not been observed between domains D2 and D3.15
The soluble form of u-PAR has been found in vitro in culture supernatants of tumor cell lines,12,16 in vivo in plasma and serum,17-20 in uterine tissue cultures from women with endometriosis,21 and in ascitic and cystic fluid from ovarian cancer patients.22,23 Recently, Chavakis et al24 reported that normal human vascular cells (smooth muscle cells and endothelial cells) also release su-PAR in the medium, especially after stimulation with phorbol myristate acetate (PMA).
Numerous studies have shown the involvement of u-PAR in the migration and tissue infiltration of cells. The u-PAR undoubtedly plays an important role in the migration and invasion of monocytes,25 smooth muscle cells,26 and tumor cells.27-30 While a number of studies indicate that u-PAR is required for angiogenesis in pathological conditions,31,32 it does seem to be dispensable for angiogenesis during development in u-PAR−/−mice33 and wound healing.34 Previously we have provided evidence for the involvement of u-PAR in angiogenesis in the formation of capillary-like structures by human foreskin microvascular endothelial cells (hMVECs) in a 3-dimensional fibrin matrix in vitro.35-37 During the investigation of the role of metalloproteinases (MMPs) in this model, we observed unexpectedly that the inhibition of MMP activity enhanced the angiogenic response in this assay (see Figure 1). This was surprising because several studies have shown that inhibition of MMPs in general leads to the blockage of cell migration,38-40angiogenesis,41,42 and inhibition of tumor growth in vivo.43 44
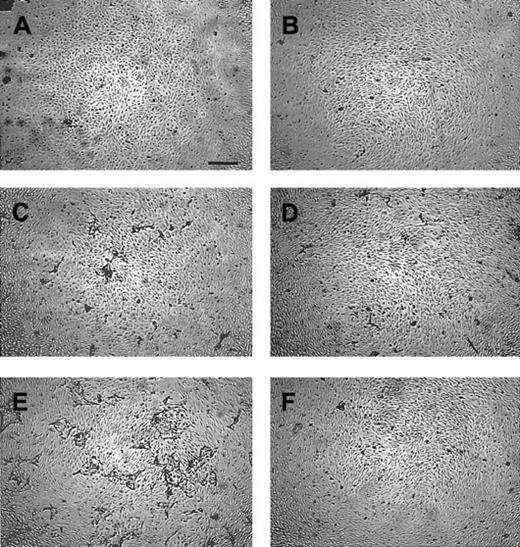
Effect of the MMP inhibitors BB94 and BB3103 on the induction of capillary-like tube formation by hMVECs in 3-dimentional fibrin matrices.
The hMVECs were cultured on top of a 3-dimensional fibrin matrix in M199 supplemented with 10% human serum and 10% NBCS and stimulated without (A,B) or with the combination of 10 ng/mL bFGF and 10 ng/mL TNF-α (C-F) in the absence (C,D) or the presence of 5 μg/mL BB94 (E,F). Simultaneous addition of 30 μg/mL neutralizing polyclonal anti–u-PA antibodies (B,D,F) inhibited tube formation by 87%. The nonphase contrast photos were taken after 7 days of culture (bar represents 500 μm) and are representative of 3 independent experiments.
Effect of the MMP inhibitors BB94 and BB3103 on the induction of capillary-like tube formation by hMVECs in 3-dimentional fibrin matrices.
The hMVECs were cultured on top of a 3-dimensional fibrin matrix in M199 supplemented with 10% human serum and 10% NBCS and stimulated without (A,B) or with the combination of 10 ng/mL bFGF and 10 ng/mL TNF-α (C-F) in the absence (C,D) or the presence of 5 μg/mL BB94 (E,F). Simultaneous addition of 30 μg/mL neutralizing polyclonal anti–u-PA antibodies (B,D,F) inhibited tube formation by 87%. The nonphase contrast photos were taken after 7 days of culture (bar represents 500 μm) and are representative of 3 independent experiments.
In the present report we show that MMP-12 is able to cleave the amino terminal domain 1 of u-PAR (D1) from the full-size receptor when hMVECs are stimulated either with a combination of basic fibroblast growth factor (bFGF) and tumor necrosis factor-α (TNF-α) or with PMA. Inhibition of the MMP-12 activity by broad-acting MMP inhibitors decreased the release of domain D1 of the receptor significantly and consequently increased the u-PA binding capacity of the hMVECs. This increase of functional u-PAR resulted in an enhanced bFGF/TNF-α–induced angiogenesis in 3-dimensional fibrin matrices in vitro.
Materials and methods
Materials
Penicillin/streptomycin, l-glutamine, and medium 199 (M199) supplemented with 20 mM HEPES were obtained from Biowhittaker (Verviers, Belgium); RPMI 1640 medium, newborn calf serum (NBCS), and fetal calf serum were obtained from Life Technologies (Grand Island, NY). Tissue culture plastics were from Costar (Cambridge, MA). A crude preparation of endothelial cell growth factor was prepared from bovine hypothalamus. Human serum was obtained from a local blood bank and was prepared from freshly obtained blood from 10 to 20 healthy donors, pooled, and stored at 4°C. Trypsin was purchased from Life Technologies; heparin and thrombin from Leo Pharmaceutic Products (Weesp, The Netherlands); and human fibrinogen from Chromogenix (Mölndal, Sweden). Factor XIII was generously provided by Dr H. Metzner and Dr G. Seeman (Aventis Behring, Marburg, Germany). The bFGF was purchased from Pepro Tech (London, England), and human recombinant TNF-α was a gift from Dr J. Travernier (Biogent, Gent, Belgium) and contained 2.45 × 107 U/mg protein and less than 40 ng lipopolysaccharide per millogram protein. Aprotinin (Trasylol) was purchased from Pentapharm (Basel, Switzerland). BB94 (batimastat, mw = 478) and BB3103 (mw = 476) were gifts from Dr E. A. Bone (British Biotech, Oxford, United Kingdom) and RS-113456-000 (RS-11, mw = 426) and RS-162813-008 (RS-16, mw = 385) from Dr R. Martin (Roche Bioscience, Palo Alto, CA). The rabbit polyclonal antibodies specific for u-PA were prepared in our laboratory.45Recombinant MMP-1, MMP-3, and MMP-9 were gifts from Dr G. Murphy (University of East Anglia, Norwich, United Kingdom); MMP-2 and MMP-13 were from Dr I. Otterness (Pfizer Central Research, Groton, CT); MMP-12 46 was from Dr S. Matsumoto (Yamanouchi Pharmaceutical, Ibaraki, Japan); MMP-14 was from Dr H. Will (Invitek, Berlin, Germany); and tumor necrosis factor–converting enzyme (TACE) was from Dr R. Black (Immunex, Seattle, WA).
Other materials used in the methods described below have been specified in detail in the related references, in the text, or were purchased from standard commercial sources.
Complementary DNA probes
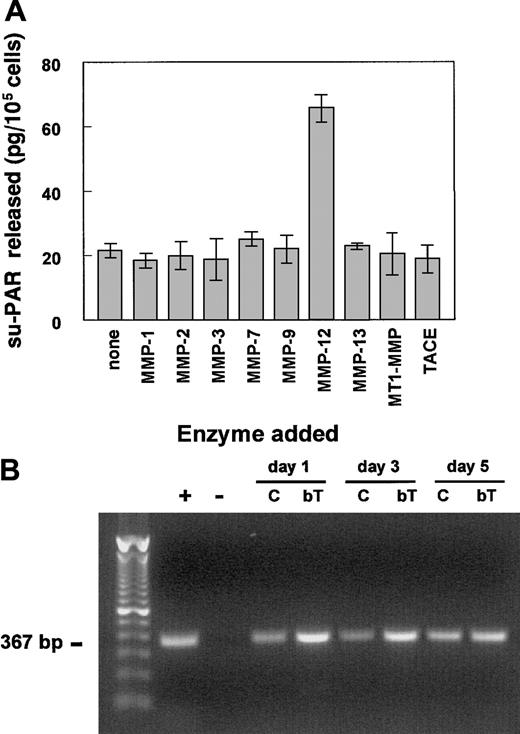
The following complementary DNA (cDNA) fragments were used as probes in the hybridization experiments: a 585–base pair (bp)BamHI fragment of the human u-PAR cDNA,47 a 1023-bp fragment of the human u-PA cDNA (a kind gift from Dr W.-D. Schleuning, Schering, Berlin, Germany),48 and a 1200-bpPstI fragment of hamster actin cDNA. For the reverse transcriptase–polymerase chain reaction (RT-PCR) experiments, the following MMP-12 specific primers (5′-3′) were used: CCACTGCTTCTGGAGCTCTT (sense) and GCGTAGTCAACATCCTCACG (antisense), resulting in a 367-bp product.
Cell culture
The hMVECs, isolated and characterized as previously described,49 50 were cultured on gelatin-coated dishes in M199 supplemented with 20 mM HEPES (pH 7.3), 10% human serum, 10% heat-inactivated NBCS, 150 μg/mL crude endothelial cell growth factor, 2 mM l-glutamine, 5 U/mL heparin, 100 IU/mL penicillin, and 100 μg/mL streptomycin at 37°C under 5% CO2/95% air atmosphere.
The messenger RNA (mRNA), binding, and su-PAR release experiments were performed after the hMVECs reached confluency (approximately 0.7 × 105 cells/cm2) and had subsequently been cultured in M199 supplemented with 20 mM HEPES (pH 7.3), 10% human serum, 2 mM l-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin for at least 24 hours. During the experiments the hMVECs were cultured in M199 supplemented with 20 mM HEPES (pH 7.3), 10% human serum, 2 mM l-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin with or without the additions indicated. For the Western blot experiments, hMVEC cell lysates were prepared. Briefly, hMVECs were washed twice with ice-cold phosphate-buffered saline, and the cells were extracted by the addition of 0.5% Triton X-100 in phosphate-buffered saline (100 μL/0.7 × 106 cells). All the supernatants and the cell extracts were stored at −20°C before use.
Determination of specific u-PA binding
Diisopropylfluorophosphate-treated u-PA (Serono, Aubonne, Switzerland) (DIP–u-PA) was labeled with Na-125I by using the Iodogen procedure (Pierce Chemical, Rockford, IL). Binding of 125I–DIP–u-PA to hMVECs was determined at 0°C as described in detail by Kroon et al.37 Specific binding was calculated by subtraction of nonspecific binding (binding in the presence of a 50-fold excess of u-PA) from the total binding.
Su-PAR fragment release induced by recombinant MMPs
For the su-PAR fragment release experiments using the recombinant MMPs, confluent hMVEC cultures were stimulated with the combination of bFGF (50 ng/mL) and vascular endothelial growth factor (VEGF)-A (25 ng/mL) for 18 hours to increase u-PAR expression, washed 3 times with M199 medium supplemented with 0.01% human serum albumin (HSA), and finally incubated with active recombinant MMP (100 ng/mL) for 2 to 4 hours. The cells were then cooled on ice, and the amount of released su-PAR fragments was determined using a su-PAR enzyme-linked immunosorbent assay (ELISA).
The u-PA and su-PAR ELISAs
Antigen levels of u-PA and su-PAR in cell culture media were measured using commercially available immunoassay kits: u-PA EIA HS (Taurus, Leiden, The Netherlands) and UR-1 (Monozyme, Hørsholm, Denmark). The configuration of the latter, using 3 mAbs determining different epitopes of the su-PAR, enables this ELISA to detect the full-length su-PAR (D1D2D3) and the truncated su-PAR fragments D2D3 and D1, although the detection of domain D1 is less efficient.18 The combination of the full-length su-PAR and the truncated su-PAR fragments D2D3 and D1 are referred to in the text as total su-PAR.
Assay of angiogenesis in vitro
Human fibrin matrices were prepared by the addition of 0.1 U/mL thrombin to a mixture of 2.5 U/mL factor XIII (final concentrations), 2 mg/mL fibrinogen, 2 mg/mL sodium citrate, 0.8 mg/mL NaCl, and 3 μg/mL plasminogen in M199 medium; 300 μL aliquots of this mixture were added to the wells of 48-well plates. After clotting at room temperature, the fibrin matrices were soaked with M199 supplemented with 10% (vol/vol) human serum and 10% (vol/vol) NBCS for 2 hours at 37°C to inactivate the thrombin. Highly confluent hMVECs (0.7 × 105 cells/cm2) were detached and seeded in a 1.25:1 split ratio on the fibrin matrices and cultured for 24 hours in M199 medium supplemented with 10% human serum, 10% NBCS, and penicillin/streptomycin. Then, the hMVECs were stimulated with the mediators and/or inhibitors for the indicated time. Fresh medium, containing the mediators and/or inhibitors, was added every second day, and the culture medium was collected. Invading cells and the formation of tubular structures of hMVECs in the 3-dimensional fibrin matrix were analyzed by phase contrast microscopy. The length of the tubelike structures was determined using an Olympus CK2 microscope equipped with a monochrome CCD camera (MX5) connected to a computer with Optimas image analysis software. Six fixed microscopic fields (7.3 mm2 per field) per well were (clockwise) analyzed and used to calculate the total length of the tubelike structures, which is expressed as mm/cm2.
RNA isolation, Northern blots, and RT-PCR
The mRNA experiments were performed after the hMVECs reached confluency and had subsequently been cultured in M199 supplemented with 20 mM HEPES (pH 7.3), 10% human serum, 2 mM l-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin for at least 24 hours. The hMVECs were then stimulated with or without (control) bFGF/TNF-α in the presence of vehicle (0.1% dimethyl sulfoxide [DMSO]) or 10 μg/mL BB94 in 0.1% DMSO for 8 hours, and total mRNA was isolated. Parallel cultures were incubated for 48 hours or 2 × 48 hours with the above-mentioned stimuli, respectively, and restimulated for another 8-hour period before mRNA was isolated. The Northern blot analysis was performed as described previously.51 The MMP-12–specific RT-PCR was performed as described in detail by Konttinen et al.52
In vitro MMP assays
The Ki values of the used MMP inhibitors were determined using MMP activity assays in the presence of the MMP inhibitors. These assays were performed with recombinant enzymes according to the method of Knight.53 The only minor modification was the buffer used: 50 mM Tris-HCl (pH 7.5), 200 mM NaCl, 10 mM CaCl2, and 0.005% Brij 35. This method employs a fluorogenic peptide substrate whose cleaved product is monitored by a PerkinElmer LS50B spectrometer.
Cell-based TACE activity assay
Human MonoMac 6 cells, at a density of 1 × 105cells/mL, were cultured at 37°C in RPMI 1640 medium supplemented with 10% fetal calf serum. The cells were incubated with varied concentrations of MMP inhibitor in DMSO for 15 minutes prior to the addition of lipopolysaccharide and PMA. Final concentrations were 10 ng/mL lipopolysaccharide, 30 ng/mL PMA, and 0.1% DMSO. The cells were then incubated for 2 hours, after which the medium was removed and analyzed for soluble TNF-α content using a TNF Quantikine Immunoassay (R&D Systems, Minneapolis, MN).
Western blot analysis of su-PAR domains
Total su-PAR in the cell culture media and cell lysates were incubated with a combination of 2 biotinylated anti–u-PAR mAbs R2 and R3, precipitated using streptavidin-coated beads as described previously.54 55 The mAbs R2 and R3 are directed against domain D3 and D1 of u-PAR, respectively. The precipitated proteins were transferred to polyvinylidene fluoride membranes (Immobilon, Millipore, Bedford, MA) and probed with rabbit polyclonal anti–u-PAR antibody. The immune complexes were subsequently visualized by incubation with a peroxidase-conjugated donkey antirabbit antibody (Amersham) and Supersignal Ultra chemoluminescent detection (Pierce Chemical).
Statistics
The data of 3 or more experiments and/or wells are expressed as the mean ± SEM and those of duplicate experiments and/or wells as the mean with the range between the error bars. Statistical analyses of the data (paired-samples t tests) were calculated using the statistic program SPSS version 8.0.
Results
Effect of the addition of MMP inhibitors on hMVEC tube formation in fibrin matrices
When hMVECs are cultured on fibrin matrices, they form capillary-like tubular structures when stimulated with the combination of bFGF and TNF-α (Figures 1C and 2A). This formation of tubular structures was accompanied by an increased u-PA accumulation in the supernatants of the tube-forming hMVECs35,36 51 (Figure 2B). To evaluate the involvement of matrix-degrading MMPs in this process, the formation of tubular structures was evaluated in the presence of the broad-spectrum MMP inhibitors BB94 (batimastat) (soluble in DMSO) or its water-soluble analog BB3103.
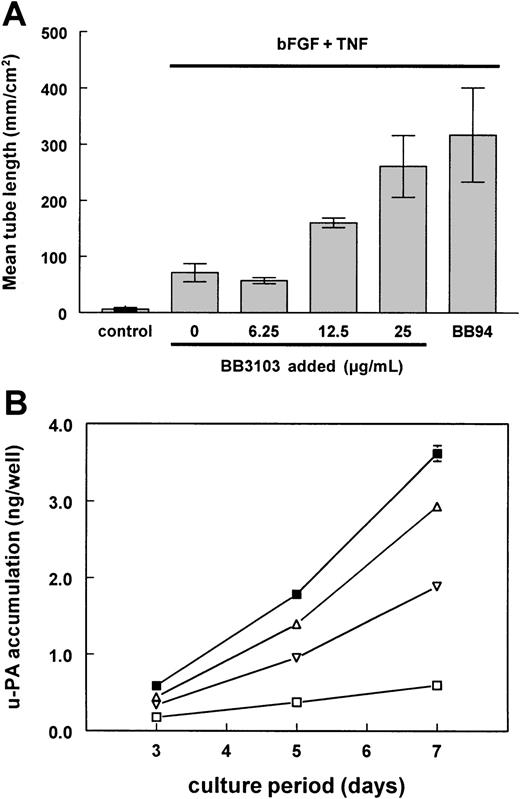
Effect of BB3103 and BB94 on bFGF/TNF-α–induced tube formation and u-PA accumulation.
The hMVECs were cultured on top of a 3-dimensional fibrin matrix in M199 supplemented with 10% human serum and 10% NBCS (control), or with bFGF (10 ng/mL) and TNF-α (10 ng/mL) in the presence of increasing amounts of BB3103 or 5 μg/mL BB94. (A) After 7 days of culture, total tube length (mm/cm2) was measured using image analysis equipment as described in “Materials and methods.” The data are expressed as the mean of duplicate wells, with the range of the 2 measurements indicated by the error bars, and are representative of 3 independent experiments, respectively. (B) Accumulation of u-PA in the supernatants of the stimulated hMVECs was measured by ELISA as described. The data of control (■), bFGF and TNF-α (▪), and bFGF/TNF-α–stimulated hMVECs in the presence of 25 μg/mL BB3103 (▵) or 5 μg/mL BB94 (▿) are expressed as the mean of duplicate wells (nanograms per well), with the range of the 2 measurements indicated by the error bars, and are representative of 3 independent experiments.
Effect of BB3103 and BB94 on bFGF/TNF-α–induced tube formation and u-PA accumulation.
The hMVECs were cultured on top of a 3-dimensional fibrin matrix in M199 supplemented with 10% human serum and 10% NBCS (control), or with bFGF (10 ng/mL) and TNF-α (10 ng/mL) in the presence of increasing amounts of BB3103 or 5 μg/mL BB94. (A) After 7 days of culture, total tube length (mm/cm2) was measured using image analysis equipment as described in “Materials and methods.” The data are expressed as the mean of duplicate wells, with the range of the 2 measurements indicated by the error bars, and are representative of 3 independent experiments, respectively. (B) Accumulation of u-PA in the supernatants of the stimulated hMVECs was measured by ELISA as described. The data of control (■), bFGF and TNF-α (▪), and bFGF/TNF-α–stimulated hMVECs in the presence of 25 μg/mL BB3103 (▵) or 5 μg/mL BB94 (▿) are expressed as the mean of duplicate wells (nanograms per well), with the range of the 2 measurements indicated by the error bars, and are representative of 3 independent experiments.
Serum- or bFGF-induced endothelial cell proliferation was not influenced by the addition of BB94, whereas BB94 slightly inhibited the serum- and bFGF-induced 2-dimensional migration of human endothelial cells into a gelatin-coated denuded area in an endothelial cell monolayer (data not shown). Surprisingly, however, an enhanced tube formation by the bFGF/TNF-α–stimulated hMVECs was observed when MMP activity was inhibited (compare Figures 1C and 1E). This enhancement of tube formation was dose-dependent for both BB3103 (Figure 2A) and BB94 (data not shown). Determination of the u-PA accumulation in the supernatants of the tube-forming hMVECs revealed that both MMP inhibitors (BB94 and BB3103) reduced the amount of u-PA antigen accumulation in the supernatants of bFGF/TNF-α–stimulated hMVECs, by 57% and 23%, respectively (Figure 2B).
Addition of the plasmin inhibitor Trasylol (data not shown) or a neutralizing polyclonal anti–u-PA antibody completely inhibited the bFGF/TNF-α–induced tube formation in the absence and in the presence of the MMP inhibitors (Figure 1D,F), indicating that the BB94-induced enhancement of tube formation was mediated via the u-PA/plasmin system.
Effect of BB94 treatment on u-PA and u-PAR expression
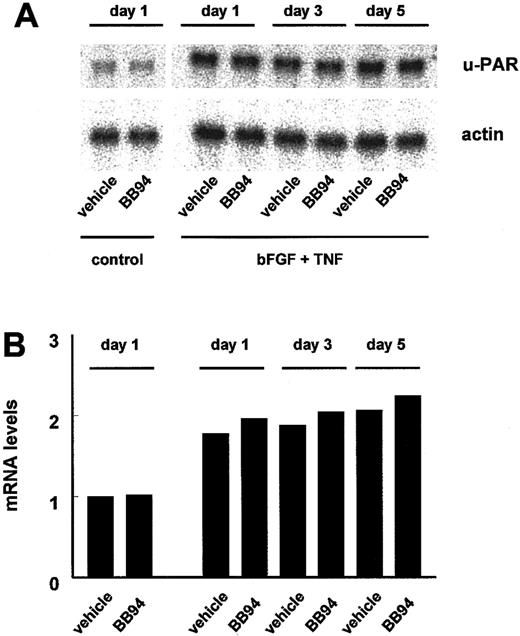
The amount of u-PA accumulation in the supernatants of cultured cells is the result of the u-PA production and the internalization of the u-PA:PAI-1 complex via the u-PAR. The effect of prolonged BB94 treatment on the bFGF/TNF-α–induced mRNA expression of u-PA and the u-PAR was investigated in hMVECs cultured on gelatin-coated culture dishes. BB94 did not influence the bFGF/TNF-α–stimulated mRNA levels of the u-PA (data not shown) and u-PAR (Figure3) during this incubation period. However, the total accumulation of u-PA antigen in the supernatants during the 5-day culture period was considerably reduced (1.7 ± 0.4 ng/well vs 5.0 ± 0.6 ng/well in BB94-treated and control cells, respectively) as previously shown in the in vitro angiogenesis experiments (Figure 2B).
Expression of u-PAR mRNA in hMVECs after BB94 treatment.
Confluent hMVECs cultured on gelatin-coated wells were preincubated without (control) or with bFGF (10 ng/mL) and TNF-α (10 ng/mL) in the presence of vehicle (0.1% DMSO) or 10 μg/mL BB94 and 0.1% DMSO for the indicated period. Then the hMVECs were restimulated, and total RNA was isolated at 8 hours and analyzed by Northern blotting for u-PAR mRNA (A). Equal loading was checked by hybridization with an actin probe. Signals for u-PAR were quantified by densimetry and adjusted for the corresponding actin signal (B). The amount of u-PAR mRNA present at the different times is expressed relative to that found under control conditions. Two independent experiments gave similar results.
Expression of u-PAR mRNA in hMVECs after BB94 treatment.
Confluent hMVECs cultured on gelatin-coated wells were preincubated without (control) or with bFGF (10 ng/mL) and TNF-α (10 ng/mL) in the presence of vehicle (0.1% DMSO) or 10 μg/mL BB94 and 0.1% DMSO for the indicated period. Then the hMVECs were restimulated, and total RNA was isolated at 8 hours and analyzed by Northern blotting for u-PAR mRNA (A). Equal loading was checked by hybridization with an actin probe. Signals for u-PAR were quantified by densimetry and adjusted for the corresponding actin signal (B). The amount of u-PAR mRNA present at the different times is expressed relative to that found under control conditions. Two independent experiments gave similar results.
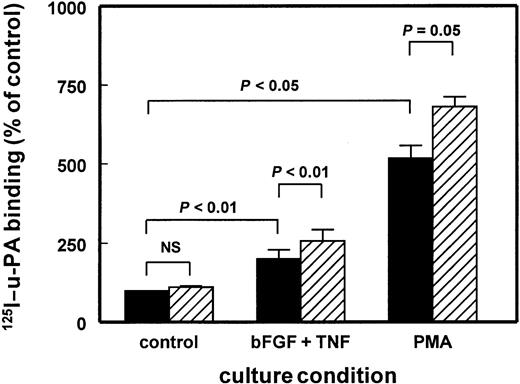
Functional u-PAR molecules on the hMVECs were evaluated by assaying the binding of 125I–DIP–u-PA. Incubation of control-, bFGF/TNF-α–, or PMA-stimulated hMVECs with BB94 for 24 hours did not show a significant effect of the MMP inhibitor on the125I–DIP–u-PA binding (data not shown). However, when the hMVECs were incubated with BB94 for 72 hours, the binding capacity of control and bFGF/TNF-α–stimulated cells for125I–DIP–u-PA was increased to 111% ± 7% (n = 5, ns) and 128% ± 6% (n = 5, P < .01), respectively (Figure 4). The effect of BB94 on hMVECs incubated with 10 nM/L PMA was similar. The binding of125I–DIP–u-PA increased to 132% ± 17% (n = 4,P = .05) (Figure 4).
Increased u-PA binding to hMVECs after BB94 treatment.
The hMVECs were preincubated for 72 hours in M199 supplemented with 10% human serum with or without PMA (10−8 M) or the combination of bFGF (10 ng/mL) and TNF-α (10 ng/mL) in the presence of vehicle (0.1% DMSO) (▪) or 10 μg/mL BB94 (▨). Subsequently, the cells were cooled on ice and the specific binding of 125I–DIP–u-PA to hMVECs was determined in triplicate wells as described in “Materials and methods.” The data represent mean ± SEM of 4 (PMA) or 5 (bFGF/TNF-α) independent experiments.
Increased u-PA binding to hMVECs after BB94 treatment.
The hMVECs were preincubated for 72 hours in M199 supplemented with 10% human serum with or without PMA (10−8 M) or the combination of bFGF (10 ng/mL) and TNF-α (10 ng/mL) in the presence of vehicle (0.1% DMSO) (▪) or 10 μg/mL BB94 (▨). Subsequently, the cells were cooled on ice and the specific binding of 125I–DIP–u-PA to hMVECs was determined in triplicate wells as described in “Materials and methods.” The data represent mean ± SEM of 4 (PMA) or 5 (bFGF/TNF-α) independent experiments.
BB94 reduces the release of total su-PAR by hMVECs
The fact that the mRNA levels of u-PAR were not affected by the treatment of BB94 and that there was an increase in functional u-PAR on the cell surface of the endothelial cells prompted us to determine the amount of soluble u-PAR (full-length su-PAR and the soluble domains D2D3 and D1) in the supernatants of these cells by ELISA. In the supernatants of bFGF/TNF-α–stimulated hMVECs, an increase in the release of total su-PAR was observed, starting after a 48-hour culture period and reaching significance after 72 hours of incubation, as shown of a representative experiment in Figure5A.
Time course of su-PAR accumulation in hMVEC supernatant.
(A) The hMVECs were stimulated with or without the combination of bFGF (10 ng/mL) and TNF-α (10 ng/mL) in the presence of vehicle (0.1% DMSO) or BB94 (10 μg/mL) for 8, 24, 48, and 72 hours. At the indicated times, supernatant was collected and total su-PAR was determined by ELISA. The data of one representative time course experiment are expressed as mean accumulative su-PAR ± SEM of triplicate wells (in nanograms per well). (B) The hMVECs were stimulated with or without the combination of bFGF (10 ng/mL) and TNF-α (10 ng/mL), or PMA (10−8 M) in the presence of vehicle (0.1% DMSO) or BB94 (10 μg/mL) for 72 hours. Supernatant was collected, and total su-PAR was determined by ELISA. Data of 5 independent experiments (mean ± SEM) are shown and expressed as nanograms per milliliter of su-PAR accumulation. (C) The hMVECs were stimulated with or without the combination of bFGF (10 ng/ml) and TNF-α (10 ng/mL) in the presence of hirudin (5 U/mL), Trasylol (100 U/ml), pepstatin A (100 μM), leupeptin (100 μM), or BB94 (10 μg/ml) for 9 days. At days 3, 6, and 9, the supernatants were collected, the cells refreshed with the same stimulators, and the su-PAR determined by ELISA. The data are expressed as mean ± SEM of triplicate wells (in nanograms per well) and are representative of 2 independent experiments.
Time course of su-PAR accumulation in hMVEC supernatant.
(A) The hMVECs were stimulated with or without the combination of bFGF (10 ng/mL) and TNF-α (10 ng/mL) in the presence of vehicle (0.1% DMSO) or BB94 (10 μg/mL) for 8, 24, 48, and 72 hours. At the indicated times, supernatant was collected and total su-PAR was determined by ELISA. The data of one representative time course experiment are expressed as mean accumulative su-PAR ± SEM of triplicate wells (in nanograms per well). (B) The hMVECs were stimulated with or without the combination of bFGF (10 ng/mL) and TNF-α (10 ng/mL), or PMA (10−8 M) in the presence of vehicle (0.1% DMSO) or BB94 (10 μg/mL) for 72 hours. Supernatant was collected, and total su-PAR was determined by ELISA. Data of 5 independent experiments (mean ± SEM) are shown and expressed as nanograms per milliliter of su-PAR accumulation. (C) The hMVECs were stimulated with or without the combination of bFGF (10 ng/ml) and TNF-α (10 ng/mL) in the presence of hirudin (5 U/mL), Trasylol (100 U/ml), pepstatin A (100 μM), leupeptin (100 μM), or BB94 (10 μg/ml) for 9 days. At days 3, 6, and 9, the supernatants were collected, the cells refreshed with the same stimulators, and the su-PAR determined by ELISA. The data are expressed as mean ± SEM of triplicate wells (in nanograms per well) and are representative of 2 independent experiments.
After a 72-hour incubation in the presence of BB94, the mean total su-PAR levels decreased by 41.0% ± 2.5% (from 0.37 ± 0.10 ng/mL to 0.21 ± 0.05 ng/mL, 5 independent experiments,P < .05) in the supernatants of control cells and by 35.5% ± 1.8% (from 0.76 ± 0.22 ng/mL to 0.51 ± 0.16 ng/mL, 7 independent experiments, P < .01) in bFGF/TNF-α–stimulated cell supernatants (Figure 5B). During the same period, the total su-PAR levels decreased by 48.3% ± 8.0% (from 9.0 ± 2.5 ng/mL to 3.4 ± 0.9 ng/ml, 5 independent experiments,P = .05) in the supernatants of PMA-stimulated cells (Figure 5B). In addition, inhibitors of aspartic or serine proteases had no significant effect on the release of su-PAR by hMVECs (Figure5C). These data suggest that MMP or related MMP-like activity is involved in the release of u-PAR or u-PAR fragments from cultured hMVECs. Of note, the inhibition of total su-PAR release by MMP inhibitor–treated cells was never blocked completely.
Release of su-PAR from hMVECs by recombinant MMP-12
To further investigate which MMP(s) were responsible for the release of the su-PAR, hMVECs were stimulated with the combination of VEGF-A and bFGF to increase u-PAR expression. The cells were then washed and incubated with various activated recombinant MMPs for 2 hours, and subsequently the release of su-PAR in the supernatants was measured by ELISA. Only MMP-12 was capable of releasing su-PAR fragments (Figure 6A). None of the other tested MMPs, including MMP-1, MMP-2, MMP-3, MMP-7, MMP-8, MMP-9, MMP-13, MMP-14 (or MT1-MMP), or TACE were able to release significant levels of su-PAR from the surface of hMVECs. The MMP-12–induced release of su-PAR fragments was completely inhibited by the addition of 1 μg/mL BB94 (data not shown).
MMP-12 releases su-PAR fragments from and is expressed in hMVECs in vitro.
(A) Confluent hMVEC cultures, stimulated with the combination of bFGF (50 ng/mL) and VEGF-A (25 ng/mL) to increase u-PAR expression, were washed 3 times with M199 medium supplemented with 0.01% HSA and incubated with the indicated (active) recombinant MMPs (100 ng/mL in M199, 0.01% HSA) for 2 to 4 hours. The supernatants were collected, and the amount of released su-PAR fragments was determined using a su-PAR ELISA and expressed as mean ± SEM (in picograms per 105 cells) of triplicate wells. The data are representative of 3 independent experiments. (B) The mRNAs of the Northern blot experiment of Figure 3 were used for RT-PCR analyses of MMP-12 expression. PCR amplification was obtained after 40 cycles as described by Konttinen et al.52 The + indicates MMP-12+ mRNA of synovial fibroblasts; -, negative control (all reagents without template); C, control hMVECs; and bT, bFGF/TNF-α–stimulated hMVECs.
MMP-12 releases su-PAR fragments from and is expressed in hMVECs in vitro.
(A) Confluent hMVEC cultures, stimulated with the combination of bFGF (50 ng/mL) and VEGF-A (25 ng/mL) to increase u-PAR expression, were washed 3 times with M199 medium supplemented with 0.01% HSA and incubated with the indicated (active) recombinant MMPs (100 ng/mL in M199, 0.01% HSA) for 2 to 4 hours. The supernatants were collected, and the amount of released su-PAR fragments was determined using a su-PAR ELISA and expressed as mean ± SEM (in picograms per 105 cells) of triplicate wells. The data are representative of 3 independent experiments. (B) The mRNAs of the Northern blot experiment of Figure 3 were used for RT-PCR analyses of MMP-12 expression. PCR amplification was obtained after 40 cycles as described by Konttinen et al.52 The + indicates MMP-12+ mRNA of synovial fibroblasts; -, negative control (all reagents without template); C, control hMVECs; and bT, bFGF/TNF-α–stimulated hMVECs.
Because MMP-12 (or macrophage elastase) is predominantly produced by macrophages, we determined whether MMP-12 was expressed by human endothelial cells. MMP-12 expression was demonstrated by RT-PCR in nonstimulated as well as in bFGF/TNF-α–stimulated hMVECs (Figure6B). The amount of MMP-12 mRNA was increased in the bFGF/TNF-α–stimulated hMVECs (Figure 6B).
Involvement of various types of proteinases in su-PAR release
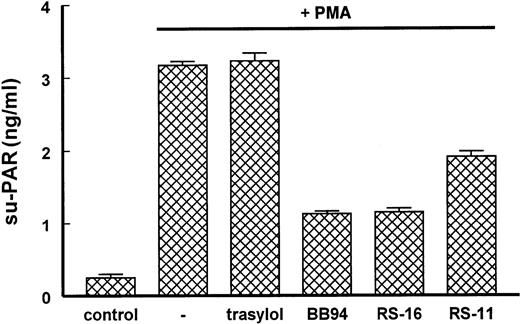
The noninvolvement of TACE activity in the su-PAR release was confirmed by the usage of MMP-specific inhibitors. The above-mentioned inhibitors BB94 and BB3103 do not only inhibit MMP activity but also TACE activity. RS-11, which is specific for MMP activity and not for TACE activity (for Ki values, see Table1), as well as RS-16 and BB94, which inhibit both MMP and TACE activities, inhibited the release of total su-PAR from PMA-stimulated hMVECs (Figure7), indicating that MMPs or a non-TACE activity largely contribute to the release of su-PAR or su-PAR fragments.
Ki values of MMP inhibitors used in this study
| . | BB94 . | RS-11 . | RS-16 . |
|---|---|---|---|
| MMP-1 | 0.4 | 70 | 110 |
| MMP-13 | 0.53 | 0.17 | 0.87 |
| MMP-2 | 0.15 | 0.054 | 0.11 |
| MMP-9 | 0.46 | 0.065 | 0.66 |
| MMP-3 | 2.3 | 5.2 | 2.6 |
| MMP-7 | 0.44 | 240 | 570 |
| MMP-12 | 0.33 | 0.033 | ND |
| TACE | 150* | > 10 000* | 94* |
| . | BB94 . | RS-11 . | RS-16 . |
|---|---|---|---|
| MMP-1 | 0.4 | 70 | 110 |
| MMP-13 | 0.53 | 0.17 | 0.87 |
| MMP-2 | 0.15 | 0.054 | 0.11 |
| MMP-9 | 0.46 | 0.065 | 0.66 |
| MMP-3 | 2.3 | 5.2 | 2.6 |
| MMP-7 | 0.44 | 240 | 570 |
| MMP-12 | 0.33 | 0.033 | ND |
| TACE | 150* | > 10 000* | 94* |
The Ki values are determined as described in “Materials and methods” and are expressed in nM.
ND indicates not determined.
Cell-based assay, not comparable with the Kivalues of the other MMPs.
Involvement of TACE activity by the release of su-PAR from hMVECs.
The hMVECs were stimulated with or without the phorbol ester PMA (10−8 M) in the presence of the MMP and TACE inhibitors BB94 (10 μg/mL), and RS-16 (10 μg/mL) or the MMP inhibitor RS-11 (10 μg/mL) for 24 hours. Supernatant was collected, and su-PAR was determined by ELISA. The data are expressed as mean ± SEM of triplicate wells (in nanograms per well) and are representative of 2 independent experiments.
Involvement of TACE activity by the release of su-PAR from hMVECs.
The hMVECs were stimulated with or without the phorbol ester PMA (10−8 M) in the presence of the MMP and TACE inhibitors BB94 (10 μg/mL), and RS-16 (10 μg/mL) or the MMP inhibitor RS-11 (10 μg/mL) for 24 hours. Supernatant was collected, and su-PAR was determined by ELISA. The data are expressed as mean ± SEM of triplicate wells (in nanograms per well) and are representative of 2 independent experiments.
Western blot analysis of su-PAR in hMVEC supernatant
The u-PAR ELISA used contains antibodies against domain D1 (mAb R3) as well as D3 (mAb R2) and in principle detects all forms of u-PAR (full-length and fragments), although probably not with the same efficiency.18 Because it had been shown recently that cleaved forms of su-PAR exist, we evaluated which forms of su-PAR were actually released by hMVECs using immunoprecipitation followed by Western blot analysis. Control cells did not release detectable amounts of su-PAR over a 48-hour incubation period. Only the 72-hour supernatant of the control cells contained enough full-size su-PAR (D1D2D3) to be precipitated and to be detected weakly by Western blot analysis after a long exposure of the blot (Figure8A, left panel).
Inhibition of the cleavage of domain D1 from domain D1D2D3 of u-PAR by BB94 in the supernatants and on the cell surface of hMVECs.
(A) The hMVECs were stimulated with or without 10−8 M phorbol ester PMA in the absence or presence of 10 μg/mL BB94 for 24, 48, or 72 hours or (B) the combination of 10 ng/mL bFGF and 10 ng/mL TNF-α in the absence or presence of 10 μg/mL BB94 for 72 hours. Supernatants were collected, and Triton X-100 cell lysates were prepared (only after 72 hours [C]). Immunoprecipitation and Western blot analysis were performed as described in “Materials and methods.” CM indicates conditioned medium.
Inhibition of the cleavage of domain D1 from domain D1D2D3 of u-PAR by BB94 in the supernatants and on the cell surface of hMVECs.
(A) The hMVECs were stimulated with or without 10−8 M phorbol ester PMA in the absence or presence of 10 μg/mL BB94 for 24, 48, or 72 hours or (B) the combination of 10 ng/mL bFGF and 10 ng/mL TNF-α in the absence or presence of 10 μg/mL BB94 for 72 hours. Supernatants were collected, and Triton X-100 cell lysates were prepared (only after 72 hours [C]). Immunoprecipitation and Western blot analysis were performed as described in “Materials and methods.” CM indicates conditioned medium.
However, in supernatant of PMA-stimulated hMVECs, full-size su-PAR was already detectable after an incubation period of 24 hours. After 48 hours of incubation, an additional su-PAR fragment was detectable, which corresponded to the soluble D2D3 form of u-PAR (Figure 8A, right panel) and could be precipitated by mAb R2 specific for D3 but not by the D1-specific mAb R3, indicating the absence of D1 in this su-PAR form.55 The appearance of the soluble D2D3 form in the supernatants of PMA-stimulated hMVECs was largely inhibited by the addition of BB94, whereas the intensity of the full-size u-PAR band was increased under these circumstances. In addition, a small band corresponding to the size of the soluble domain D1 was observed in the 72-hour supernatant of PMA-stimulated hMVECs (Figure 8A, right panel). This domain D1 was not present when the hMVECs were cultured in the presence of BB94. Evaluation of Triton X-100 extracts of hMVECs showed 2 forms of u-PAR in the extracts from control and PMA-stimulated hMVECs: full-size u-PAR (D1D2D3) and the D2D3 form but not that of the D1 fragment (Figure 8C, PMA long exposure). Incubation of the cells with BB94 changed the ratio between these 2 forms in favor of the full-size form (Figure 8C, PMA short exposure), suggesting that an MMP or MMP-like proteinase is involved in the cleavage of (s)u-PAR at a site located between the domains D1 and D2. Notably, the change in ratio between the 2 su-PAR forms was not complete, indicating that not only MMPs or MMP-like molecules are involved. When hMVECs were stimulated with bFGF and TNF-α, the release of the D2D3 form was observed in the supernatants of the long incubation periods (72 hours), which could be inhibited by the addition of BB94 (Figure 8B). The D1 fragment was never observed after stimulation of the hMVECs with bFGF and TNF-α (data not shown).
Discussion
An increasing number of plasma membrane proteins are found that have one or more soluble forms. These soluble forms are produced either by a separate biosynthetic pathway or by shedding from the plasma membrane by proteolytic or hydrolytic activity.56Different classes of proteases are involved in these shedding processes, among which are several MMPs as well as members of MMP-related protein families, such as ADAMS and TACE.57 In addition to the release of full-size proteins from the plasma membrane, MMP-like proteolytic activity is also implicated in the activation of membrane-bound proteins and in the cleavage of proteins and the subsequent release of peptide fragments with biologic activity.
Here we show that MMP-12 is able to release the u-PA-binding domain D1 of u-PAR from endothelial cells. Inhibition of the MMP-12 activity by the broad-acting inhibitors BB94 or BB3103 prevented the release of D1. This effect was accompanied by an increased u-PA binding capacity of the hMVECs and, subsequently, an enhanced capacity of these cells to form capillary-like tubular structures in a 3-dimensional fibrin matrix.
Two forms of shedding of u-PAR have been described in in vitro and in vivo studies. The first is caused by the removal of almost the entire receptor by a phospholipase D,11 which results in full-size soluble u-PAR containing the domains D1D2D3. The second reflects the removal of the D1 domain of u-PAR by u-PA, either directly or via the activation of plasminogen.12 13 In the present study we detected both forms of su-PAR in the supernatant of hMVECs and showed that the release of domain D1 was largely attributed to a novel mechanism, namely by the cleavage of MMP-12 activity (Figure9).
Schematic representation of the enzymatic pathways involved in the release of su-PAR from activated hMVECs in the absence or presence of MMP inhibitors.
(1) Indicates cleavage of D1D2D3 by phospholipase D11; (2) indicates cleavage of D1 from the u-PAR by u-PA/plasmin12 13; and (3) indicates cleavage of D1 from the u-PAR by MMP-12 activity (this paper).
Schematic representation of the enzymatic pathways involved in the release of su-PAR from activated hMVECs in the absence or presence of MMP inhibitors.
(1) Indicates cleavage of D1D2D3 by phospholipase D11; (2) indicates cleavage of D1 from the u-PAR by u-PA/plasmin12 13; and (3) indicates cleavage of D1 from the u-PAR by MMP-12 activity (this paper).
Initially, MMP-12, or macrophage metalloelastase, was described as being exclusively expressed by macrophages.58 However, the expression of MMP-12 recently was shown for other cell types, eg, synovial fibroblast (synoviocytes) and some tumor cell lines.52,59 MMP-12 is thought to be an antiangiogenic protease. Recently, Dong et al showed that MMP-12 was involved in the generation of the angiogenesis inhibitor angiostatin from plasminogen,60 and tumor cell lines transfected with MMP-12 showed a reduced capacity in growth and metastasis.61 In addition to the ability to generate angiostatin, we now provide evidence that the antiangiogenic capacity is also able to reduce the number of functional u-PARs on endothelial cells. The same may be true for tumor cells and monocytes and macrophages. An increased MMP-12 activity will decrease the number of functional u-PARs on the tumor cells. This will lead to a decrease in u-PA binding to the u-PAR on the cell surface, resulting in a decreased tumor invasion and metastasis.62
At present nothing is known about the MMP-12 cleavage site in (s)u-PAR. Furthermore, it is not known whether MMP-12 acts directly on the u-PAR or indirectly via proteolysis (and activation) of other proteins. However, the ineffectiveness of the inhibitors of aspartic and serine proteases suggests that MMP-12 acts directly on (s)u-PAR. Of note, MMP inhibition never completely blocked the release of the D1 domain (both as measured by ELISA as well as Western blot analysis). This leaves open the possibility that u-PA or elastase activity may additionally make a minor contribution to D1 cleavage in hMVECs under our experimental conditions.
Western blot analysis showed that the release of domain D1 by endothelial cells was prevented by MMP inhibition, which was particularly visible after an increase of the u-PAR level by PMA. The amount of detected soluble D1 was very low compared with the corresponding soluble D2D3 band. This is due to the lower efficacy of mAb R3 to precipitate D1-containing su-PAR forms compared with the detection by mAb R2 to precipitate D2-containing forms of the su-PAR.55 However, the increases of soluble D2D3 fragment in the supernatant and cell-bound D2D3 u-PAR in the cell extracts after stimulation indicates a considerable cleavage of the D1 fragment.
The functions of the soluble D1, D2D3, and D1D2D3 forms are not known, but it is likely that their formation affects u-PAR occupation and availability. The release of domain D1 of u-PAR may compete with cell membrane–expressed u-PAR for u-PA–mediated cellular activation.3 It also may affect cell binding to the matrix: u-PAR can act as an adhesion molecule via its interaction with vitronectin in the extracellular matrix.7,10 Because domain D1 of u-PAR is involved in this interaction,10cleavage of this domain of u-PAR will affect cell adhesion. Enhanced expression of both u-PAR and u-PA is found at the leading edge of migrating cells.63 On these sites, proteolysis of matrix proteins occurs, which is kept in balance with the formation of new cell-matrix interactions. Removal of domain D1 by a u-PA–independent mechanism may thus reduce cell-matrix interactions and thereby alter cell migration and invasion.3
Both u-PA and u-PAR play an important regulatory role in the invasion and formation of capillary-like tubular structures by microvascular endothelial cells in fibrin matrices.35,37,64 Our observation that broad-acting MMP inhibitors, such as BB94, enhance angiogenesis in a fibrin matrix is unexpected and probably caused by the increased availability of full-size u-PAR on the cell membrane. Indeed, Kroon et al37 showed that small changes in the availability of u-PAR have large effects on angiogenesis in vitro.
Because angiogenesis and growth of certain tumors can be inhibited by competitors of u-PAR, as has been shown for the amino-terminal fragment of u-PA,32,37 the effect of elevation of functional u-PAR by MMP inhibitors requires further investigation, especially in light of the possible use of MMP inhibitors in the clinical treatment of tumor patients. Especially, because the stroma of many tumors is a fibrinous exudate,65 66 protection of the u-PAR by MMP inhibition may counteract the favorable effects of these inhibitors on the cell migration and invasion of cells into this fibrinous stroma. Future studies have to elucidate whether cleavage of u-PAR indeed occurs at these sites and affects cellular behavior in vivo.
The release of the u-PA-binding domain D1 of the u-PAR by an MMP-like activity underpins the tight connection between the u-PA/plasmin and the MMP systems. Many studies showed a close immunolocalization of the 2 systems in vivo.67-69 In addition, plasmin is able to activate several MMPs, such as MMP-1, MMP-3, and MMP-9, in vitro.70-72 In this respect, the release of domain D1 of the u-PAR by an MMP activity may be relevant as feedback regulation. The decreased expression of functional u-PAR results in a decreased u-PA activity on the cell surface and may thereby cause a decreased activation of some of the enzymes of the MMP family.
In conclusion, we have provided evidence for a new aspect of interregulation of the u-PA/plasmin and MMP cascades in pericellular proteolysis, namely the cleavage of domain D1 of u-PAR by MMP-12 activity. The increase of the formation of capillary-like tubular structures by hMVECs in a fibrin matrix is primarily the result of the protection of u-PAR by broad-acting MMP inhibitors.
The authors thank Mario Vermeer and Natascha van Lent for their excellent technical assistance, Drs H. Metzner and G. Seeman for providing factor XIII; Dr E. A. Bone for providing BB94 and BB3103, and Dr R. Martin for providing RS-113456-000 and RS-162813-008 and the data of the experiments on the Ki values of the inhibitors used.
P.K. and E.P. supported by the Dutch Cancer Society (grant TNOP-97-1511) and C.F.M.S. supported by the Italian Association for Cancer Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
P. Koolwijk, Gaubius Laboratory TNO-PG, Zernikedreef 9, 2333 CK Leiden, The Netherlands; e-mail:p.koolwijk@pg.tno.nl.


![Fig. 8. Inhibition of the cleavage of domain D1 from domain D1D2D3 of u-PAR by BB94 in the supernatants and on the cell surface of hMVECs. / (A) The hMVECs were stimulated with or without 10−8 M phorbol ester PMA in the absence or presence of 10 μg/mL BB94 for 24, 48, or 72 hours or (B) the combination of 10 ng/mL bFGF and 10 ng/mL TNF-α in the absence or presence of 10 μg/mL BB94 for 72 hours. Supernatants were collected, and Triton X-100 cell lysates were prepared (only after 72 hours [C]). Immunoprecipitation and Western blot analysis were performed as described in “Materials and methods.” CM indicates conditioned medium.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/10/10.1182_blood.v97.10.3123/6/m_h81011057008.jpeg?Expires=1769345428&Signature=GHvMIEXL6TTm2PcTuyTM5n-zhPcEjjEx8ffq1M2f541q4TYO4VFPc4tAiYdYuVZgETkfMqOQEc31HxHJW0sSQRBPCN2CCy9zM-zo8zugUtddhSDhuY0j6Q8Ax8NkYKwaCM0G1oAWPVcKAY2tJDqwzWwGkbFNiBRVU88-DSziL88Vet7cen~3FQyyOpIv~gBZ8YltUtG~dXjvYUjFFEMy~Qi3CZnVoFlNfJx~lN~moYXZYVnPkqdhDXX8dY-0MLVXD5BQ9ZO-X-bqmuzQFkRT1pI74Vx53UCOVe~OOguPDboY0N-5KLYmtT2TIzjBHV9RVvpS-8dQPcIQYK2GFjnX~g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal