Abstract
The CC chemokine receptor 8 (CCR8) is expressed on monocytes and type 2 T lymphocytes. CCR8 is the sole receptor for the human CC chemokine I-309, as well as for viral monocyte inflammatory protein-I (vMIP-I), a human chemokine homologue induced in human cells by the Kaposi sarcoma-related human herpesvirus-8. Recently it was found that I-309 messenger RNA and protein are expressed by human umbilical vein endothelial cells (HUVECs) and that the secretion of endothelial I-309 is stimulated by apolipoprotein(a). I-309, vMIP-I, and the conditioned medium from apolipoprotein(a)-stimulated HUVECs induce endothelial chemotaxis. A polyclonal anti-CCR8 antibody and a newly developed murine monoclonal antibody against CCR8 inhibited this activity. The G-protein inhibitor pertussis toxin also inhibited endothelial chemotaxis, providing further evidence for a chemokine receptor-mediated effect. Endothelial cells contain CCR8 mRNA as shown by RNA blot analysis as well by direct sequence analysis. Immunohistochemical studies identified CCR8 and I-309 on the endothelium of human atherosclerotic plaques and in endothelial-derived spindle cells of Kaposi sarcoma. These results indicate that CCR8 is an endothelial receptor that may modulate endothelial function.
Introduction
The CC chemokine receptor-8 (CCR8) has recently been characterized.1-3 CCR8 is expressed on monocytes and T lymphocytes1-3 and is preferentially expressed on type 2 T lymphocytes.4 CCR8 is a G-protein–coupled 7-transmembrane receptor that is the sole receptor for the human CC chemokine I-309 and for the viral monocyte inflammatory protein-I (vMIP-I), a human chemokine homologue induced by the human herpesvirus-8 (HHV-8) that has been directly linked to Kaposi sarcoma.5-7 CCR8 receptor is a coreceptor for diverse human immunodeficiency virus-1 (HIV-1) strains, and I-309 has been found to inhibit HIV-1 envelope-mediated cell-cell fusion and virus infection.8
I-309 is secreted by activated monocytes and lymphocytes9,10 and is a potent chemoattractant for both these cell types.11,12 Our prior studies have shown that the apolipoprotein(a) (apo[a]) portion of the atherogenic lipoprotein Lp(a) stimulates human vascular endothelial cells to produce a monocyte chemotactic activity.13 We have recently identified I-309 as the principal monocyte chemoattractant secreted by human umbilical vein endothelial cells (HUVECs) when incubated with apo(a).14 I-309 was found in human atheromatous plaques in the luminal endothelium, in macrophage-rich areas of the plaque, and in acellular areas of the vessel wall matrix.
We now report that I-309, vMIP-I, and the endothelial conditioned medium (CM) produced by incubating Lp(a) or a recombinant apo(a) derivative with HUVECs induce endothelial chemotaxis. That CCR8 is the receptor responsible for mediating endothelial chemotaxis was supported by the demonstration that both a polyclonal and monoclonal antibody directed against CCR8 inhibited endothelial chemotaxis and that HUVECs expressed CCR8 messenger RNA (mRNA). Immunohistochemical studies have identified CCR8 in the endothelium of human atherosclerotic plaques and in the endothelial-derived spindle cells of Kaposi sarcoma. These findings suggest that CCR8 may play a role in vascular biology and participate in the genesis of Kaposi sarcoma.
Materials and methods
Proteins and antibodies
Recombinant I-309, monocyte chemotactic protein-1 (MCP-1), vMIP-I, goat polyclonal antibody against I-309 and CCR2, as well a murine monoclonal antibody against CCR2 and the isotype controls were obtained from R&D Systems (Minneapolis, MN). Goat antibody against a 26-amino acid-containing peptide from the N-terminal portion of CCR8 (VTDYYYPDIFSSPCDALEIQTNGKLC)8 15 (single-letter amino acid code) was from Alexis Biochemicals (San Diego, CA). We have produced a murine monoclonal antibody against this peptide using standard hybridoma methodology. Additional antibodies used in immunohistochemical studies included rabbit antihuman von Willebrand factor (vWF; A0082, Dako, Carpenteria, CA), monoclonal antihuman CD-68 panmacrophage antibody (KP-1, M814, Dako), and monoclonal antibody to smooth muscle α-actin (1A4, Dako).
Purification of Lp(a) and a recombinant apo(a) derivative
Culture of HUVECs
The HUVECs were harvested and cultured as described.13 For generating endothelial cell CM, cells from passages 2 through 5 were grown in 6-well plates. Either Lp(a) (100 μg/mL; 100 nM) or 6K apo(a) (20 μg/mL; 100 nM) were incubated with HUVECs for 6 hours at 37°C, and the CM frozen at −80°C until tested. Cell incubation mixtures contained 20 μg/mL polymyxin B (Sigma, St Louis, MO) to inhibit endotoxin.
Endothelial cell transmigration activity
Cell transmigration was measured as described for mononuclear cells13,18 using a Neuroprobe chamber (Nucleopore, Pleasanton, CA) containing a polycarbonate filter with 5-μm pores. The filter was treated with 0.2% gelatin for 30 minutes at 37°C before the assay. For each assay, 29 μL of the chemokine to be tested or of HUVEC-conditioned culture medium was added to the wells of the lower chamber. Then 50 μL of HUVECs (1 × 106 cells/mL) was added to each well of the upper chamber. After a 4-hour incubation in 5% CO2 at 37°C, nonmigrating cells were scraped from the upper surface of the filter. Migrating cells on the lower surface were fixed with methanol and stained with Diff-Quik (Baxter Healthcare, Miami, FL). The number of HUVECs on the lower surface of the filter was determined by counting 3 microscopic fields per well under oil immersion (400 ×). To establish the effect of antibodies against CCR8, the purified antibodies or isotype controls were added to confluent HUVECs (1 μg IgG/mL) in 6-well tissue culture plates and incubated for 30 minutes at 37°C before the assay. After washing, the treated endothelial cells were harvested. To study the effects of pertussis and cholera toxins on endothelial cell transmigration induced by I-309, vMIP-I, and the CM from HUVECs incubated with Lp(a), the cells were cultured in the presence of 0.1 μg/mL pertussis toxin or 0.4 μg/mL cholera toxin for 16 hours before the assays.3All samples were tested for endothelial cell transmigration in triplicate wells in the Neuroprobe chamber.
Sequence analysis of HUVEC CCR8 complementary DNA
Total RNA was isolated using the RNAeasy kit (Qiagen, Chatsworth, CA). Oligonucleotide primers (Operon Technologies, Alameda, CA) for CCR8 complementary DNA (cDNA) were designed as described.2 The sense 5′-TTATGTGTCTCTGTGACCAG and the antisense 5′-TAGTCTTCATTGATCCTCAC primers corresponded to positions 327-346 and 1425-1444 in the published sequence and span the entire coding region of TER1(CCR8) of 355 amino acids.19 For cDNA synthesis, the following reagents were added to Eppendorf tubes (20 μL total): RNA (1 μg), oligoT (500 μg/mL), DTT (0.01 M), dNTP (500 μM each), Superscript II (200 U) in buffer (50 mM Tris-HCl, pH 8.3; 75 mM KCl). The reaction mixture was incubated for 1 hour at 42°C. The cDNA (5 μL) products were added to tubes containing the following reaction mixtures at the indicated final concentrations: 200 μM dNTPs, 1.5 mM MgCl2, 0.2 μM sense primer, 0.2 μM antisense primer, 2.5 U AmpliTaq, and buffer (10 mM Tris-HCl, pH 8.3, 50 mM KCl) to a total volume of 50 μL. The mixture was preheated to 95°C for 5 minutes followed by 30 cycles of incubations at 94°C for 15 seconds, 62°C for 30 seconds, and 72°C for 15 seconds followed by a final extension step of 72°C for 10 minutes. The 1118-bp DNA fragment obtained was cloned in T/A cloning vector (InVitrogene, San Diego, CA) and sequenced using sequenase (US Biochemical, Cleveland, OH). The identity of the fragment as CCR8 mRNA was confirmed by sequence analysis (GenBank accession number U62556). Superscript II was excluded from the control reverse transcriptase-polymerase chain reaction (RT-PCR) to ensure that no DNA contamination was present.
Northern blot analysis
Samples of RNA were electrophoresed through 1.8% agarose gels containing formaldehyde, blotted, and cross-linked to Hybond-N nylon membranes (Amersham, Arlington Heights, IL). Levels of CCR8 mRNA were analyzed by prehybridization in buffer containing 5 × standard sodium citrate (SSC), 5 × Denhardts, 10 × dextran sulfate, 10% sodium dodecyl sulfate (SDS), and 1 μg/mL salmon sperm DNA for 2 hours at 42°C followed by hybridization with 32P-labeled probes and analyzed by autoradiography. The cDNA probes were labeled with [32P]dCTP by random priming to a specific activity of greater than 108 cpm/μL and used at 2 × 106 cpm/mL. The CCR8 probe was a 1118-kb RT-PCR fragment amplified from stimulated HUVECs. As a control, filters were hybridized with cDNA encoding the constitutively expressed glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Immunohistochemistry
Tissue samples from human coronary endarterectomy specimens and from Kaposi sarcoma biopsies were routinely processed and immunostained for CCR8, I-309, vWF, CD-68, and α-actin using an avidin-biotin technique.20 Tissue sections were blocked with ovalbumin or, in some instances, with normal rabbit or horse serum before the addition of the specific detecting antibody. Antigen retrieval was used on some specimens.21 A positive control, nonimmune negative, and processing controls were performed for each antigen stain. Preabsorption of the CCR8 and I-309 antibody yielded negative staining.
Statistical analysis
Results of data are reported as the mean + SD. Levels of significance were determined by 2-tailed Studentt test.
Results
I-309, vMIP-I, and the CM from HUVECs incubated with 6K apo(a) induces chemotaxis of HUVECs
The transmigration of endothelial cells in response to the human CC chemokine I-309 and to the Kaposi sarcoma-associated CC chemokine vMIP-I was studied in Neuroprobe chemotactic chambers using a 5-μm membrane coated with gelatin. In a preliminary study, concentrations of I-309 and vMIP-I from 0.01 to 500 ng/mL were used to induce endothelial transmigration. The response to increasing concentrations of I-309 and vMIP-I was bimodal (data not shown), a finding characteristic for a chemotactic response to CC chemokines. The maximum response occurred at 100 ng/mL (∼10 nM) of the chemokines, a concentration that also induced maximum chemotactic response in cell lines transfected with CCR8.2
To establish that the transmigration of endothelial cells in response to the CC chemokines was chemotactic (directed), rather than chemokinetic (nondirected), a checkerboard analysis was performed (Figure 1). I-309 (100 ng/mL), vMIP-I (100 ng/mL), or the CM from 6K apo(a)-stimulated endothelial cells (KCM) was placed in the bottom wells, in the top wells with the indicator endothelial cells, or in both top and bottom wells of the chemotaxis chamber. All 3 agonists increased endothelial cell transmigration approximately 5-fold when placed in the bottom wells of the chemotaxis chamber, as compared to the medium 199 control. In contrast, the migration of endothelial cells was 3- to 4-fold less when the agonists were added either to the top or to both top and bottom wells. This checkerboard analysis indicates that the transmigration of HUVECs in response to the agonists tested was primarily chemotactic. This study shows for the first time that I-309, vMIP-I, and apo(a) CM are chemotactic for HUVECs.
I-309, vMIP-I, and the CM from 6K apo(a) derivative incubated with HUVECs are chemotactic for endothelial cells.
I-309 or vMIP-I (100 ng/mL) was placed in both top and bottom wells, only in the top well (containing the HUVECs), or only in the bottom wells of the chemotaxis chamber. A recombinant apo(a) derivative containing 6 type 4 kringles, kringle V, and the protease domain of apo(a) (40 μg/mL) was incubated 6 hours with HUVECs and the CM (K) was tested for chemotactic activity as described for the purified chemokines. The − indicates medium 199; I, I-309; v, vMIP-I; K, medium from HUVECs treated with 6K apo(a) derivative. Significant activity was observed when the agonist was in the bottom chamber only indicating that the endothelial transmigration was due to chemotaxis and not due to chemokinesis. hpf, high power field.
I-309, vMIP-I, and the CM from 6K apo(a) derivative incubated with HUVECs are chemotactic for endothelial cells.
I-309 or vMIP-I (100 ng/mL) was placed in both top and bottom wells, only in the top well (containing the HUVECs), or only in the bottom wells of the chemotaxis chamber. A recombinant apo(a) derivative containing 6 type 4 kringles, kringle V, and the protease domain of apo(a) (40 μg/mL) was incubated 6 hours with HUVECs and the CM (K) was tested for chemotactic activity as described for the purified chemokines. The − indicates medium 199; I, I-309; v, vMIP-I; K, medium from HUVECs treated with 6K apo(a) derivative. Significant activity was observed when the agonist was in the bottom chamber only indicating that the endothelial transmigration was due to chemotaxis and not due to chemokinesis. hpf, high power field.
Polyclonal antibody against CCR8 inhibits chemotaxis of HUVECs induced by I-309 and vMIP-I
To examine the effect of blocking polyclonal antibodies against CCR8, I-309, and vMIP-I receptor, HUVECs were pretreated for 30 minutes at 37°C with 1 μg/mL normal goat IgG, goat anti-CCR8, or goat anti-CCR2, the MCP-1 receptor. These cells were then added to the top of the chemotaxis chamber. I-309 or vMIP-I (100 ng/mL each) was added to the bottom wells of the chamber and incubated as detailed in “Materials and methods.” There was no significant difference in the chemotactic response of HUVECs in the presence of normal IgG or of antibody against CCR2. In contrast, antibody against CCR8 significantly inhibited endothelial chemotaxis (P < .001; Figure2).
Polyclonal goat antibody against CCR8 inhibits the chemotaxis of HUVECs in response to I-309 and vMIP-I.
I-309 (▨; 100 ng/mL) and vMIP-I (▪; 100 ng/mL) were tested for endothelial cell chemotactic activity as detailed in “Materials and methods.” Prior to assay, the indicator HUVECs were incubated either with normal goat IgG, goat polyclonal antibody against CCR8 (anti-CCR8), or goat polyclonal antibody against CCR2 (anti-CCR2) (1 μg/mL) for 30 minutes at 37°C. The anti-CCR8 significantly inhibited endothelial chemotaxis as induced by I-309 and vMIP-I (*P < .001). hpf, high power field.
Polyclonal goat antibody against CCR8 inhibits the chemotaxis of HUVECs in response to I-309 and vMIP-I.
I-309 (▨; 100 ng/mL) and vMIP-I (▪; 100 ng/mL) were tested for endothelial cell chemotactic activity as detailed in “Materials and methods.” Prior to assay, the indicator HUVECs were incubated either with normal goat IgG, goat polyclonal antibody against CCR8 (anti-CCR8), or goat polyclonal antibody against CCR2 (anti-CCR2) (1 μg/mL) for 30 minutes at 37°C. The anti-CCR8 significantly inhibited endothelial chemotaxis as induced by I-309 and vMIP-I (*P < .001). hpf, high power field.
Polyclonal antibodies against I-309 or CCR8 inhibit endothelial chemotaxis induced by the conditioned medium from HUVECs incubated with Lp(a) or 6K apo(a)
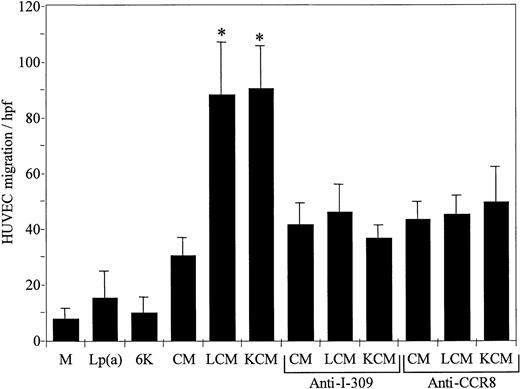
We tested the capacity of the CM from HUVECs incubated with either Lp(a) (LCM) or with recombinant 6K apo(a) (KCM) to induce endothelial chemotaxis. Lp(a) or the 6K apo(a) in the absence of incubation with HUVECs did not have significant endothelial chemotactic activity. LCM and KCM induced a 3-fold increase in endothelial transmigration as compared to the CM from endothelial cells incubated with medium 199 (P < .001; Figure 3). Blocking polyclonal antibodies directed against I-309 or CCR8 significantly inhibited chemotaxis induced by LCM or KCM, whereas the control goat IgG had no inhibitory effect.
The CM resulting from incubation of Lp(a) (LCM) or recombinant 6K apo(a) (KCM) with HUVECs induces chemotaxis of HUVECs that is inhibited by polyclonal antibodies against I-309 or CCR8.
The CM obtained following incubation of medium 199 (CM), Lp(a) (150 μg/mL; LCM), or a recombinant apo(a) derivative containing 6 type 4 kringles, kringle V, and the protease domain (40 μg/mL; KCM) with HUVECs for 6 hours at 37°C, were tested for HUVECs transmigration as described in “Materials and methods.” Medium 199 (M), Lp(a) (150 μg/mL), or the apo(a) derivative (6K; 40 μg/mL) was used as a control. Polyclonal goat anti-I-309 (1 μg/mL) was added to the CM, or polyclonal goat anti-CCR8 (1 μg/mL) was added to the indicator endothelial cells prior to testing as indicated in the figure. Neither Lp(a) nor the 6K apo(a) derivative directly induced HUVECs transmigration as compared to the medium 199 control. LCM and KCM induced endothelial cell transmigration as compared to the CM (*P < .001). Anti-I-309 and anti-CCR8 inhibited endothelial cell transmigration induced by LCM and KCM to the constitutive activity induced by the HUVEC CM. hpf, high power field.
The CM resulting from incubation of Lp(a) (LCM) or recombinant 6K apo(a) (KCM) with HUVECs induces chemotaxis of HUVECs that is inhibited by polyclonal antibodies against I-309 or CCR8.
The CM obtained following incubation of medium 199 (CM), Lp(a) (150 μg/mL; LCM), or a recombinant apo(a) derivative containing 6 type 4 kringles, kringle V, and the protease domain (40 μg/mL; KCM) with HUVECs for 6 hours at 37°C, were tested for HUVECs transmigration as described in “Materials and methods.” Medium 199 (M), Lp(a) (150 μg/mL), or the apo(a) derivative (6K; 40 μg/mL) was used as a control. Polyclonal goat anti-I-309 (1 μg/mL) was added to the CM, or polyclonal goat anti-CCR8 (1 μg/mL) was added to the indicator endothelial cells prior to testing as indicated in the figure. Neither Lp(a) nor the 6K apo(a) derivative directly induced HUVECs transmigration as compared to the medium 199 control. LCM and KCM induced endothelial cell transmigration as compared to the CM (*P < .001). Anti-I-309 and anti-CCR8 inhibited endothelial cell transmigration induced by LCM and KCM to the constitutive activity induced by the HUVEC CM. hpf, high power field.
A murine monoclonal antibody against the N-terminal extracellular domain of CCR8 inhibits HUVECs chemotaxis induced by I-309, vMIP-I, Lp(a) CM, but not by stromal cell-derived factor
A murine monoclonal antibody was produced against a synthetic peptide from the N-terminal extracellular domain of CCR8 and tested for its effect on HUVEC chemotaxis. The monoclonal anti-CCR8 inhibited the endothelial chemotaxis induced by I-309, vMIP-I, and the Lp(a)-HUVEC CM (LCM), but did not inhibit endothelial chemotaxis induced by stromal cell-derived factor 1 (SDF-1; Figure4). The murine IgG isotype control, IgG1, did not inhibit endothelial transmigration in response to the 3 chemokines. The HUVECs used in this experiment to study the endothelial response to LCM were derived from a different donor and demonstrated a lower background activity than did the HUVECs used for the 3 chemokines.
Murine monoclonal antibody against CCR8 inhibits chemotaxis of HUVECs in response to I-309, vMIP-I, and LCM.
I-309, vMIP-I, or SDF-1 (100 ng/mL each) was used to induce endothelial chemotaxis. The HUVECs were used without treatment (No treatment) or were incubated 30 minutes with 1 μg murine monoclonal antibody against CCR8 (Mab > CCR8), an isotype monoclonal IgG1 control (IgG1), or with a murine monoclonal antibody against CCR2 (isotype IgG1), the MCP-1 receptor (Mab > CCR2). The CM obtained following incubation of Lp(a) (150 μg/mL) with HUVECs for 6 hours (LCM) was also tested using HUVECs from a different donor. These cells had lower background activity when tested using medium 199 (Control). The monoclonal anti-CCR8 inhibited endothelial transmigration stimulated by I-309, vMIP-I, and LCM, but did not inhibit migration in response to SDF-1 (*P < .001). Neither the control murine immunoglobulin nor the monoclonal anti-CCR2 inhibited endothelial chemotaxis in response to the 3 chemokines tested. hpf, high power field.
Murine monoclonal antibody against CCR8 inhibits chemotaxis of HUVECs in response to I-309, vMIP-I, and LCM.
I-309, vMIP-I, or SDF-1 (100 ng/mL each) was used to induce endothelial chemotaxis. The HUVECs were used without treatment (No treatment) or were incubated 30 minutes with 1 μg murine monoclonal antibody against CCR8 (Mab > CCR8), an isotype monoclonal IgG1 control (IgG1), or with a murine monoclonal antibody against CCR2 (isotype IgG1), the MCP-1 receptor (Mab > CCR2). The CM obtained following incubation of Lp(a) (150 μg/mL) with HUVECs for 6 hours (LCM) was also tested using HUVECs from a different donor. These cells had lower background activity when tested using medium 199 (Control). The monoclonal anti-CCR8 inhibited endothelial transmigration stimulated by I-309, vMIP-I, and LCM, but did not inhibit migration in response to SDF-1 (*P < .001). Neither the control murine immunoglobulin nor the monoclonal anti-CCR2 inhibited endothelial chemotaxis in response to the 3 chemokines tested. hpf, high power field.
Pertussis toxin inhibits HUVEC chemotaxis induced by I-309, vMIP-I, and LCM
To provide further evidence that the induction of endothelial chemotaxis by I-309, vMIP-I, and LCM was associated with a G-coupled chemokine receptor, the HUVECs were treated with the protein inhibitor pertussis toxin. Pertussis toxin inhibited endothelial transmigration induced by I-309, vMIP-I, and LCM (P < .001; Figure5). Cholera toxin, in contrast, had no inhibitory effect on endothelial chemotaxis.
Pertussis toxin inhibits HUVECs chemotaxis induced by I-309, vMIP-I, and LCM.
I-309, vMIP-I, and LCM, prepared as detailed in the legend for Figure3, were tested for chemotactic activity using HUVECs pretreated with either pertussis or cholera toxin as described in “Materials and methods.” Pertussis toxin inhibited HUVEC chemotaxis in response to all 3 agonists (*P < .001), whereas cholera toxin did not inhibit. hpf, high power field.
Pertussis toxin inhibits HUVECs chemotaxis induced by I-309, vMIP-I, and LCM.
I-309, vMIP-I, and LCM, prepared as detailed in the legend for Figure3, were tested for chemotactic activity using HUVECs pretreated with either pertussis or cholera toxin as described in “Materials and methods.” Pertussis toxin inhibited HUVEC chemotaxis in response to all 3 agonists (*P < .001), whereas cholera toxin did not inhibit. hpf, high power field.
CCR8 mRNA is expressed in HUVECs
RNA blot analysis with CCR8 cDNA demonstrated a single band in Jurkat cells as well as in confluent HUVECs (Figure6). The identity of the DNA transcript as CCR8 was confirmed by sequence analysis as detailed in the “Materials and methods” (GenBank accession number U62556).
RNA blot analysis of CCR8 mRNA in HUVECs.
Aliquots (10 μg) of total RNA from Jurkat cells (Ju) or from confluent HUVECs (Hu) were size-fractionated on agarose gels and hybridized to 32P-labeled human CCR8 cDNA as described in “Materials and methods.” GAPDH (GAP) is shown as a control for loading of samples. The cDNA was cloned as detailed in “Materials and methods” and the identity of the fragment as CCR8 mRNA was confirmed by sequence analysis.
RNA blot analysis of CCR8 mRNA in HUVECs.
Aliquots (10 μg) of total RNA from Jurkat cells (Ju) or from confluent HUVECs (Hu) were size-fractionated on agarose gels and hybridized to 32P-labeled human CCR8 cDNA as described in “Materials and methods.” GAPDH (GAP) is shown as a control for loading of samples. The cDNA was cloned as detailed in “Materials and methods” and the identity of the fragment as CCR8 mRNA was confirmed by sequence analysis.
The endothelium in human atherosclerotic plaques contains CCR8 and I-309
The luminal endothelium of coronary endarterectomy specimens stained positively for CCR8 with both the polyclonal anti-CCR8 (Figure7A) and the murine monoclonal anti-CCR8 (Figure 7C). The endothelium was identified by positive staining for vWF antigen (Figure 7D). Control goat IgG (not shown) and murine IgG1 used as an isotype control did not stain the arterial specimens (Figure 7F). I-309, as we have previously shown,14 stained the endothelium (Figure 7B). CCR8 and I-309 antigen was also detected in subendothelial tissue (Figure 7A-C). The luminal endothelium did not stain for the monocyte/macrophage antigen CD-68; however, some cells in the immediate subendothelium stained positively for CD-68 (Figure 7E). The staining of the endothelium by the CCR8 antisera was inhibited by absorption with the N-terminal peptide of CCR8, and the staining for I-309 was inhibited by absorption of the antibody with immobilized recombinant I-309.
CCR8 is found on the endothelium in human coronary endarterectomy specimens.
Sections of human coronary endarterectomy specimens were stained with polyclonal antibody for CCR8 (A), I-309 (B), murine monoclonal antibody against CCR8 (C), vWF antigen (D), monocyte/macrophage antigen CD-68 (E), and the IgG1 isotype control for the monoclonal antibody against CCR8 (F). Endothelial cells in the lesion, identified by their staining for vWF antigen (D, arrow) stain positively for CCR8 with both polyclonal and monoclonal antibodies (A,C, arrows), and for I-309 (B, arrow). Anti-CD-68 did not stain the endothelium (E, arrow); however, it stained cells in the subendothelial neointima. The murine IgG1 isotype control did not stain the endothelial surface (F, arrow) or the underlying tissue. (DAB-peroxidase, hematoxylin counterstain, original magnification × 400).
CCR8 is found on the endothelium in human coronary endarterectomy specimens.
Sections of human coronary endarterectomy specimens were stained with polyclonal antibody for CCR8 (A), I-309 (B), murine monoclonal antibody against CCR8 (C), vWF antigen (D), monocyte/macrophage antigen CD-68 (E), and the IgG1 isotype control for the monoclonal antibody against CCR8 (F). Endothelial cells in the lesion, identified by their staining for vWF antigen (D, arrow) stain positively for CCR8 with both polyclonal and monoclonal antibodies (A,C, arrows), and for I-309 (B, arrow). Anti-CD-68 did not stain the endothelium (E, arrow); however, it stained cells in the subendothelial neointima. The murine IgG1 isotype control did not stain the endothelial surface (F, arrow) or the underlying tissue. (DAB-peroxidase, hematoxylin counterstain, original magnification × 400).
Kaposi sarcoma contains CCR8 and I-309
Ten human biopsy specimens of Kaposi sarcoma were immunostained for multiple antigens including CCR8, I-309, macrophage antigen CD-68 (KP-1), smooth muscle cell α-actin, and vWF antigen. Sections from a representative biopsy specimen are shown in Figure8. The tissue section shown is comprised mainly of endothelial-derived spindle cells that stain intensely and diffusely for CCR8 with both the polyclonal antibody (Figure 8A) and the murine monoclonal anti-CCR8 (Figure 8D). Similar positive staining was documented for I-309 (Figure 8B). vWF antigen stained diffusely indicating endothelial cell origin for the Kaposi tumor cells (Figure8C). The Kaposi spindle cells also stained for CD-68 antigen (Figure8E). Microvessels as identified by pericytes staining positively for α-actin are also scattered throughout the biopsy specimen (Figure8F). Neither the secondary antibody (control, Figure 8G) nor the murine IgG1 (Figure 8H) significantly stained the Kaposi tissue. In addition to blocking the tissue sections with ovalbumin, the inclusion of horse serum or rabbit serum did not inhibit immunostaining for CCR8 or I-309. Absorption of the CCR8 with the N-terminal peptide of CCR8 blocked the immunostaining (data not shown).
Kaposi sarcoma shows diffuse staining for CCR8 and I-309.
Biopsies of Kaposi sarcoma were stained for multiple antigens as detailed in “Materials and methods.” Shown is a Kaposi sarcoma patch stained with goat polyclonal anti-CCR8 (A), anti-I-309 (B), anti-vWF (C), murine monoclonal anti-CCR8 (Mab > CCR8; D), anti-CD-68 (E), anti-α-actin (F), secondary antibody alone (Control, G), and isotype control IgG1 (H). The Kaposi sarcoma spindle cells stain intensely for CCR8 with both the polyclonal and monoclonal antibodies (A,D). The spindle cells also stain diffusely for vWF and CD-68 (C,E). Pericytes in small vessels stain for α-actin (F). There is no staining when the primary antibody is omitted (G), and only a trace staining with the mouse IgG1 isotype control (H). (DAB-peroxidase, hematoxylin counterstain, original magnification × 200).
Kaposi sarcoma shows diffuse staining for CCR8 and I-309.
Biopsies of Kaposi sarcoma were stained for multiple antigens as detailed in “Materials and methods.” Shown is a Kaposi sarcoma patch stained with goat polyclonal anti-CCR8 (A), anti-I-309 (B), anti-vWF (C), murine monoclonal anti-CCR8 (Mab > CCR8; D), anti-CD-68 (E), anti-α-actin (F), secondary antibody alone (Control, G), and isotype control IgG1 (H). The Kaposi sarcoma spindle cells stain intensely for CCR8 with both the polyclonal and monoclonal antibodies (A,D). The spindle cells also stain diffusely for vWF and CD-68 (C,E). Pericytes in small vessels stain for α-actin (F). There is no staining when the primary antibody is omitted (G), and only a trace staining with the mouse IgG1 isotype control (H). (DAB-peroxidase, hematoxylin counterstain, original magnification × 200).
Discussion
Endothelial cell migration in response to chemotactic signals appears to be required for angiogenesis, wound repair, and atherogenesis.22-25 A variety of factors including vascular endothelial growth factor23 and basic fibroblast growth factor26 can stimulate endothelial migration. Chemokines, which are low-molecular-weight proteins (8-10 kd), that have the capacity to direct leukocyte trafficking into areas of inflammation27,28 may also participate in endothelial cell migration. Chemokines are classified into 2 major families that are distinguished by the position of the first 2 of 4 conserved cysteines (CXC and CC). A CXC chemokine, SDF-1, is a potent inducer of endothelial chemotaxis,29 and its receptor CXCR4 has been reported as essential for vascularization of the gastrointestinal tract.30 The CC chemokine receptor CCR2 was recently identified on human endothelial cells, and its agonist, the CC chemokine MCP-1, was found to stimulate endothelial chemotaxis.31
In the present study we document for the first time that the CC chemokine I-309 and the HHV-8 encoded CC chemokine vMIP-I stimulate the transmigration of HUVECs. The results of checkerboard analysis indicate that the endothelial response to these agonists was due to chemotaxis rather than to chemokinesis. The CM from Lp(a)- or from 6K apo(a)-stimulated HUVECs, previously shown to contain I-309 and to be chemotactic for monocytes,14 was also chemotactic for endothelial cells. That the endothelial agonist was I-309 in the CM was supported by the observation that anti-I-309 antibody inhibited endothelial migration.
It has been reported recently that vMIP-I is a specific agonist for CCR8.5,6 In a study using 65 recombinant chemokines to characterize the recognition properties of CCR8, only 2, I-309 and vMIP-I, acted as agonists as they bound to CCR8 and induced calcium flux.5 In another study, cell lines transfected with known or suspected chemokine receptors were tested for calcium flux response to 40 different chemokines. Only cells transfected with CCR8 (CCR8-Y3 cells) responded to vMIP-I and I-309.6 Cell lines stably transfected with 12 other chemokine receptors were tested and were found not to respond to either vMIP-I or I-309 emphasizing the specificity of CCR8. Thus our finding that I-309 and vMIP-I induce endothelial chemotaxis provides strong evidence that functional CCR8 is present on the endothelial surface. This concept is also supported by our finding that 2 different antibodies, a goat polyclonal antibody and a newly developed murine monoclonal antibody directed against the N-terminal extracellular portion of CCR8, inhibited the endothelial migration induced by I-309 and vMIP-I. In contrast, a polyclonal goat and a murine monoclonal antibody directed against CCR2, the MCP-1 receptor, did not inhibit endothelial chemotaxis induced by I-309, vMIP-I, or the LCM. The demonstration that the G-coupled protein inhibitor pertussis toxin inhibited the chemotaxis of HUVECs induced by I-309, vMIP-I, and LCM provides additional evidence that the endothelial chemotaxis induced by these agonists is mediated by a chemokine receptor.
We have also documented by RNA blot analysis and more importantly by direct sequence analysis the presence of CCR8 cDNA in HUVECs. Our immunohistochemical studies of human coronary endarterectomy specimens demonstrate that CCR8 and I-309 are present on the luminal endothelium of the atherosclerotic plaque. We have also observed CCR8, I-309, and vWF antigen in subendothelial areas of the atherosclerotic plaque. The cellular source of these antigens in the intima are unknown at present. These findings support the possibility that the I-309 and its receptor, CCR8, may contribute to the atherogenic process.
Kaposi sarcoma HHV-8 is causally linked to hematologic malignancies including all types of Kaposi sarcoma, primary effusion lymphomas, and to multicentric Castleman disease.7,32,33 HHV-8 has been found in Kaposi sarcoma associated with acquired immunodeficiency syndrome, classic Kaposi sarcoma, endemic Kaposi sarcoma, and posttransplant Kaposi sarcoma32,33 indicating that Kaposi sarcoma can occur independently of HIV-1 infection. A hallmark of Kaposi sarcoma is intense angiogenesis, proliferation of endothelial cell-derived spindle cells and inflammation.34 The Kaposi sarcoma genome encodes 4 viral proteins including the CC chemokines vMIP-I, -II, and -III that share homology with human macrophage inflammatory protein (MIP-1), viral interleukin 6, and a G protein-coupled receptor (KSHV-GPR).35 The role of these viral chemokines in the etiology of Kaposi sarcoma is not known; however, both vMIP-I and vMIP-II were highly angiogenic in a chorioallantoic assay, suggesting a possible role in Kaposi sarcoma.36 The mechanisms underlying their angiogenic effect have not been defined; however, because CCR8 is preferentially expressed on Th2 lymphocytes,4 it was concluded that expression of vMIP-I by KSHV might alter the Th1/Th2 balance of the host immune response.6
The findings in the present study indicate that vMIP-I is directly chemotactic for endothelial cells. Immunohistochemical studies of Kaposi sarcoma biopsy specimens document diffuse staining for CCR8 associated with Kaposi sarcoma spindle cells. We also found intense and diffuse staining for I-309 in the Kaposi sarcoma lesion in a similar distribution to that of CCR8. Spindle cells appear to represent a mixed population of activated endothelial cells and macrophages,37 and these cells have been shown to stain for CD-68 antigen, as observed in the present study.38These findings raise questions as to the possible role of I-309 and CCR8 in the formation of the Kaposi sarcoma lesion. Endothelial CCR8 may be in part responsible for the vasculogenic activity of vMIP-I previously documented in the chorioallantoic assay.36
Lipoprotein(a) has been shown to be an independent risk factor for atherosclerosis (reviewed in reference 39). Lp(a) consists of a low-density lipoprotein particle disulfide linked to apo(a),40,41 a glycoprotein of variable size that shares partial homology with plasminogen.42,43 The mechanisms by which Lp(a) participates in atherosclerosis are not defined. Our findings that Lp(a) stimulates endothelial chemotaxis by means of its capacity to induce endothelial I-309 production adds to our understanding of the pathophysiologic role for this lipoprotein.14 Because I-309 has been shown to be a chemoattractant for monocytes and for Th2 lymphocytes, it is reasonable to postulate that vessel wall-associated I-309 would attract these inflammatory cells into the forming atherosclerotic plaque. The present demonstration that CCR8 is present on vascular endothelial cells and that its stimulation by I-309 leads to endothelial migration indicates that I-309 may also function as an autocrine modulator of endothelial function. Two different angiogenesis inhibitors were found to reduce both intimal neovascularization and plaque growth in the apolipoprotein E-deficient mouse, providing evidence that new vessel formation is an important determinant of plaque growth.25 We have shown previously that I-309 is distributed widely in the human atheromatous plaque, appearing in the endothelium, in macrophages, and in the acellular matrix.14 We propose that I-309 in the plaque may provide an endothelial chemotactic stimulus, in addition to other inflammatory cytokines, to induce new vessel growth.
The identification of CCR8 as an I-309 and vMIP-I receptor on vascular endothelial cells may be important to our understanding of the mechanisms underlying endothelial function in atherogenesis, in the formation of the lesions in Kaposi sarcoma, and as a novel target for the modulation of angiogenesis.
Supported in part by National Institutes of Health grant HL-54469.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Peter C. Harpel, Box 1079, Mount Sinai School of Medicine, 1 Gustave L. Levy Place, New York, NY 10029; e-mail:peter.harpel@mssm.edu.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal