The c-Abl gene encodes a widely expressed tyrosine kinase that is important for development, particularly of the nervous system.1 But the signaling pathways that employ c-Abl in normal cells are not well defined. Abl protein is located in both the nucleus and the cytoplasm, and recent studies suggest that a substantial component of Abl resides in the actin cytoskeleton and mediates signaling related to integrin activation.2 Other studies have linked Abl to the cellular response to DNA damage, including cell cycle arrest, DNA repair, and apoptosis.3-7Abl tyrosine kinase is activated in response to ionizing radiation, and fibroblasts from Abl knockout mice have been reported to have reduced sensitivity to ionizing radiation.7
Abl kinase activity is activated in the Bcr/Abl andTel/Abl oncogene products, resulting in leukemia. STI571 is a novel tyrosine kinase inhibitor (Novartis, Basel, Switzerland) that inactivates Bcr/Abl, c-Abl, platelet-derived growth factor receptor, and c-kit, but not members of the Src or Jak families of tyrosine kinases.8 STI571 inhibits growth of cell lines transformed by Bcr/Abl or primary cells from patients with chronic myeloid leukemia (CML) and induces apoptosis of CML cells in tissue culture studies.9 Importantly, STI571 inhibits Abl kinase activity at concentrations that are otherwise nontoxic to normal hematopoietic cells, and has shown impressive activity in early-phase clinical trials in patients with CML.10
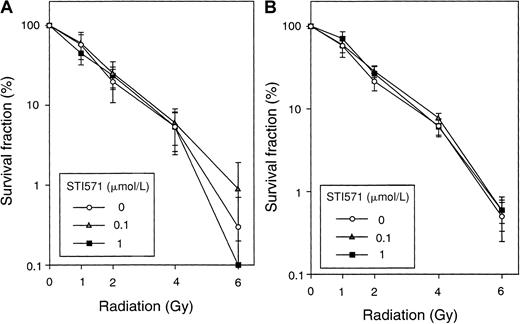
Because ionizing radiation activates Abl kinase and loss of theAbl gene has been associated with reduced sensitivity to ionizing radiation, it is possible that inhibition of Abl by STI571 would reduce sensitivity to ionizing radiation. To test this hypothesis, normal bone marrow cells from BALB/c mice exposed to 0, 0.1, or 1 μmol/L STI571 for 24 hours before being exposed to 0-6 Gy of gamma radiation, using a Gammacell 1000 (Atomic Energy of Canada, Mississagua, Canada). One μmol/L STI571 is sufficient to completely inhibit Bcr/Abl and c-Abl tyrosine kinase activity when assayed by the sensitive technique of immune complex kinase assay (Uemura, unpublished data, 1999). After irradiation, granulocyte/macrophage colony-forming cells (CFU-GMs) were measured. The methylcellulose medium contained STI571 at the same concentration as pretreatment (Figure1A). The survival of CFU-GMs at each dose of radiation was the same with or without STI571.
Effects of STI571 on survival after ionizing radiation.
Bone marrow cells from BALB/c mice were treated with 0, 0.1, or 1 μmol/L STI571 for 24 hours before being exposed to 0-6 Gy gamma irradiation. After irradiation, cells (5 × 104 to 2 × 105 cells) were plated in triplicate in 1 mL Iscove modified Dulbecco medium containing 1% methylcellulose, 20% fetal bovine serum, 5% WEHI-3B conditioned medium as a source of murine IL-3, 20 ng/mL recombinant human G-CSF, and STI571 (at the same concentration as pretreatment). (A) CFU-GMs (> 50 cells) were enumerated on day 8 to determine radiation response. The results are presented as the percentage of surviving colonies relative to an unirradiated control. (B) U937 cells were treated with 0, 0.1, or 1 μmol/L STI571 for 24 hours before being exposed to 0- 6 Gy gamma irradiation. After irradiation, cells (1 × 103 cells) were plated in 1 mL of RPMI 1640 containing 1% methylcellulose, 20% FCS, and STI571 (the same concentrations as pretreatment). Colonies were counted on day 8. Shown here is 1 of 2 independent experiments.
Effects of STI571 on survival after ionizing radiation.
Bone marrow cells from BALB/c mice were treated with 0, 0.1, or 1 μmol/L STI571 for 24 hours before being exposed to 0-6 Gy gamma irradiation. After irradiation, cells (5 × 104 to 2 × 105 cells) were plated in triplicate in 1 mL Iscove modified Dulbecco medium containing 1% methylcellulose, 20% fetal bovine serum, 5% WEHI-3B conditioned medium as a source of murine IL-3, 20 ng/mL recombinant human G-CSF, and STI571 (at the same concentration as pretreatment). (A) CFU-GMs (> 50 cells) were enumerated on day 8 to determine radiation response. The results are presented as the percentage of surviving colonies relative to an unirradiated control. (B) U937 cells were treated with 0, 0.1, or 1 μmol/L STI571 for 24 hours before being exposed to 0- 6 Gy gamma irradiation. After irradiation, cells (1 × 103 cells) were plated in 1 mL of RPMI 1640 containing 1% methylcellulose, 20% FCS, and STI571 (the same concentrations as pretreatment). Colonies were counted on day 8. Shown here is 1 of 2 independent experiments.
The responses to genotoxic stresses are believed to be mediated by both p53-dependent and -independent pathways. The possibility that Abl kinase activity contributes to radiation sensitivity in cell lines in which p53 is mutated was, therefore, considered. Thep53 mutant hematopoietic cell line U937 was exposed to ionizing radiation (0-6 Gy), and the fraction of surviving cells was again measured by colony formation. Again, the addition of STI571 did not alter the surviving fraction or size of surviving colonies, after radiation (Figure 1B). Overall, our results suggest that, with the techniques used here, inhibition of c-Abl tyrosine kinase with STI571 at concentrations in culture of up to 1 μmol/L does not affect radiation response where survival is the endpoint.
In a previous study, Dan et al treated U937 cells with etoposide and found that 10 μmol/L STI571 (previously known as CGP57148B) reduced apoptosis of U937 cells without interfering with JNK1/SAPK activation.11 It was concluded that c-Abl acts downstream of caspases during the development of p53- independent apoptosis. Our study differs from that of Dan et al by the use of a lower dose of STI571. We found that 1 μmol/L STI571 was sufficient to essentially completely inhibit Bcr/Abl, Tel/Abl, or c-Abl kinase activity but, unlike 10 μmol/L STI571, was not associated with any nonspecific toxicity either on normal murine marrow cells or on U937 cells. It is also possible that Abl kinase is necessary for DNA repair responses to etoposide, but not to ionizing radiation.
Whereas c-Abl seems to be activated in response to ionizing radiation, the requirement for Abl tyrosine kinase activity to mediate cellular responses to DNA damage is less clear. For example, while Abl −/− fibroblasts from mice have been reported to have increased survival after radiation, Abl −/− avian cells had a normal survival curve.7,12 Also, the role of Abl in DNA-damage–induced G1 arrest is controversial.1,6,12 In the present study, we found no evidence that pharmacologic kinase inhibition of c-Abl with STI571 altered radiation sensitivity in either primary murine hematopoietic cells or in a human p53 mutant cell line. However, some functions of c-Abl are kinase-independent,13 14 and it is possible that kinase activity is not required for some cellular responses to radiation. In any event, these results suggest that the effects of ionizing radiation in patients should not be altered significantly by STI571 treatment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal