Abstract
Chronic myelogenous leukemia (CML), a malignancy of a hematopoietic stem cell, is caused by the Bcr-Abl tyrosine kinase. STI571(formerly CGP 57148B), an Abl tyrosine kinase inhibitor, has specific in vitro antileukemic activity against Bcr-Abl–positive cells and is currently in Phase II clinical trials. As it is likely that resistance to a single agent would be observed, combinations of STI571 with other antileukemic agents have been evaluated for activity against Bcr-Abl–positive cell lines and in colony-forming assays in vitro. The specific antileukemic agents tested included several agents currently used for the treatment of CML: interferon-alpha (IFN), hydroxyurea (HU), daunorubicin (DNR), and cytosine arabinoside (Ara-C). In proliferation assays that use Bcr-Abl–expressing cells lines, the combination of STI571 with IFN, DNR, and Ara-C showed additive or synergistic effects, whereas the combination of STI571 and HU demonstrated antagonistic effects. However, in colony-forming assays that use CML patient samples, all combinations showed increased antiproliferative effects as compared with STI571 alone. These data indicate that combinations of STI571 with IFN, DNR, or Ara-C may be more useful than STI571 alone in the treatment of CML and suggest consideration of clinical trials of these combinations.
Introduction
Chronic myelogenous leukemia (CML) is a hematopoietic stem cell disorder. In the chronic phase of the disease, there are excess numbers of myeloid cells; however, these cells differentiate and function normally. Over time, there is a progressive loss of terminal differentiation, and the disease terminates in an acute leukemia, known as blast crisis. Blast crisis is usually of myeloid phenotype, but, in up to one third of patients, a lymphoid phenotype is seen. In all phases of the disease, leukemic cells contain the Philadelphia (Ph) chromosome.1 In addition, approximately 20% of adults and 5% of children with acute lymphoblastic leukemia (ALL) are Ph chromosome positive.1On a molecular level, the Ph chromosome results in the juxtaposition of Bcr and Abl sequences, leading to a chimeric messenger RNA and protein termed Bcr-Abl.2,3 Virtually all cases of CML express a 210-kd form of Bcr-Abl, whereas 50% of adults and 95% of children with Ph-positive ALL express a shorter version of Bcr-Abl, termed p185 or 190 Bcr-Abl.1 4
Current standard treatments for CML include stem cell transplantation, interferon (IFN)-alpha-containing regimens, and hydroxyurea (HU). Allogeneic stem cell transplantation is the only curative therapy. Long-term survival from this procedure is approximately 65%; however, only one third of patients are eligible for this procedure because of the lack of availability of a donor or because of concerns regarding mortality with advancing age. Thus, the overall cure rate for CML is less than 20%.4-6 IFN can prolong survival by an average of up to 2 years as compared with HU, and there is evidence that the addition of cytosine arabinoside (Ara-C) to IFN improves response rate and survival.7-9 In the blast phase of the disease, patients are typically refractory to standard induction chemotherapy regimens, particularly with myeloid disease phenotype.4,5Patients with Ph chromosome–positive ALL have a worse prognosis than do patients with normal cytogenetics.10 11
Bcr-Abl is a constitutively activated tyrosine kinase that has been shown to be the cause of CML with tyrosine kinase activity being essential to the function of the Bcr-Abl protein.12-14Recent clinical trials with STI571, a specific inhibitor of the Bcr-Abl tyrosine kinase, have shown this compound to have significant activity in all phases of CML as well as Ph chromosome–positive acute leukemias.15,16 However, in patients with acute leukemias, relapses have been a major problem.15 This situation raises the possibility that later relapses will also be seen in chronic phase patients because of resistance to STI571. In this study, combinations of STI571 with several antileukemic agents were investigated, including IFN, HU, daunorubicin (DNR), and Ara-C. These studies were performed to determine whether these combinations would enhance the activity of STI571 and whether these combinations might be useful in an attempt to circumvent resistance.
Materials and methods
Reagents
STI571, provided by E. Buchdunger (Novartis, Basel, Switzerland), was prepared as a 10 mmol/L stock solution in sterile phosphate-buffered saline and was diluted in RPMI-1640 medium before use. DNR (Bedford Labs, Bedford, OH), IFN-α (Schering, Kenilworth, NJ), Ara-C (Pharmacia & Upjohn, Kalamazoo, MI), and HU (Sigma, St Louis, MO) were dissolved in sterile water, diluted in RPMI-1640 medium (Gibco BRL, Rockville, MD), and used within 24 hours of reconstitution. 3-(4,5-Dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma) was stored as a 10 mg/mL sterile stock solution and used at a final concentration of 1 mg/mL.
Cell lines and culture conditions
MO7e, a human megakaryoblastic cell line,17 was grown in RPMI-1640 medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS; Gibco BRL), 2% (v/v) l-glutamine (Gibco BRL), 1% (v/v) penicillin-streptomycin (pen/strep), and 10 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) (Immunex, Seattle, WA).
MO7p210, a derivative of MO7e engineered to express Bcr-Abl,18 and K562, a Bcr-Abl–positive CML blast crisis patient cell line,19 were grown in RPMI-1640 medium supplemented with 10% (v/v) FBS, 2% l-glutamine, and 1% pen/strep. All cell lines were grown in 5% CO2/95% O2 air, in a 37°C fully humidified incubator.
MTT assays
Cells were plated at a concentration of 5 × 103cells per well. Each concentration of drug alone and in combination with STI571 was assayed in quadruplicate. Controls were performed, using identical dilutions of medium with identical concentrations of solvent used for STI571 and the antileukemic agents. Each plate contained serial dilutions of cells to ensure that a linear relationship between optical density (OD) and cell number was maintained. Wells were assayed for uptake of MTT at daily intervals as described.20 Plates were read with a 96-well scanning spectrophotometer at 570 nm and reported as ODs. The mean and standard deviation were calculated for each concentration and combination and were reported as the percentage of growth versus control. Cell proliferation curves were generated from these data, and results from day 3 were used to assess for activity. The percentage of inhibition of proliferation is calculated as 1 − (OD MO7p210 + drug/OD MO7p210) × 100. To evaluate antagonistic, additive, or synergistic effects of drug combinations, isobolograms were generated from the inhibitory concentrations with the highest number of data points and plotted as a function of the concentration of IFN, DNR, Ara-C, or HU versus the concentration of STI571.
Colony-forming assays
Colony-forming assays were performed on bone marrow and peripheral blood samples of 4 CML patients. Each of these patients was in late chronic phase or early accelerated phase of the disease, had failed a trial of IFN, and were off all therapy for at least 1 week. Bone marrow or blood samples were collected after obtaining informed consent. Five milliliters of bone marrow or 10 mL of peripheral blood was diluted 1:4 with warmed Iscove modified Dulbecco media (IMDM; Gibco BRL), layered over Ficoll-Paque (Amersham Pharmacia, Uppsala, Sweden), and centrifuged at 1300 rpm for 20 minutes at 20°C. Mononuclear cells were aspirated from the density interface, resuspended in 15 mL of IMDM, and pelleted by centrifugation at 1000 rpm for 10 minutes at 20°C. After resuspension in IMDM, viable cells were counted by trypan blue exclusion and diluted to a concentration of 5 × 105cells/mL. Cells were diluted 1:10 in methylcellulose media containing erythropoietin and interleukin-3 (IL-3) for burst-forming unit, erythroid (BFU-E) assays (Methocult GF H4434, Stem Cell Technologies, Vancouver, BC), or GM-CSF and IL-3 for colony-forming unit–granulocyte-macrophage (CFU-GM) assays (Methocult GF H4534, Stem Cell Technologies). Cells (5 × 104) were plated in 35-mm cell culture dishes. A range of concentrations and combinations of STI571 and other antileukemic agents were assayed in duplicates. After a 2-week incubation at 37°C, BFU-E and CFU-GM were counted. Results were calculated as the percentage of growth versus control.
Statistical analysis
BFU-E and CFU-GM are expressed as the percentage of inhibition versus control, and the mean and standard deviation of colony inhibition for each dose of drug and each combination were calculated across all patient samples. Nonpaired, single-tail t tests were used to evaluate efficacy of STI571 and drugs versus STI571 alone, as well as STI571 and drugs versus drug alone.
Results
Cellular proliferation studies
Three cell lines were analyzed for inhibition of proliferation by STI571 and a variety of antileukemic agents, HU, IFN, Ara-C, and DNR. The 3 cell lines chosen for this analysis were MO7e cells, a human megakaryoblastic cell line that requires either GM-CSF, IL-3, or steel factor (SF) for survival or proliferation; MO7p210 cells, a derivative of the MO7e cell line, engineered to express Bcr-Abl; and K562 cells, a Bcr-Abl–positive human cell line derived from a CML patient in blast crisis. Initial experiments were performed with a wide range of concentrations of STI571 and each of the antileukemic agents to establish an approximation of a 50% inhibitory concentration (IC50). Additional experiments were then performed by using a narrower range of concentrations to determine whether additive, synergistic, or antagonistic effects of STI571 were seen with each of the antileukemic agents.
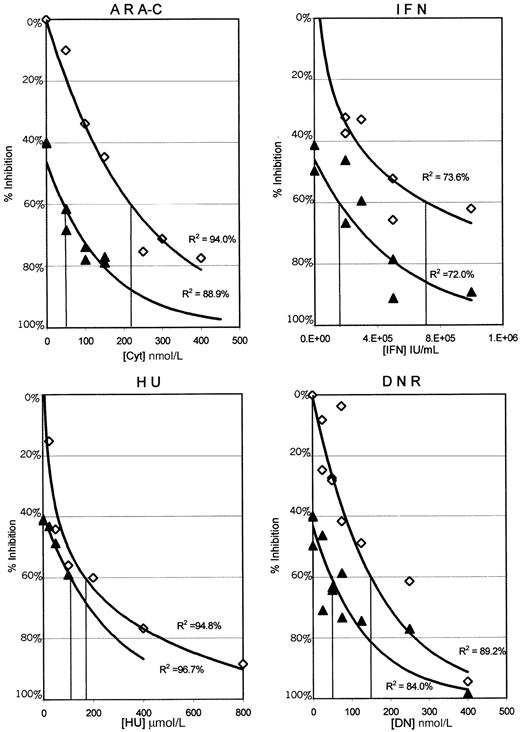
The results presented in Figure 1 plot the percentage of inhibition of proliferation of the MO7p210 cells after 3 days in the continuous presence of HU, IFN, DNR, or Ara-C alone as compared with 0.05 μmol/L STI571 combined with each of these agents. In these graphs, the antileukemic activity of STI571 alone is indicated by the intersection with the y-axis. Each data point represents the mean of 4 wells from 1 of 3 separate experiments. To determine the relationship between the percentage of inhibition of proliferation and drug concentrations, a best fit regression line was generated. R2 values indicate the percentage of data that can be accounted for by the regression line. For all of the antileukemic agents, except HU, there is a substantial shift in the curves, consistent with increased antiproliferative activity of the combinations. Similar data were obtained by using 0.025 and 0.1 μmol/L STI571 (data not shown). As the highest clustering of data points in Figure 1 was at or near an IC60 for the combinations, these regression lines were used to estimate an IC60 for each of the combinations as compared with each of the antileukemic agents alone (Table 1).
MO7p210 dose response curves for each of the antileukemic agents alone and in combination with 0.05μmol/L STI571.
Cells were analyzed daily for uptake of MTT in the presence or absence of various antileukemic agents. Data from day 3 is plotted as the concentration of antileukemic drug versus inhibition of proliferation, comparing each antileukemic agent alone (⋄) to each antileukemic agent with 0.05 μmol/L STI571 (▴). Each data point represents a mean across 4 identical wells from 1 of 3 separate experiments. Standard deviations ranged from ± 0% to 13%, with a median of 2%. Regression lines generated in Microsoft Excel represent the best fit relationship between drug concentration and the percentage of inhibition of proliferation. R2 values indicate the percentage of data that can be accounted for by the regression line. IC60 concentrations for the MO7p210 with each antileukemic alone and in combination with STI571 were determined from these graphs and are listed in Table 1. Identical analyses were performed for both the MO7e and K562 cell lines, with the results summarized in Table 1.
MO7p210 dose response curves for each of the antileukemic agents alone and in combination with 0.05μmol/L STI571.
Cells were analyzed daily for uptake of MTT in the presence or absence of various antileukemic agents. Data from day 3 is plotted as the concentration of antileukemic drug versus inhibition of proliferation, comparing each antileukemic agent alone (⋄) to each antileukemic agent with 0.05 μmol/L STI571 (▴). Each data point represents a mean across 4 identical wells from 1 of 3 separate experiments. Standard deviations ranged from ± 0% to 13%, with a median of 2%. Regression lines generated in Microsoft Excel represent the best fit relationship between drug concentration and the percentage of inhibition of proliferation. R2 values indicate the percentage of data that can be accounted for by the regression line. IC60 concentrations for the MO7p210 with each antileukemic alone and in combination with STI571 were determined from these graphs and are listed in Table 1. Identical analyses were performed for both the MO7e and K562 cell lines, with the results summarized in Table 1.
IC60 concentrations of antileukemic agents alone and in combination with STI571
| Agent(s) . | MO7 . | MO7p210 . | K562 . |
|---|---|---|---|
| STI571 | >10 μmol/L | 0.07 μmol/L | 0.27 μmol/L |
| HU | 63 μmol/L | 167 μmol/L | 367 μmol/L |
| STI571 + HU | 59 μmol/L | 110 μmol/L | 340 μmol/L |
| DNR | 59 nmol/L | 154 nmol/L | 164 nmol/L |
| STI571 + DNR | 57 nmol/L | 47 nmol/L | 72 nmol/L |
| IFN | 7.6e5 IU/mL | 7.2e5 IU/mL | 6.0e5 IU/mL |
| STI571 + IFN | 7.3e5 IU/mL | 1.5e5 IU/mL | 3.2e5 IU/mL |
| Ara-C | 45 nmol/L | 218 nmol/L | 159 nmol/L |
| STI571 + Ara-C | 40 nmol/L | 42 nmol/L | 53 nmol/L |
| Agent(s) . | MO7 . | MO7p210 . | K562 . |
|---|---|---|---|
| STI571 | >10 μmol/L | 0.07 μmol/L | 0.27 μmol/L |
| HU | 63 μmol/L | 167 μmol/L | 367 μmol/L |
| STI571 + HU | 59 μmol/L | 110 μmol/L | 340 μmol/L |
| DNR | 59 nmol/L | 154 nmol/L | 164 nmol/L |
| STI571 + DNR | 57 nmol/L | 47 nmol/L | 72 nmol/L |
| IFN | 7.6e5 IU/mL | 7.2e5 IU/mL | 6.0e5 IU/mL |
| STI571 + IFN | 7.3e5 IU/mL | 1.5e5 IU/mL | 3.2e5 IU/mL |
| Ara-C | 45 nmol/L | 218 nmol/L | 159 nmol/L |
| STI571 + Ara-C | 40 nmol/L | 42 nmol/L | 53 nmol/L |
IC60 concentrations were determined as described in Figure 1.
HU indicates hydroxyurea; DNR, daunorubicin; IFN, interferon; Ara-C, cytosine arabinoside.
MO7e cell line
When grown in GM-CSF, MO7e cells were not inhibited by concentrations of STI571 of up to 10 μmol/L. In contrast, the growth of MO7e cells was easily inhibited by various concentrations of antileukemic agents, IFN, DNR, HU, and Ara-C. As predicted, there was no change in the inhibition of proliferation of MO7e cells when STI571 was added to IFN, DNR, HU, or Ara-C compared with each of the antileukemic agents alone (Table 1).
MO7p210 cell line
MO7p210 cells have previously been shown to be highly sensitive to STI571.21 Consistent with the known resistance to antileukemic agents imparted by Bcr-Abl expression, MO7p210 cells required higher concentrations of each of the antileukemic agents, except IFN, for inhibition of cellular proliferation, as compared with MO7 cells (Table 1). However, when STI571 was combined with IFN, DNR, or Ara-C, the IC60s for these agents dropped to concentrations lower or equal to those of the MO7e parental cell line. There was no change in the IC60 when STI571 was combined with HU (Table 1).
K562 cell line
Identical experiments were performed with K562 cells, a Bcr-Abl–positive human cell line derived from a CML patient in blast crisis. Concentrations of 0.1, 0.25, and 0.5 μmol/L STI571 were combined with each of the antileukemic agents. A 40% to 45% inhibition of proliferation was seen with the use of 0.25 μmol/L STI571. Thus, this dose was selected to construct graphs similar to those in Figure 1 for the K562 cell lines. Regression lines were generated for each of the combinations, and IC60 concentrations were determined from these graphs (Table 1). Similar to the MO7p210 cells, K562 cells demonstrated substantially higher IC60s for DNR, HU, and Ara-C than the MO7e cell line (Table 1). Again, with the exception of HU, combining each of the antileukemic agents with STI571 resulted in a substantial decrease in the IC60s (Table 1).
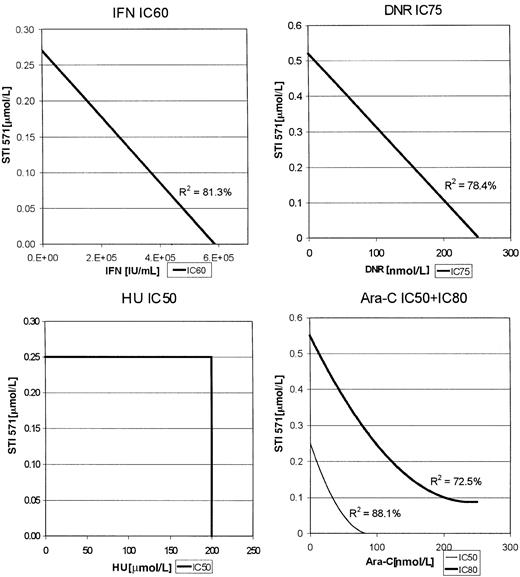
For the K562 cells, enough data points were clustered at various inhibitory concentrations of each of the combinations to construct isobolograms (Figure 2). Thus, for the combination of Ara-C and STI571, the IC50s and IC80s were used, whereas IC75s were used for the combination of DNR with STI571 as indicated in Figure 2. In this analysis, a straight line would represent an additive effect, a downward bowing curve a synergistic effect, and an upward bowing curve an antagonistic effect. As with the MO7p210 cells, the combinations of STI571 plus IFN or DNR produced additive antileukemic effects, whereas STI571 plus Ara-C produced the most substantial increase in inhibition of proliferation, consistent with a synergistic effect. R2 values, indicating the degree of fit for the generation of the regression lines, were all more than 0.74, demonstrating a high degree of confidence in these regression lines.
K562 isobolograms.
Isobolograms are generated from the inhibitory concentrations with the highest number of data points. Concentrations producing the indicated inhibition are plotted as a function of antileukemic drug versus STI571 concentration.
K562 isobolograms.
Isobolograms are generated from the inhibitory concentrations with the highest number of data points. Concentrations producing the indicated inhibition are plotted as a function of antileukemic drug versus STI571 concentration.
Colony-forming assays
Colony-forming assays that used bone marrow or peripheral blood samples from 4 CML patients were performed, and BFU-E and CFU-GM were counted. In these assays, STI571 was again analyzed in combination with HU, IFN, DNR, or Ara-C. All 4 of the patients were in late chronic phase or early accelerated phase of the disease. Blood or marrow samples were obtained with patients who had been off all therapies for at least 1 week; thus, circulating drug levels were not likely to be a confounding variable in these assays.
In Figure 3, the data for CFU-GM and BFU-E are shown. In this figure, data are plotted that compare STI571 alone as the basis for comparison with each additional line, representing a different dose of the various antileukemic agents. Data were plotted in this manner, as there was consistent inhibition of colony formation for all patients at each dose of STI571, whereas there was significant interpatient variability with the different antileukemic agents. As can be seen in Figure 3, STI571 in combination with IFN, DNR, or Ara-C produced substantial decreases in colony formation. This increased inhibition of colony formation was seen in both CFU-GM and BFU-E and is consistent with the cell line proliferation assays. In most of these combinations, the decrease in colony formation by adding STI571 to either IFN, DNR, or Ara-C was statistically significant. However, in contrast to the results of the MO7p210 and K562 cell line assays, combinations of STI571 with HU also demonstrated significant inhibition of colony formation.
Compiled BFU-E and CFU-GM colony-forming assay data.
Results are plotted as a function of STI571 concentration versus the percentage of colony-formation inhibition. Each data line represents a dose of antileukemic agent, with data points representing averaged colony-formation responses across all 4 patients. Combinations of antileukemic agents and STI571 that produced a statistically significant decrease in colony formation are denoted as significant (*, .005 < P < .05) or highly significant (+,P < .005) in comparison to STI571 alone.
Compiled BFU-E and CFU-GM colony-forming assay data.
Results are plotted as a function of STI571 concentration versus the percentage of colony-formation inhibition. Each data line represents a dose of antileukemic agent, with data points representing averaged colony-formation responses across all 4 patients. Combinations of antileukemic agents and STI571 that produced a statistically significant decrease in colony formation are denoted as significant (*, .005 < P < .05) or highly significant (+,P < .005) in comparison to STI571 alone.
Discussion
In this study, the combination of STI571 with various antileukemic agents was investigated. The major conclusion from the data is that the addition of standard agents used for the treatment of various stages of CML adds to the antiproliferative activities of STI571. The only exception to this conclusion was that HU appeared antagonistic in cell lines; however, it did improve on STI571 when analyzed in colony-forming assays that used CML patient samples. This discrepancy could be due to the difference in the assay systems, differences in cellular phenotype, or other unknown factors.
The possibility of antagonism between STI571 and antileukemic agents was a major reason for undertaking these studies. In colony-forming assays that used chronic phase CML patient samples, we have previously demonstrated that STI571 selects for the growth of benign hematopoietic progenitors.21 However, attempts to eliminate the Bcr-Abl–positive clone by incubating STI571 with CD34+cells in the presence of a variety of cytokines have failed (B.J.D., unpublished data, June 1996). The most likely explanation for this discrepancy is that colony-forming assays require cellular proliferation, whereas, for the in vitro purging experiments, cells may remain quiescent. Thus, we hypothesized that inhibition of cellular proliferation with antileukemic agents may protect Bcr-Abl–expressing cells from STI571. As seen, with the exception of HU, this situation was not the case.
Having demonstrated that STI571 improves the in vitro benefits from antileukemic agents, further studies are warranted to elucidate the mechanism of this enhancement. STI571 is known to induce apoptosis of Bcr-Abl–expressing cell lines.21-23 Thus, it is possible that the same or different apoptotic pathways are used by STI571 as compared with the other antileukemic agents to explain additive or synergistic effects. Whether cell cycle–dependent effects of these agents are also operational is also worthy of investigation.
The results presented in this study are consistent with the findings that Bcr-Abl expression renders cells resistant to chemotherapeutic agents.24 25 As seen, both MO7p210 and K562 cells required higher doses of antileukemic agents for cell killing than did M07 cells. However, treatment of the Bcr-Abl–expressing cells with STI571 rendered these cells susceptible to cell killing at concentrations of the antileukemic agents similar to the concentration required to kill MO7 cells. These data suggest that the Bcr-Abl–resistance phenotype can be completely reversed by treating cells with STI571.
We chose low-dose, continuous exposure to the antileukemic agents rather than investigating all possible combinations of doses and schedules. This investigation was done specifically to evaluate whether STI571 would increase the sensitivity of Bcr-Abl–expressing cells to antileukemic agents. Thus, the experiments presented in the study are most obviously applicable to chronic phase patients whose current treatment regimens include low-dose, continuous exposure to agents, such as IFN and Ara-C. In particular, the colony-forming data validate the rationale for clinical trials of these combinations in chronic phase patients. In acute leukemia patients, antileukemic agents are typically given as high-dose bolus infusions. By reversing the chemotherapy resistance phenotype of Bcr-Abl–expressing cell lines, it is also possible that STI571 will enhance the benefits from standard chemotherapy regimens used for the treatment of Bcr-Abl acute leukemias. As relapses in blast crisis patients treated with STI571 are a major problem,15 and blast crisis patients are highly resistant to standard chemotherapy, these data suggest that combinations of STI571 with standard antileukemic agents are a viable approach to the treatment of Bcr-Abl acute leukemias.
Despite the dramatic results with the use of STI571 to treat patients with chronic phase CML who have failed IFN therapy,16 the major questions being addressed in ongoing clinical trials of STI571 in these chronic phase patients are the duration of responses and whether it will be possible to completely eradicate the leukemic clone. As Bcr-Abl is thought to contribute to the genetic instability responsible for disease progression,26 it is possible that long-term therapy with STI571 without eradication of Bcr-Abl could also improve survival. In any case, it is possible that resistance would develop with long-term administration of STI571 without elimination of the leukemic clone or that side effects from long-term administration would be observed. Thus, the preferable approach would be to combine STI571 with other agents to either prevent the emergence of resistant clones or to enhance the eradication of the leukemic clone.
Although the data suggests that Ara-C may be the best partner with STI571 in terms of synergy, caution in overinterpreting this finding is warranted. In particular, all of the patients whose samples were used in colony-forming assays had failed a trial of IFN. Thus, the benefits from the STI571/IFN combination may be underestimated from these in vitro studies. However, these data suggest that clinical trials with these combinations are worth pursuing. On the basis of these in vitro data, clinical trials are planned that use the combination of STI571 with IFN and STI571 with low-dose Ara-C. In addition, trials of combinations of STI571 with standard induction chemotherapy are planned for patients with CML blast crisis and Ph + ALL.
Supported by National Institutes of Health grant CA65823. B.J.D. is a recipient of a Translational Research Award from the Leukemia and Lymphoma Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Brian J. Druker, Division of Hematology and Medical Oncology, L592, Oregon Health Sciences University, 3181 SW Sam Jackson Park Rd, Portland, OR 97201; e-mail: drukerb@ohsu.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal