Abstract
JAB/suppressor of cytokine signaling 1 (SOCS1) STAT-induced STAT inhibitor–1 (SSI-1) (JAB/SOCS1/SSI-1) is an SH2-domain–containing protein that is induced by and negatively regulates signaling by a number of cytokines including interleukin-4 (IL-4), IL-6, interferon (IFN)-γ, prolactin, growth hormone, and erythropoietin. The role of JAB/SOCS1/SSI-1 in IL-2 signaling has been analyzed. JAB/SOCS1/SSI-1 is strongly induced by IL-2 in peripheral blood T cells, and JAB/SOCS1/SSI-1 overexpression strongly inhibits IL-2–induced signal transducer and activator of transcription–5 (Stat5) phosphorylation and transcriptional activity. In cotransfection experiments, JAB/SOCS1/SSI-1 associates with both Jak1 and Jak3; however, JAB/SOCS1/SSI-1 had a greater effect on Jak1 tyrosine phosphorylation and kinase activity. JAB/SOCS1/SSI-1 also interacts with IL-2Rβ, and this interaction requires the A region (residues 313-382) of IL-2Rβ. However, this interaction was not essential for the inhibitory action of JAB. Thus, JAB/SOCS1/SSI-1 is an IL-2–induced inhibitor of IL-2 signaling that functions by inhibiting Jak kinase activity. This suggests an important role for JAB/SOCS1/SSI-1 in regulating T-cell responses.
Introduction
Interleukin-2 (IL-2) is the major growth factor regulating the clonal expansion of antigen-activated T cells. IL-2 can also regulate activation, proliferation, and differentiation of other hematopoietic cells including B cells and natural killer (NK) cells.1,2 Moreover, genetic evidence indicates a pivotal role for IL-2 in the maintenance of peripheral tolerance.3,4 The high-affinity receptor for IL-2 is composed of 3 chains: the IL-2 receptor α chain (IL-2Rα), IL-2Rβ, and the common cytokine receptor γ chain (γc).2 IL-2 induces heterodimerization of IL-2Rβ and γc,5,6 which is followed by the rapid activation of the Janus family tyrosine kinases, Jak1 and Jak3.7,8 Jak1 is constitutively associated with IL-2Rβ, and Jak3 is associated primarily with γc,9-11 but Jak3 also can interact with IL-2Rβ following stimulation with IL-2.12 The activation of Jak1 and Jak3 is pivotal for the activation of downstream signaling pathways including the recruitment and rapid tyrosine phosphorylation of Stat5a, Stat5b, and Stat3.2,13-16 These STAT proteins can then dimerize and translocate into the nucleus, where they regulate transcription of IL-2 target genes.2,17 18
Many studies have focused on the mechanisms by which cytokines, such as IL-2, exert their actions. Pathways including the Jak-STAT, MAP kinase, and phosphoinositol (PI) 3-kinase pathways have been shown to be activated by IL-2.2 Much less is known about how IL-2 signaling is inhibited. The SH2 domain–containing phosphotyrosine phosphatase Shp-1 has been shown to negatively regulate signaling in response to a number of stimuli including IL-2.19 Negative regulation of IL-2 signaling by calpain-mediated cleavage of the cytoplasmic portion of γc has also been suggested.20 Protein inhibitor of activated STAT (PIAS) proteins specifically bind to certain activated STAT proteins and inhibit STAT DNA binding activity21,22; however, their role in IL-2 signaling has not yet been demonstrated. Over the past few years, another family of cytokine-inducible proteins has been described.23-29 Several of these proteins, which are variably called cytokine-inducible SH2-containing (CIS) proteins, SOCS, or SSIs, have been shown to be involved in the negative regulation of the Jak-STAT pathway.28 29
JAB/SOCS1/SSI-1 was identified both as a Jak2-binding protein and as an inhibitor of IL-6 signaling.24-26 JAB/SOCS1/SSI-1 has been shown to interact with all 4 Jak kinases (Jak1, Jak2, Jak3, and Tyk2) and inhibit signaling by IL-3, IL-4, IL-6, growth hormone, LIF, prolactin, interferon (IFN)-γ, and erythropoietin. The inhibitory mechanism of JAB/SOCS1/SSI-1 has been proposed to involve, in the context of Jak2, a direct interaction with the kinase activation loop, thereby resulting in decreased catalytic activity.25,30Recently, 2 JAB/CIS/SOCS/SSI family members, CIS1 and SOCS3, have been shown to inhibit IL-2 signaling.31-33 IL-2 hyperresponsiveness of JAB/SOCS1/SSI-1–deficient murine splenocytes has been reported,34 35 which suggests that IL-2 signaling might also be negatively regulated by JAB/SOCS1/SSI-1. Here we have analyzed the role of JAB/SOCS1/SSI-1 in IL-2 signaling. We demonstrate that JAB/SOCS1/SSI-1 is strongly induced by IL-2, can associate with IL-2Rβ, and potently inhibits IL-2–induced Stat5 function, all of which suggest a significant role for this molecule in regulating T-cell immune responses.
Materials and methods
Cells, transfections, and reporter assays
Peripheral blood lymphocytes (PBLs) were prepared from healthy donors using standard methods. To generate preactivated PBLs, freshly isolated PBLs were cultured for 48 hours in the presence of 2 μg/mL PHA-L (Boehringer Mannheim GmbH, Germany) in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, and 100 U/mL each of penicillin and streptomycin and then rested overnight in the absence of IL-2, as previously described.14 We cultured 293T+ cells in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS, 2 mM L-glutamine, and 100 U/mL each of penicillin and streptomycin. Transient transfections of 293T+ cells were performed using a calcium-phosphate transfection kit (5-prime 3-prime, Inc.). Lysates for luciferase assays were prepared using the Dual Luciferase Assay System kit (Promega Company, Madison, WI). Protein concentrations were measured with a protein assay kit (Bio-Rad Laboratories, Hercules, CA), and 0.1 μg protein was used for luciferase assays.
RNA preparation and Northern blot analysis
Messenger RNA (mRNA) was extracted from PBLs using the FastTrack 2.0 Kit (InVitrogen, Westbrook, ME). Northern blotting was performed according to standard procedures using JAB/SOCS1/SSI-1 or pHe7 (32P)–labeled complementary DNA (cDNA) probes.pHe7 is a housekeeping gene36 whose expression is not affected by a wide range of stimuli.
cDNA constructs
The constructs used were Myc epitope–tagged arginine-105-lysine (R105K) and arginine-105-glutamic acid (R105E) mutant forms as well as wild type (WT) murine JAB/SOCS1/SSI-1 cDNA constructs30; WT, mutant, and truncation human IL-2Rβ constructs12; WT human γccDNA11,37; and the β-casein reporter construct.33,38 39 The murine Jak1 and human Jak3 cDNAs were provided by Drs J. Ihle and J. O'Shea, respectively.
Reagents and antibodies
We used anti-Myc epitope mAb 9E10 anti-Jak1, and anti-Jak3 (all from Santa Cruz Biotechnology, Inc, Santa Cruz, CA); antiphosphotyrosine (PY20) and anti-Stat5 (pan-Stat5) (Upstate Biotechnology, Inc, Lake Placid, NY); and anti–IL-2Rβ monoclonal antibody (mAb) Mikβ1 (gift from Dr M. Tsudo). The generation of anti-Stat5a, anti-Stat5b,39 and anti–IL-2Rβ5 (anticytoplasmic domain, ErdA) has been described.
Immunoprecipitation and Western blotting
Cells were harvested and washed with phosphate-buffered saline (PBS); lysed in lysis buffer comprising 50 mmol/L Tris (tris[hydroxymethyl] aminomethane (pH 7.5), 150 mM sodium chloride (NaCl), 0.5% NP-40, 1 mM Na3VO4, 5 mM NaF, 10 μg/mL each of leupeptin and aprotinin, and 1 mM AEBSF; and centrifuged at 14 000g at 4°C for 10 minutes. Lysates were either boiled immediately in reducing sodium dodecyl sulfate (SDS) sample buffer or immunoprecipitated for 1-3 hours at 4°C using specific antibodies and protein A Sepharose beads (Amersham Pharmacia Biotech). The immune complexes were washed 4 times with lysis buffer. The samples were boiled in reducing SDS sample buffer and analyzed by SDS-PAGE (polyacrylamide gel electrophoresis).
Jak1 and Jak3 in vitro kinase assay
cDNAs for murine Jak1 or human Jak3 in expression vectors (9 μg or 3 μg per transfection, respectively) were transiently transfected into 293T+ cells with or without pcDNA3 carrying Myc-JAB/SOCS1/SSI-1. Cells were lysed in 0.5 mL buffer A comprising 0.5% NP-40, 150 mM NaCl, 50 mM HEPES (4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid) buffer (pH 7.0), 1 mM DTT, 50 mM NaF, 1 mM sodium vanadate, 1 mM phenylmethylsulphonyl fluoride, and 1% aprotinin. Cell extracts were immunoprecipitated with 5 μL anti-Jak1 or anti-Jak3 antibodies, then washed twice with buffer A. One-half of each immunoprecipitate was subjected to immunoblotting with PY20, anti-Jak1, anti-Jak3, and anti-Myc antibodies. For in vitro kinase assays, the other half of each immunoprecipitate was washed twice with kinase reaction buffer comprising 50 mM HEPES buffer (pH 7.5), 50 mM NaCl, 5 mM magnesium dichloride (MgCl2), 5 mM manganese dichloride (MnCl2), and 0.1 mM sodium vanadate). The beads were resuspended in 20 μL kinase reaction buffer and then incubated with γ–32P-ATP (adenosine 5′-triphosphate) 0.37 MBq [10 μCi] per sample; final concentration, 10 μM ATP) for 5 minutes at room temperature. After washing twice with PBS, the beads were subjected to 10% SDS-PAGE and autoradiography.
Results
JAB/SOCS1/SSI-1 mRNA and protein expression are induced by IL-2
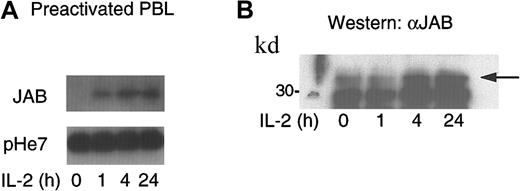
To elucidate the role of JAB/SOCS1/SSI-1 in IL-2 signaling, we first examined the expression of JAB/SOCS1/SSI-1 mRNA in human PBLs. PBLs were preactivated with PHA to induce high-affinity IL-2R expression and thus to prime cells for IL-2 responsiveness. As shown in Figure 1A, significant JAB/SOCS1/SSI-1 mRNA could be detected within one hour of IL-2 stimulation, and the signal was sustained for at least 24 hours. JAB/SOCS1/SSI-1 protein was potently expressed in these cells within 4 hours of treatment with IL-2 and sustained at a high level for at least 24 hours (Figure1B).
Induction of JAB/SOCS1/SSI-1 mRNA and protein expression in preactivated PBLs by IL-2.
(A) Cells were either left untreated or stimulated with IL-2 for 1, 4, or 24 hours. mRNA was extracted and analyzed by Northern blotting using32P-labeled JAB/SOCS1/SSI-1 cDNA as a probe. The blot was stripped and reprobed using pHe7 cDNA to control variations in loading. (B) Preactivated PBLs were either left untreated or stimulated with IL-2 for 1, 4, or 24 hours, and JAB/SOCS1/SSI-1 protein expression was analyzed by Western blotting of cell lysates.
Induction of JAB/SOCS1/SSI-1 mRNA and protein expression in preactivated PBLs by IL-2.
(A) Cells were either left untreated or stimulated with IL-2 for 1, 4, or 24 hours. mRNA was extracted and analyzed by Northern blotting using32P-labeled JAB/SOCS1/SSI-1 cDNA as a probe. The blot was stripped and reprobed using pHe7 cDNA to control variations in loading. (B) Preactivated PBLs were either left untreated or stimulated with IL-2 for 1, 4, or 24 hours, and JAB/SOCS1/SSI-1 protein expression was analyzed by Western blotting of cell lysates.
JAB/SOCS1/SSI-1 inhibits IL-2–induced Jak1 activation and Stat5 phosphorylation, but only slightly inhibits activation of Jak3
Phosphorylation and activation of Jak1, Jak3, and Stat5 during IL-2 signaling is essential for the induction of Stat5 DNA binding and transcriptional activity.2,17 Therefore, we studied the effect of JAB/SOCS1/SSI-1 on the tyrosine phosphorylation and functional activity of these molecules in 293T+ cells. In these cells, overexpression of Jak1 and Jak3 results in the activation and phosphorylation of these Jak kinases. 293T+ cells were transiently transfected with Jak1 in the presence or absence of wild type JAB/SOCS1/SSI-1 (JAB/SOCS1/SSI-1WT). Jak1 phosphorylation was reduced in the presence of the JAB/SOCS1/SSI-1WT (Figure 2A, top panel, lane 4 vs lane 2 and lane 8 vs lane 6), as was the kinase activity of Jak1 (Figure 2A, second panel, lane 4 vs lane 2). An inhibitory effect on Jak1 tyrosine phosphorylation was seen with 4 μg transfected JAB/SOCS1/SSI-1WT DNA but not with 0.4 μg (lanes 3, 4, 7, and 8). JAB/SOCS1/SSI-1 had less of an inhibitory effect on Jak3 phosphorylation (Figure 2B, top panel, lane 4 vs lane 2 and lane 8 vs lane 6) and had only a minor effect on Jak3 kinase activity (Figure 2B, second panel, lane 4 vs lane 2). The reason why JAB/SOCS1/SSI-1 less potently affects Jak3 than Jak1 is unclear; however, it may be relevant that Jak1 catalytic activity has been shown to be strictly dependent on phosphorylation of tyrosine (Y)1033 in the kinase activation loop, whereas Jak3 is only partially dependent on Y980, the corresponding tyrosine.40 41Interestingly, an SH2-domain mutant of JAB/SOCS1/SSI-1 (JAB/SOCS1/SSI-1R105K) that is not able to bind phosphotyrosine did not affect tyrosine phosphorylation of Jak1 or Jak3 (data not shown). We also investigated the ability of JAB/SOCS1/SSI-1 to interact with Jak1 and Jak3 and found that JAB/SOCS1/SSI-1WT associated with both Jak1 (Figure 2A, bottom panel, lanes 7 and 8) and Jak3 (Figure 2B, bottom panel, lanes 7 and 8). Taken together, the results suggest that Jak kinases are the main targets of JAB/SOCS1/SSI-1 action in the IL-2–signaling pathway.
Inhibition of Jak1 and Jak3 tyrosine phosphorylation and kinase activity and Stat5 phosphorylation by JAB/SOCS1/SSI-1.
293T+ cells were transiently transfected with the indicated amounts of Myc-tagged JAB/SOCS1/SSI-1 in the presence (+) or absence (−) of Jak1 or Jak3 cDNAs. Cells were lysed, and total cellular extracts (TCLs) were either directly analyzed on Western blots or were first immunoprecipitated with either (A) anti-Jak1 or (B) anti-Jak3. One-half of each immunoprecipitate was blotted with antiphosphotyrosine (αPY), anti-Jak1 or Jak3, and anti-Myc mAb 9E10 to detect JAB/SOCS1/SSI-1. The other half of each immunoprecipitate was subjected to in vitro kinase assay and then analyzed by SDS-PAGE and autoradiography.
Inhibition of Jak1 and Jak3 tyrosine phosphorylation and kinase activity and Stat5 phosphorylation by JAB/SOCS1/SSI-1.
293T+ cells were transiently transfected with the indicated amounts of Myc-tagged JAB/SOCS1/SSI-1 in the presence (+) or absence (−) of Jak1 or Jak3 cDNAs. Cells were lysed, and total cellular extracts (TCLs) were either directly analyzed on Western blots or were first immunoprecipitated with either (A) anti-Jak1 or (B) anti-Jak3. One-half of each immunoprecipitate was blotted with antiphosphotyrosine (αPY), anti-Jak1 or Jak3, and anti-Myc mAb 9E10 to detect JAB/SOCS1/SSI-1. The other half of each immunoprecipitate was subjected to in vitro kinase assay and then analyzed by SDS-PAGE and autoradiography.
We next evaluated whether JAB/SOCS1/SSI-1 could inhibit IL-2–induced signaling events. For these experiments, we first used a previously established 293T+-cell IL-2–signaling reconstitution system and studied the effect of JAB/SOCS1/SSI-1 overexpression on IL-2–induced tyrosine phosphorylation of Stat5. As shown in Figure3, IL-2 could induce Stat5 tyrosine phosphorylation (lane 2), and this phosphorylation was inhibited by the expression of JAB/SOCS1/SSI-1WT (lane 4). This effect was specific as neither JAB/SOCS1/SSI-1R105E or JAB/SOCS1/SSI-1R105K SH2-domain mutants inhibited Stat5 tyrosine phosphorylation (Figure 3, lanes 6 and 8). Instead, if anything, these mutant constructs augmented tyrosine phosphorylation of Stat5, which is consistent with their acting as dominant negative constructs that could compete with endogenous JAB/SOCS1/SSI-1 and perhaps other family proteins as well.
Inhibition of IL-2–induced Stat5 phosphorylation by JAB/SOCS1/SSI-1.
We transiently transfected 293T+ cells with IL-2Rβ, IL-2Rγ, Jak3, Stat5a, and Stat5b in the presence or absence of WT or R105K or R105E SH2-domain mutants of JAB/SOCS1/SSI-1. After 24 hours, cells were split and plated in duplicate. Another 24 hours later, one set was stimulated with 2 nM IL-2 for 15 minutes, and the other set was left untreated. Cells were then lysed and immunoprecipitated with antibodies against Stat5a and Stat5b, and the immunocomplexes were subjected to SDS-PAGE and Western blotting with antiphosphotyrosine (PY20), anti-Stat5, or anti-Myc 9E10 antibodies.
Inhibition of IL-2–induced Stat5 phosphorylation by JAB/SOCS1/SSI-1.
We transiently transfected 293T+ cells with IL-2Rβ, IL-2Rγ, Jak3, Stat5a, and Stat5b in the presence or absence of WT or R105K or R105E SH2-domain mutants of JAB/SOCS1/SSI-1. After 24 hours, cells were split and plated in duplicate. Another 24 hours later, one set was stimulated with 2 nM IL-2 for 15 minutes, and the other set was left untreated. Cells were then lysed and immunoprecipitated with antibodies against Stat5a and Stat5b, and the immunocomplexes were subjected to SDS-PAGE and Western blotting with antiphosphotyrosine (PY20), anti-Stat5, or anti-Myc 9E10 antibodies.
JAB/SOCS1/SSI-1 inhibits IL-2–induced Stat5-dependent transcription
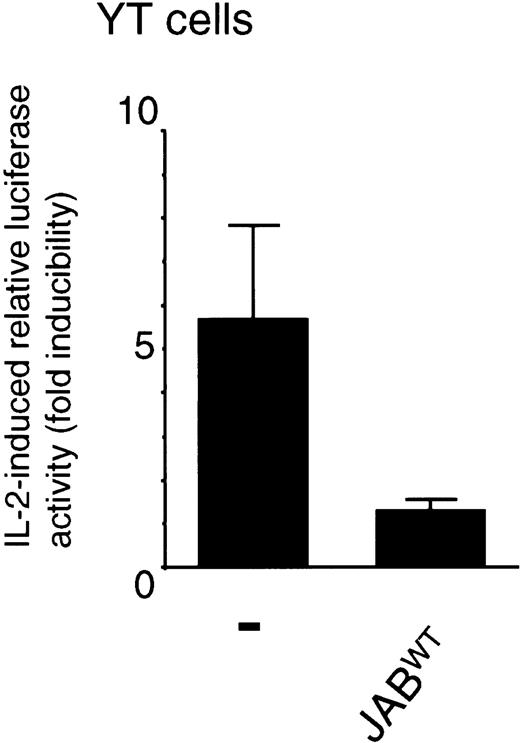
Because JAB/SOCS1/SSI-1WT could inhibit tyrosine phosphorylation of Jak1 and Stat5, we next investigated the effect of JAB/SOCS1/SSI-1WT on IL-2–induced Stat5-dependent transcription. In YT cells, a lymphoid cell line that expresses both endogenous IL-2Rs and the receptor-associated signaling molecules, transfection of JAB/SOCS1/SSI-1WT potently inhibited IL-2–induced luciferase activity of a Stat5-dependent reporter (derived from the β-casein gene promotor region) (Figure4). JAB/SOCS1/SSI-1WT also potently inhibited IL-2–induced Stat5-dependent reporter activity in a 293T+ IL-2–signaling reconstitution system (data not shown).
IL-2–induced Stat5-dependent transcription is inhibited by JAB/SOCS1/SSI-IWT.
YT cells were transfected using the DEAE dextran method according to standard protocols with 2 μg β-casein luciferase reporter, 20 ng pRL-TK control reporter, and with or without 5 μg JAB/SOCS1/SSI-1WT cDNA. Twenty-four hours later the cells were either left untreated or stimulated with 10 nM IL-2 for 24 hours. The data are the mean ± SD of normalized luciferase values of 4 experiments. The normalized luciferase values (average ± SD) of cells transfected with control plasmid were 2.3 ± 0.7 and 12.6 ± 4.3, and the values of JAB/SOCS1/SSI-1WT–transfected cells were 1.8 ± 0.1 and 2.2 ± 0.4.
IL-2–induced Stat5-dependent transcription is inhibited by JAB/SOCS1/SSI-IWT.
YT cells were transfected using the DEAE dextran method according to standard protocols with 2 μg β-casein luciferase reporter, 20 ng pRL-TK control reporter, and with or without 5 μg JAB/SOCS1/SSI-1WT cDNA. Twenty-four hours later the cells were either left untreated or stimulated with 10 nM IL-2 for 24 hours. The data are the mean ± SD of normalized luciferase values of 4 experiments. The normalized luciferase values (average ± SD) of cells transfected with control plasmid were 2.3 ± 0.7 and 12.6 ± 4.3, and the values of JAB/SOCS1/SSI-1WT–transfected cells were 1.8 ± 0.1 and 2.2 ± 0.4.
JAB/SOCS1/SSI-1 associates with IL-2Rβ
The above results show that IL-2 induces JAB/SOCS1/SSI-1 expression and that JAB/SOCS1/SSI-1 can inhibit IL-2 signaling in YT and 293T+ cells. Recently, it was observed that the related CIS1 protein associates with the IL-2Rβ.33 To determine if JAB/SOCS1/SSI-1 is also able to associate with IL-2Rβ, we performed cotransfection and immunoprecipitation experiments in 293T+ cells using WT IL-2Rβ and IL-2Rβ mutants. When JAB/SOCS1/SSI-1WT and WT IL-2R components were cotransfected into 293T+ cells, JAB/SOCS1/SSI-1 efficiently coprecipitated with WT IL-2Rβ (Figure5A,B, top 2 panels, lane 1). To map the region of IL-2Rβ required for this association, we transiently transfected JAB/SOCS1/SSI-1WT and different IL-2Rβ mutants containing various internal deletions or C-terminal truncations and performed coimmunoprecipitation experiments. Deletion of the A region (residues 313-382) greatly diminished the interaction of IL-2Rβ with JAB/SOCS1/SSI-1WT (Figure 5A, 2 top panels, lane 2). Deletion of the S region (residues 267-323) did not influence the interaction (Figure 5A, lanes 3).
JAB/SOCS1/SSI-1 associates with IL-2Rβ.
293T+ cells were transiently transfected with JAB/SOCS1/SSI-1WT and either WT IL-2Rβ or IL-2Rβ internal deletion mutants, IL-2RβΔA and IL-2RβΔS (A) or the indicated C-terminal truncation mutants (B), or the indicated tyrosine mutants (C). Lysates were immunoprecipitated with anti-IL-2Rβ or anti-Myc epitope antibody 9E10 and then Western blotted with the indicated antibodies. (D) 293T+ cells were transfected with WT IL-2Rβ or IL-2RβΔA, γc, Jak3, β-casein–luciferase reporter construct and the pRL-TK reporter construct, with or without 20-1000 ng of the JAB/SOCS1/SSI-1WT expression vector. After 24 hours, cells were split and plated in duplicate and stimulated with 20 nM IL-2 for another 24 hours or left untreated. Activity of the β-casein–luciferase reporter construct was normalized against pRL-TK control luciferase. The data shown are the mean ± SD of 6 experiments.
JAB/SOCS1/SSI-1 associates with IL-2Rβ.
293T+ cells were transiently transfected with JAB/SOCS1/SSI-1WT and either WT IL-2Rβ or IL-2Rβ internal deletion mutants, IL-2RβΔA and IL-2RβΔS (A) or the indicated C-terminal truncation mutants (B), or the indicated tyrosine mutants (C). Lysates were immunoprecipitated with anti-IL-2Rβ or anti-Myc epitope antibody 9E10 and then Western blotted with the indicated antibodies. (D) 293T+ cells were transfected with WT IL-2Rβ or IL-2RβΔA, γc, Jak3, β-casein–luciferase reporter construct and the pRL-TK reporter construct, with or without 20-1000 ng of the JAB/SOCS1/SSI-1WT expression vector. After 24 hours, cells were split and plated in duplicate and stimulated with 20 nM IL-2 for another 24 hours or left untreated. Activity of the β-casein–luciferase reporter construct was normalized against pRL-TK control luciferase. The data shown are the mean ± SD of 6 experiments.
To verify these data and to further define the region of IL-2Rβ that mediates the interaction, we studied the ability of C-terminal IL-2Rβ truncation mutants to interact with JAB/SOCS1/SSI-1. The sequences distal to amino acid 371 were not required for this association (Figure 5B, top panel, lanes 4-5 vs lane 1), while truncation at amino acid 330 greatly diminished the interaction (lane 2 vs lane 1). Interaction was seen with the IL-2Rβ350, although the interaction was less than that seen with WT IL-2Rβ. These results define residues 330-350 within the A region of IL-2Rβ as critical for its interaction with JAB/SOCS1/SSI-1WT, which is analogous to the findings for CIS1.33 The bases for the interactions of CIS1 and JAB with IL-2Rβ are unclear. However, because this region of IL-2Rβ contains Y338, we investigated whether tyrosine mutants of IL-2Rβ could still associate with JAB/SOCS1/SSI-1. Indeed, we found that IL-2Rβ mutants in which all 6 tyrosines or only Y338 were mutated to phenylalanines could still associate with JAB/SOCS1/SSI-1 (Figure 5C). Moreover, these interactions occurred under conditions of no IL-2 stimulation. Although it is conceivable that the IL-2Rβ–JAB/SOCS1/SSI-1 interaction is in part phosphotyrosine based, it is clear that substantial interaction is independent of tyrosine phosphorylation of IL-2Rβ.
We next sought to clarify whether the interaction between IL-2Rβ and JAB/SOCS1/SSI-1 is required for the inhibitory action of JAB/SOCS1/SSI-1. We therefore studied the effect of JAB/SOCS1/SSI-1WT overexpression on IL-2–induced luciferase activity in an IL-2R reconstitution system by using either WT IL-2Rβ or IL-2Rβ with the A region deleted (Figure 5D). In these experiments, the activity mediated by the IL-2Rβ WT construct was more potent than the IL-2Rβ-ΔA mutant. Nevertheless, JAB/SOCS1/SSI-1 was able to potently inhibit IL-2–induced luciferase activity even when the A region was deleted, presumably because it can directly bind to Jaks. Interestingly, the degree of inhibition of reporter activity mediated by the IL-2Rβ-ΔA mutant by a range of concentrations of JAB/SOCS1/SSI-1 was if anything greater than that seen with WT IL-2Rβ. This suggests that the ability of JAB/SOCS1/SSI-1 to interact with IL-2Rβ is not important for its inhibitory function. This is contrary to CIS1, which cannot associate with Jak kinases and whose inhibitory function requires the A region of IL-2Rβ.33
Discussion
Cytokine signaling is regulated by a balance between activating and inhibitory signals. Genetic alterations causing deleterious mutations in different components of the IL-2R are associated with abnormal lymphocyte function and impaired lymphoid homeostasis. Mice lacking IL-23 or mice or humans lacking IL-2Rα4,42 exhibit lymphocyte activation and severe autoimmunity; IL-2Rβ–deficient mice exhibit both enhanced T-cell and B-cell activation and NK-cell deficiency43,44; and γc deficiency causes X-linked severe combined immune deficiency (SCID) in humans37 and deregulated proliferation and accumulation of CD4+ lymphocytes in mice.45,46 Much less is known about negative regulators of IL-2 signaling. It has been suggested that decreased expression of Shp-1 might result in augmented IL-2–independent growth of human T lymphotropic virus–I (HTLV-I)–infected T cells, thereby suggesting a negative regulatory role for Shp-1 in IL-2 signaling.19Recently, lymphocytes from JAB/SOCS1/SSI-1–deficient mice were shown to exhibit enhanced IL-2 responsiveness, suggesting a negative regulatory role for JAB/SOCS1/SSI-1 in IL-2 signaling.34 35 Here we have studied the role of JAB/SOCS1/SSI-1 in IL-2 signaling and have found that JAB/SOCS1/SSI-1 is rapidly induced in PBLs following IL-2 stimulation. Furthermore, we show that JAB/SOCS1/SSI-1 inhibits IL-2–induced Stat5 tyrosine phosphorylation and Stat5-dependent transcription, thereby suggesting a role for JAB/SOCS1/SSI-1 as a negative regulator in IL-2 signaling.
JAB/SOCS1/SSI-1 was originally characterized in the context of IL-6 and LIF signaling. Subsequently, JAB/SOCS1/SSI-1 was shown to be induced also by prolactin, growth hormone, and IFN-γ. In stable overexpressing cell lines and transient expression systems, JAB/SOCS1/SSI-1 has been shown to inhibit signaling by these cytokines as well as by IL-4, which does not induce JAB/SOCS1/SSI-1 expression.47 This suggests that JAB/SOCS1/SSI-1 and possibly other CIS/SOCS/SSI family members are not only involved in classical negative feedback circuits but can also mediate cross-talk between different cytokine receptors.48 49 Our results show that IL-2 potently induces JAB/SOCS1/SSI-1, and we hypothesize that JAB/SOCS1/SSI-1 represents a Stat5-dependent negative feedback loop in IL-2 signaling.
The mechanism of inhibition by JAB/SOCS1/SSI-1 and other family members is being extensively studied. It is clear that JAB/SOCS1/SSI-1 can bind to several cytokine receptor-associated signaling molecules and modulate their activity. In overexpression systems, JAB/SOCS1/SSI-1 has been shown to bind to and/or modulate the activity of all the Jak family kinases as well as Grb-2, Vav, and Tec.25,50,51 The functional activity of JAB/SOCS1/SSI-1 is phosphotyrosine dependent, and mutations in the SH2 domain of JAB/SOCS1/SSI-1 block its inhibitory action52,53 (Figure 3). The exact mechanism of inhibition by this protein is not fully understood; however, there is convincing evidence that JAB/SOCS1/SSI-1 can bind to the kinase activation loop of Jak2, thereby possibly interfering with the phosphotransfer function of the kinase domain.25 30 It is reasonable to hypothesize that JAB/SOCS1/SSI-1 might inhibit the activity of other kinases by the same mechanism. As previously reported, we found that JAB/SOCS1/SSI-1 was able to associate with both Jak1 and Jak3, and at least for Jak1, JAB/SOCS1/SSI-1 efficiently inhibited the tyrosine phosphorylation and kinase activity. We also found that like CIS1, JAB/SOCS1/SSI-1 also interacts with the A region of IL-2Rβ. However, deleting the A region of IL-2Rβ only affects the inhibitory action of CIS1.
These results suggest that in the IL-2 pathway, a critical target of JAB/SOCS1/SSI-1 action is Jak1. Jak1 has also been implicated as the target of JAB/SOCS1/SSI-1 action in the IL-4–signaling system,47 which shares some signaling components with the IL-2R system. Recent studies suggest a second possible mechanism of inhibition. The SOCS box of JAB/SOCS1/SSI-1 has been shown to associate with elongins B and C, suggesting that the SOCS box may function as a linker protein that directs signaling molecules to proteosomal degradation.54 However, this remains unclear, as the SOCS box has also been implicated in maintaining the stability of CIS/SOCS/SSI family proteins.52 55 Whether this mechanism contributes to the inhibition of IL-2 signaling needs to be resolved.
In conclusion, our data, together with the earlier observation that T cells from JAB/SOCS1/SSI-1 KO mice exhibit enhanced IL-2 responsiveness, suggest that JAB/SOCS1/SSI-1 is part of the IL-2 pathway in T lymphocytes and that JAB/SOCS1/SSI-1 may have a role in regulating T-cell responses. However, it is also clear that more than one CIS/SOCS/SSI protein regulates IL-2 signaling, and more studies are needed to clarify the in vivo role(s) of these proteins.
Supported in part by a grant (B.S.) from the Swiss National Science Foundation, Bern, Switzerland; by grants (P.E.K) from the Academy of Finland and the Finnish Cancer Society, Finland; by a grant from the Emil Aaltonen Foundation, Finland; and by a grant from the Maud Kuistila Foundation, Finland.
B.S. and P.E.K. contributed equally to this study.
Submitted March 7, 2000; accepted September 8, 2000.
References
Author notes
Warren J. Leonard, Lab of Molecular Immunology, NHLB1, NIH, Bethesda, MD 20892-1674; e-mail:wjl@helix.nih.gov. © 2001 by The American Society of Hematology




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal