Abstract
The malignant Reed-Sternberg cell of Hodgkin disease is an aberrant B cell that persists in an immunolgically mediated inflammatory infiltrate. Despite its nonproductive immunoglobulin genes, the Reed-Sternberg cell avoids the usual apoptotic fate of defective immune cells through an unknown mechanism. A likely candidate is the surface receptor, CD40, consistently expressed by Reed-Sternberg cells, and the first link in the pathway to NF-κB activation, the central regulator of cytokine production and apoptosis. CD40 signaling in B lymphocytes coordinates the immune response, including immunoglobulin isotype switch and Fas-mediated apoptosis. CD40-induced NF-κB activation is mediated by adapter proteins, the TNF receptor (TNFR)-associated factors (TRAFs), especially TRAFs 2, 3, and 5. Using a Hodgkin cell line, this study demonstrates that CD40 activation of NF-κB is mediated by proteolysis of TRAF3. Results further demonstrate that the pathway can be blocked by treatment with pharmacologic doses of a specific protease inhibitor, pepstatin-A, even in the presence of a mutated NF-κB inhibitor, I-κBα. The stability of TRAF3 regulates CD40/NF-κB–mediated control of the immune response, which is central to the biologic activity of the Reed-Sternberg cell. Prevention of TRAF3 proteolysis may be an entry point for design of novel pharmaceuticals to treat Hodgkin disease and immune system disorders.
Introduction
The Reed-Sternberg (RS) cell of Hodgkin disease is a malignant germinal center B cell1,2 with rearranged but nonproductive immunoglobulin genes.1 However, through as yet unknown mechanisms, RS cells resist the apoptotic fate normally suffered by defective B cells with crippled immunoglobulin genes. Intervention in this antiapoptotic mechanism might be an approach for the development of targeted, pharmacologic management of Hodgkin disease.
The RS cells survive and thrive in a milieu of an intense cellular inflammatory infiltrate. A complex array of secreted factors and surface receptors are elaborated by the RS cell to recruit and nurture this infiltrate. The transcription factor, NF-κB, is central to the regulation of many of these factors and to apoptosis itself. In B cells, NF-κB is highly regulated through the surface receptor, CD40, a member of the tumor necrosis factor receptor (TNFR) superfamily.3,4 CD40 is virtually always expressed by RS cells in all cases and its ligand, CD40L, is expressed by the many T cells that surround RS cells.5 Therefore, we chose to dissect the CD40–NF-κB axis in RS cells to identify vulnerable points of the signaling pathway.
In B cells, CD40 regulates the progression from immunoglobulin isotype switch to cytokine secretion and, ultimately, terminates in Fas-mediated apoptosis to shut down the immune response.6-9 CD40 signal transduction pathways result in transcriptional events: CD40 activates both NF-κB and JNK/SAPK pathways.10 The transcription of several cytokine genes, including interleukin (IL)-2, IL-6, IL-8, tumor necrosis factor (TNF), and granulocyte-macrophage colony-stimulating factor, are up-regulated in response to NF-κB activity.11 Such cytokines exert widespread effects on the proliferation and activation of all components of the immune system and most are readily found in Hodgkin tissues.2
Proximal events in CD40-mediated transcription factor activation depend on the family of TNFR-associated factors (TRAFs), especially TRAFs 2, 3, and 5.12-14 We have shown these genes to be expressed in single RS cells from patient samples.15 These proteins do not have enzymatic activity themselves but serve as adapters to downstream kinases. Recently, more links in the pathway were discovered: TRAF3 interaction with CD40 increases during CD40 ligation, whereas TRAF2 binding decreases,16,17 the TRAFs bind to TRAF-associated activator of NF-κB (TANK)18,19 and NF-κB-inducing kinase (NIK),10,20 which are necessary for transduction of the signal toward NF-κB activation. TRAFs 2, 5, and 6 mediate activation of the NF-κB pathway via CD40.12,13,20 TRAF3, in contrast, binds to CD40, TANK, and NIK, but does not activate NF-κB10,18 and overexpression of TRAF3 actually blocks the activation by the other TRAFs.12,13 The mechanism of this blockade may be competitive because all TRAFs share the binding domain for CD40 and the downstream activators.18,21 22
In an RS cell-derived line,23 we show that, following CD40 ligation, TRAF3 is degraded by a protease, thus removing the block in NF-κB activation. These observations were further supported when treatments that maintained TRAF3 levels served to restore the block of NF-κB activation in stimulated cells. Thus, one role of TRAF3 is to maintain the “NF-κB off ” position in the resting state. We exploited this regulatory step using protease inhibitors as well as genetic deletion mutants of TRAF3 to show that TRAF3 must be degraded to release the mediator and activate NF-κB. These findings demonstrate the feasibility of denying the RS cell a critical, life-supporting physiologic event with a specific, pharmacologic protease inhibitor.
Materials and methods
Cell culture
The KMH223 cells were maintained at a density of 0.3 × 106 to 0.5 × 106 cells/mL in RPMI 1640 medium (Life Technologies, Gaithersburg, MD) supplemented with 10% qualified, heat-inactivated fetal bovine serum (FBS; Life Technologies), 100 U/mL penicillin, and 100 mg/mL streptomycin. Where indicated, cells were treated with CD40 ligand (CD40L) (Immunex, Seattle, WA) at a final concentration of 500 ng/mL. For protease inhibition, pepstatin A (Sigma, St Louis, MO) was reconstituted at 1 μg/mL in 0.1% deoxycholic acid. The drug was added to cells in culture for 30 minutes before addition of CD40L.
Isolation of membrane, cytosolic, and nuclear fractions
The membrane and cytosolic extracts were prepared as described.24 Briefly, cells were pelleted by centrifugation at 500g for 5 minutes. The pellet was rinsed in 1 mL of ice cold Dulbecco phosphate-buffered saline (DPBS; Life Technologies) and resuspended in 50 μL of homogenization buffer (10 mmol/L HEPES, pH 7.5, 0.3 mol/L sucrose) supplemented with carboxypolypeptidase (PMSF) (0.5 mmol/L), pepstatin A (1 μg/mL), aprotinin (1 mg/mL), leupeptin (1 mg/mL), Na3VO4 (2 mmol/L), and NaF (10 mmol/L). The cells were allowed to swell for 20 minutes on ice, after which they were transferred to a 1.5-mL microcentrifuge tube and homogenized with 15 to 20 strokes of the Deltaware pellet pestle (Kimble Glass Company, Vineland, NJ). The cell lysate was pelleted by centrifugation at 3000g for 10 minutes to isolate the nuclear pellet, which was stored at −70°C. The supernatant was centrifuged again at 5000g to remove mitochondria. The supernatant from this step was centrifuged at 100 000g; the resulting pellet represented the membrane fraction, and the supernatant was the cytoplasmic fraction. The membrane pellet was resuspended in homogenization buffer without sucrose.
Electrophoretic mobility shift assay (EMSA)
The nuclear pellet, isolated as described above, was resuspended in an equal volume of buffer C (20 mmol/L HEPES, pH 7.5, 20% glycerol, 0.42 mol/L NaCl, 1.5 mmol/L MgCl2, 0.2 mmol/L EDTA, 0.25 mmol/L DTT) supplemented with PMSF (0.5 mmol/L), pepstatin A (1 mg/mL), aprotinin (1 mg/mL), leupeptin (1 mg/mL), Na3VO4 (2 mmol/L), and NaF (10 mmol/L). Nuclei were lysed by vortexing for 30 minutes at 4°C followed by centrifugation at 10 000g for 45 minutes at 4°C. The supernatant containing nuclear protein was harvested and stored at −70°C. Ten nanograms of an oligonucleotide containing the NF-κB consensus sequence (Santa Cruz Biotechnologies, Santa Cruz, CA) was end-labeled with 50 μCi γ-32P-adenosine triphosphate (NEN Life Science Products, Boston, MA), using T4 polynucleotide kinase (Life Technologies) according to the manufacturer's instructions. The radiolabeled probe was separated from unincorporated nucleotides by passing it through a G-25 Sephadex spin column (Boehringer Mannheim, Indianapolis, IN). One microgram of nuclear extract, in 2 μL of buffer C, was combined with 0.1 ng of 32P–end-labeled double-stranded DNA containing the NF-κB consensus sequence in the presence of 1.5 μg poly dI:dC (Sigma), 10 ng of an irrelevant 25-nucleotide single-stranded oligonucleotide, 12 mmol/L HEPES, pH 7.5, 1 mmol/L EDTA, 1 mmol/L 2-mercaptoethanol, 6% glycerol, 10 mmol/L KCl, 0.05 mmol/L DTT, 0.05 mmol/L PMSF, 0.1% NP-40 in a final volume of 10 μL. In competition experiments, an unlabeled oligonucleotide containing either the wild-type or a mutant NF-κB binding site (Santa Cruz Biotechnologies) was added at 30-fold excess (3 ng). The mixture was incubated at 25°C for 30 minutes, after which 1 mL of 1% bromophenol blue dye was added. The sample was immediately loaded onto a 4% polyacrylamide gel and separated by electrophoresis at 150 V for 1 hour. The gel was transferred to 3M Whatman paper and dried under vacuum at 80°C, followed by autoradiography. Autoradiographs were scanned using densitometry and evaluated with Quantity One software (PDI, Huntington Station, NY) to quantitate fold-activation of NF-κB.
Western blotting
TRAF3 was detected by immunoblotting with the H122 rabbit polyclonal antibody (Santa Cruz Biotechnologies) and goat antirabbit-horseradish peroxidase (HRP) conjugate. The Renaissance HRP substrate was applied (NEN Life Science Products) and the membrane was exposed to film (Eastman Kodak, Rochester, NY). The membrane was stripped by incubating in stripping buffer (60 mmol/L Tris, pH 6.8, 2% sodium dodecyl sulfate [SDS], 50 mmol/L 2-mercaptoethanol) for 30 minutes at 70°C with constant agitation. The stripped membrane was washed 5 times DPBS (Life Technologies) and 0.1% Tween-20. Membranes were then re-probed with either anti-CD40 antibody (Santa Cruz Biotechnologies), anti-CD30 antibody (BerH2; Dako Corporation, Carpentiera, CA), or antihuman glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Trevigen, Inc, Gaithersburg, MD).
Expression vectors and transfections
The N-terminal deletion mutant of TRAF3 was constructed from the plasmid expression vector pSG5FLAGLAP1 (generous donation from Dr G. Mosialos, reference 25) containing the full-length human TRAF3 (F-TRAF3) complementary DNA (cDNA) by cleaving within the gene with BssH2 (Life Technologies) and Sfu1 (Boehringer Mannheim), which removed nucleotides 39 to 927. The 3′-recessed ends were filled in using the large (Klenow) fragment of DNA polymerase I (Life Technologies), according to the manufacturer's instructions. The plasmid DNA was recircularized with T4 DNA ligase (Life Technologies). This expression construct containing a TRAF3 deletion mutant, pSG5FLAGTRAF3δ300 (here called δ300TRAF3) was replicated in Escherichia colistrain DH5α (Life Technologies), and the plasmid DNA was isolated by alkaline lysis and column purification procedure (Qiagen, Chatsworth, CA). For stable transfection, F-TRAF3 or δ300TRAF3 and the selectable marker pSV2neo (Stratagene, La Jolla, CA) (4:1) were co-precipitated, and resuspended at a concentration of 0.5 mg/mL. Ten micrograms of the plasmid mix was added to 107 cells in 80 mL DPBS (Life Technologies) and the mixture was placed into a Gene Pulser cuvette (BioRad, Hercules, CA). The cells were electroporated at 200 mV, and placed directly into 10-mL nonselective medium for 3 days, followed by selection medium containing 250 μg/mL Geneticin (Life Technologies). The medium was changed every 4 days until log-phase growth was attained. The cells were then placed into culture medium containing 500 μg/mL Geneticin and split into 4 pools. Following additional log-phase growth, expression of the transfected TRAF3 cDNA was confirmed by Western blotting.
Radiolabeling and immunoprecipitation
Cellular proteins were metabolically labeled by the addition of 40 μCi/mL 35S-EasyTag Labeling Mix (NEN Life Science Products) to KMH2 cells in culture without cysteine and methionine. The cells were cultured in the presence of the radiolabeled amino acids for 5 hours. CD40L (Immunex) was added during the last hour of incubation. So that labeling time did not vary between samples, the different time points of CD40L stimulation were separated into different flasks and stimulated such that all time points were harvested at the same time (ie, labeling began at time [0 hour:00 minute], stimulation of flask 1 began at 4:00, flask 2 at 4:30, flask 3 at 4:55, flask 4 left untreated, and all were harvested at 5:00). The cells were harvested in 1 mL lysis buffer (50 mmol/L HEPES, pH 7.5,10% glycerol,0.5% NP-40). Protein content was normalized to 2 mg. The protein and antibody were mixed with protein A Sepharose beads in 1 ml lysis buffer (50 mmol/L HEPES, pH 7.5,10% glycerol, 0.5% NP-40) under constant agitation at 4°C overnight. For untransfected KMH2 cells, the antihuman TRAF3 antibody H20 (Santa Cruz Biotechnologies) was used. For transfected KMH2 cell lines, the anti-FLAG monoclonal antibody M2 (Eastman Kodak) was used. The protein A Sepharose beads were washed 3 times with 1 mL lysis buffer and resuspended in 200 μL SOL buffer (50 mmol/L TEA-HCl, pH 7.4, 100 mmol/L NaCl, 2 mmol/L EDTA, 0.4% SDS, 2 mmol/L β-mercaptoethanol). Samples were boiled for 2 minutes and allowed to cool to 25°C, after which 4 μL of 0.5 mol/L iodoacetamide was added. The beads were then pelleted and the supernatant was added to 700 μL of lysis buffer with clean beads and antihuman TANK antibody C20 (Santa Cruz Biotechnologies). Samples were incubated at 4°C for 2 hours with constant agitation. Immune complexes were separated on 10% SDS-ppolyacrylamide gel electrophoresis (PAGE). The gel was dried onto Whatman 3M paper and exposed to film (Eastman Kodak) using the BioMax TranScreen LE intensifying screen (Eastman Kodak).
Quantitative reverse transcriptase-polymerase chain reaction (RT-PCR)
Cells were lysed in Trizol reagent (Life Technologies) and RNA was isolated according to the manufacturer's instructions. cDNA was generated from the RNA by reverse transcription. Briefly, 1 μg of total RNA was denatured in the presence of random hexamer primers by heating at 70°C. A mixture of 10 mmol/L dNTPs was added to the denatured RNA and primers. The reverse transcription was performed with Superscript II RT enzyme (Life Technologies) in a final reaction volume of 20 μL according to the manufacturer's recommendations. The interleukin (IL)-6 gene expression was quantitated using the IL-6 PCR-MIMIC kit (Clontech, Palo Alto, CA) with the IL-6 amplimer set according to the manufacturer's protocol. Briefly, the PCR mixture contained 0.2 pmol/μL of each IL-6 primer, 1 mmol/L dNTPs, 20 mmol/L Tris-HCl (pH 8.4), 50 mmol/L KCl, 1.5 mmol/L MgCl2, 1 μL of the MIMIC dilution, 1 μL of the above RT reaction mixture, and 2.5 U Taq DNA polymerase in a final volume of 50 μL. Thermal cycling was carried out as follows: 1 cycle of 94°C for 3 minutes, 60°C for 1 minute, 72°C for 1 minute, followed by 34 cycles of 94°C for 30 seconds, 60°C for 45 seconds, 72°C for 30 seconds, and an additional extension period at 72°C for 5 minutes. For actin PCR, the following primer pair used: 5′-GAACGGTGAAGG TGACAG CAG T-3′, 5′-TGGGGGACAAAAAGG GGGAAG G-3′.
Results
TRAF 3 depletion precedes NF-κB activation
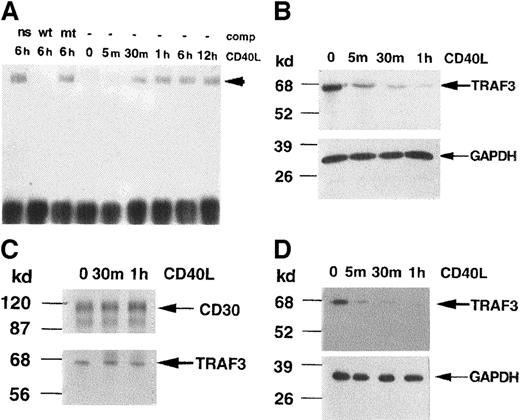
Activation of NF-κB is a known consequence of CD40 ligation, resulting in the nuclear translocation of the p50/p65 dimer and c-Rel proteins.26 CD40 ligation in KMH2 cells causes increased cell survival and secretion of NF-κB-dependent cytokines such as IL-8.27 28 We determined the time-course of activation of NF-κB in stimulated KMH2 cells. As shown in Figure1A, no change in DNA binding activity of NF-κB activity was noted within 5 minutes, but activity increased at 30 minutes and reached a maximum level (∼2.2-fold unstimulated) by 1 hour after CD40 ligation.
Time course of CD40-mediated activation of NF-κB and depletion of TRAF3 in KMH2 cells.
KMH2 cells were stimulated with human CD40L for the indicated times. (A) Nuclear extracts were prepared, followed by EMSA. One microgram of each extract was incubated with radiolabeled double-stranded oligonucleotide containing the NF-κB DNA binding motif. Binding reaction was performed either without a competitor or in the presence of 30-fold excess of unlabeled competitor oligonucleotide (comp) containing wild-type NF-κB binding site (wild-type, wt), mutant NF-κB binding site (mt) or a nonspecific sequence estrogen response element (ns). The arrowhead indicates the position of the protein-DNA complex. (B-D) The level of TRAF3 was detected by Western blot using antihuman TRAF3 antibody in total cell (B), membrane (C), and cytoplasmic extracts (D). The position of TRAF3 (67 kd) is indicated by an arrow. In panels B and C, the level of the “housekeeping” enzyme GAPDH is detected to demonstrate equal protein content in each lane. In panel D, the membrane-bound CD30 is used for this purpose. The results are representative of 3 independently performed experiments.
Time course of CD40-mediated activation of NF-κB and depletion of TRAF3 in KMH2 cells.
KMH2 cells were stimulated with human CD40L for the indicated times. (A) Nuclear extracts were prepared, followed by EMSA. One microgram of each extract was incubated with radiolabeled double-stranded oligonucleotide containing the NF-κB DNA binding motif. Binding reaction was performed either without a competitor or in the presence of 30-fold excess of unlabeled competitor oligonucleotide (comp) containing wild-type NF-κB binding site (wild-type, wt), mutant NF-κB binding site (mt) or a nonspecific sequence estrogen response element (ns). The arrowhead indicates the position of the protein-DNA complex. (B-D) The level of TRAF3 was detected by Western blot using antihuman TRAF3 antibody in total cell (B), membrane (C), and cytoplasmic extracts (D). The position of TRAF3 (67 kd) is indicated by an arrow. In panels B and C, the level of the “housekeeping” enzyme GAPDH is detected to demonstrate equal protein content in each lane. In panel D, the membrane-bound CD30 is used for this purpose. The results are representative of 3 independently performed experiments.
In looking at TRAF3 levels within this time period, there was a notable decline in the total level of endogenous TRAF3 protein in KMH2 cells within 5 minutes of stimulation of CD40 by its ligand (Figure 1B). Depletion of TRAF3 continued for the duration of the study (1 hour). We asked whether TRAF3 is in the membrane and if changes in the protein level could be detected in the membrane and/or cytosolic component(s) of TRAF3 following CD40 ligation. By Western blotting, TRAF3 was observed in the membrane fraction of unstimulated cells and there was no significant change in the level of membrane-associated TRAF 3 on activation of CD40 within 1 hour (Figure 1C). In contrast, a decrease in the cytoplasmic pool of TRAF 3 occurred within 5 minutes and cytosolic TRAF3 levels continued to decline for at least up to 1 hour of CD40 stimulation (Figure 1D). Because membrane content of TRAF3 did not change at these time points (5 minutes to 1 hour), depletion of the cytosolic pool of TRAF3 did not correlate with its otherwise anticipated translocation to the membrane. In Figure 1B-C, the level of the “housekeeping” enzyme GAPDH is detected to demonstrate equal protein content in each lane. In Figure 1D, the membrane-bound CD30 is used for this purpose. These results suggested that CD40 ligation resulted in rapid elimination of the cytosolic TRAF3 in KMH2 cells. These data indicate that CD40 ligation results in depletion of TRAF3, followed by NF-κB activation in KMH2 cells.
CD40-generated signals are blocked by a protease inhibitor
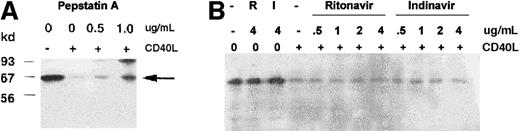
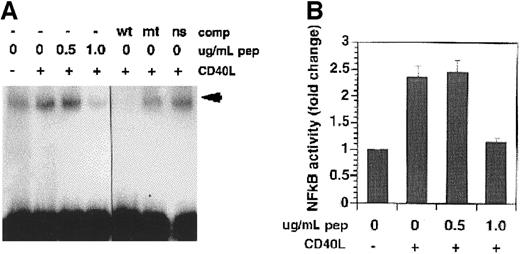
We hypothesized that depletion of TRAF3 following CD40 ligation may be due to activation of a protease(s) in KMH2 cells, and protease inhibition may then restore the level of TRAF3 in stimulated cells. Several different classes of protease inhibitors (serine-aprotinin, leupeptin, PMSF; thiol-leupeptin, PMSF; lysosomal-ALLN; and acid/aspartate-pepstatin A, ritonavir, indinavir) were tested for their ability to protect TRAF3 in stimulated KMH2 cells. Treatment with the aspartate protease inhibitor, pepstatin A, resulted in the partial restoration of TRAF3 level in CD40L-treated cells (Figure2A). Under the conditions of the experiment, none of other inhibitors had any effect on CD40-induced depletion of TRAF3 in these cells (data not shown), including the 2 other aspartate protease inhibitors, ritonavir and indinavir (Figure2B). This indicates that partial recovery of TRAF3 observed in the presence of pepstatin A was not due to nonspecific effects of a protease inhibitor on CD40L binding or CD40 stimulation. Pepstatin A treatment also caused the marked inhibition of NF-κB activity in CD40-stimulated KMH2 cells (Figure 3A,B). Taken together, these data suggest a role of a protease(s) in CD40 signaling leading to NF-κB activation.
Pepstatin A blocks CD40-mediated TRAF3 depletion and NF-κB activation.
Western blot analysis of cytosolic TRAF3. KMH2 cells were stimulated with CD40L for 45 minutes in the presence of the indicated concentrations of pepstatin A (A), or ritonavir or indinavir (B). The cytoplasmic fractions were analyzed by Western blotting with anti-TRAF3 antibody. The arrow indicates the position of TRAF3. Pep indicates pepstatin A; R, ritonavir; I, indinavir.
Pepstatin A blocks CD40-mediated TRAF3 depletion and NF-κB activation.
Western blot analysis of cytosolic TRAF3. KMH2 cells were stimulated with CD40L for 45 minutes in the presence of the indicated concentrations of pepstatin A (A), or ritonavir or indinavir (B). The cytoplasmic fractions were analyzed by Western blotting with anti-TRAF3 antibody. The arrow indicates the position of TRAF3. Pep indicates pepstatin A; R, ritonavir; I, indinavir.
EMSA of NF-κB activity in pepstatin-treated cells.
(A) Nuclear extracts were prepared from cells treated with CD40L in the presence of pepstatin A for 45 minutes and NF-κB DNA binding activity was determined. The arrowhead indicates the position of the protein-DNA complex. (B) Quantitation of the effect of pepstatin A on CD40-mediated NF-κB activation. Data shown are mean ± SE from 3 independent experiments performed as in panel C. Pep indicates pepstatin A.
EMSA of NF-κB activity in pepstatin-treated cells.
(A) Nuclear extracts were prepared from cells treated with CD40L in the presence of pepstatin A for 45 minutes and NF-κB DNA binding activity was determined. The arrowhead indicates the position of the protein-DNA complex. (B) Quantitation of the effect of pepstatin A on CD40-mediated NF-κB activation. Data shown are mean ± SE from 3 independent experiments performed as in panel C. Pep indicates pepstatin A.
A TRAF3 amino-terminus deletion mutant is resistant to CD40-mediated degradation and blocks NF-κB activation
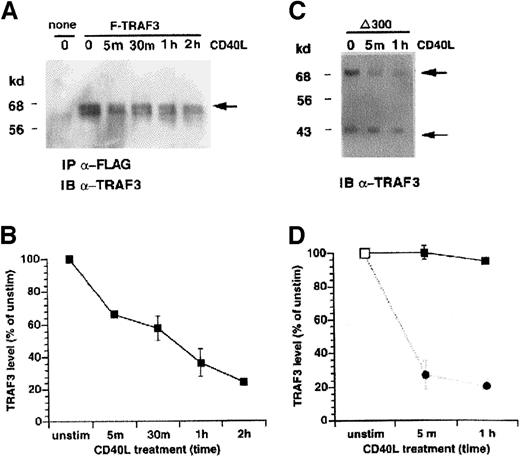
It is possible that pepstatin A inhibited additional steps in the CD40-initiated pathway in addition to TRAF3 degradation. To more directly assess the role of TRAF3 in CD40 signaling, we examined the effect of modulation of TRAF3 expression/activity on CD40-mediated NF-κB activation. An expression vector (pSG5) containing FLAG-tagged F-TRAF3 cDNA25 was used to construct the amino-terminus deletion mutant of TRAF3 cDNA (δ300TRAF 3) similar to the previously reported dominant negative TRAF3 mutant14 as explained in “Materials and methods.” KMH2 cells were stably cotransfected with F-TRAF3, δ300TRAF3, and a selectable marker pSV2neo plasmid DNA, or control vector pSV2neo alone. Expression of the epitope-tagged TRAF3 protein was examined at various times following CD40 stimulation (Figure 4). In the first experiment, the FLAG-tagged F-TRAF3 construct was selectively immunoprecipitated with anti-FLAG antibody to distinguish it from endogenous TRAF3. The immunoprecipitate was then immunoblotted with anti-TRAF3 antibody. Although F-TRAF3 was degraded after CD40 ligation (Figure 4A,B), the deletion mutant (δ300TRAF3, aa13-aa310) appeared to be resistant to degradation (Figure 4C,D). The presence of δ300TRAF3, however, did not prevent degradation of the endogenous TRAF3 protein in δ300TRAF3 transfectants (Figure 4C,D). In these experiments, TRAF3 was detected by immunoblotting total protein samples because its molecular size distinguishes it from endogenous TRAF3. These data imply that the amino-terminus of TRAF3 is a potential target site for proteolytic degradation following CD40 stimulation.
The full-length TRAF3, but not the N-terminal deletion mutant, is depleted in stimulated cells.
(A) Stable KMH2 transfectants expressing the full-length epitope-tagged TRAF3 cDNA (F-TRAF3) were stimulated with human CD40L for the indicated times. The FLAG-tagged protein was immunoprecipitated from total cell extract and analyzed by Western blotting using anti-TRAF3 antibody. The position of F-TRAF3 is indicated by the arrow. The reason for a doublet band representing TRAF3 in anti-FLAG may be due to either alternatively processed exogenous protein or cross-reacting and coprecipitating endogenous TRAF3. (B) Quantification of 2 independent experiments (mean ± SE) performed as in panel A. The ▪ represents the level of the transfected F-TRAF3 protein. (C) Stable KMH2 transfectants expressing the deletion mutant of TRAF3 (δ300TRAF3) were stimulated with human CD40L for the indicated times. Total cell extracts were analyzed by Western blotting using anti-TRAF3 antibody. The position of endogenous TRAF3 (68 kd) is indicated by the heavy arrow and the TRAF3 deletion mutant (δ300TRAF3, ∼ 35 kd) by the thin arrow. (D) Resistance of mutant (δ300TRAF3) TRAF3 to proteolytic degradation, mean ± SE of 3 independent experiments. Here, the ▪ represents the level of the transfected δ300TRAF3, and the ● represents the endogenous, full-length TRAF3. Unstim indicates unstimulated; IP, immunoprecipitated; IB, immunoblotted.
The full-length TRAF3, but not the N-terminal deletion mutant, is depleted in stimulated cells.
(A) Stable KMH2 transfectants expressing the full-length epitope-tagged TRAF3 cDNA (F-TRAF3) were stimulated with human CD40L for the indicated times. The FLAG-tagged protein was immunoprecipitated from total cell extract and analyzed by Western blotting using anti-TRAF3 antibody. The position of F-TRAF3 is indicated by the arrow. The reason for a doublet band representing TRAF3 in anti-FLAG may be due to either alternatively processed exogenous protein or cross-reacting and coprecipitating endogenous TRAF3. (B) Quantification of 2 independent experiments (mean ± SE) performed as in panel A. The ▪ represents the level of the transfected F-TRAF3 protein. (C) Stable KMH2 transfectants expressing the deletion mutant of TRAF3 (δ300TRAF3) were stimulated with human CD40L for the indicated times. Total cell extracts were analyzed by Western blotting using anti-TRAF3 antibody. The position of endogenous TRAF3 (68 kd) is indicated by the heavy arrow and the TRAF3 deletion mutant (δ300TRAF3, ∼ 35 kd) by the thin arrow. (D) Resistance of mutant (δ300TRAF3) TRAF3 to proteolytic degradation, mean ± SE of 3 independent experiments. Here, the ▪ represents the level of the transfected δ300TRAF3, and the ● represents the endogenous, full-length TRAF3. Unstim indicates unstimulated; IP, immunoprecipitated; IB, immunoblotted.
To address the possibility of a negative regulatory effect of the nondegradable TRAF3 deletion mutant on CD40 signaling, DNA binding activity of NF-κB was compared in various transfectants on CD40 ligation. None of the transfectants had a significant effect on the low constitutive level of NF-κB activity in unstimulated KMH2 cells (data not shown). However, CD40 stimulation caused NF-κB activation in F-TRAF3 and vector transfectants, but failed to induce NF-κB activity in δ300TRAF3 transfectants (Figure5A-C). Because CD40 stimulation causes depletion of endogenous TRAF3 in δ300TRAF3 transfectants (Figure 4), these data provide strong evidence for the δ300TRAF3-mediated dominant negative inhibition of CD40 signaling and, ultimately, activation of NF-κB.
Constitutive expression of the N-terminal deletion mutant of TRAF3 blocks NF-κB activation following CD40 ligation.
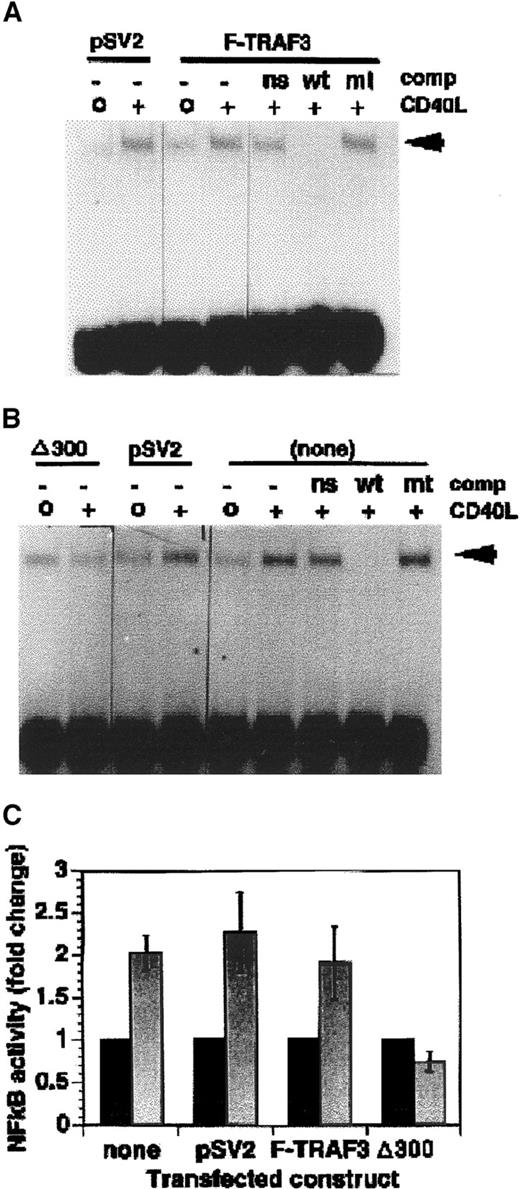
(A) KMH2 transfectants expressing the full-length TRAF 3 construct (F-TRAF3) or the selectable marker alone (pSV2) were stimulated for 1 hour with CD40L. Nuclear extracts were analyzed for NF-κB DNA-binding activity EMSA. (B) KMH2 transfectants expressing the deletion mutant of TRAF3 (δ300TRAF3) or the selectable marker alone (pSV2neo) were stimulated for 1 hour with CD40L and nuclear extracts were analyzed for NF-κB DNA-binding activity. (C) Mean ± SE of densitometric results from 3 independent experiments as performed in panels A and B. Comp indicates competitor; ns, nonspecific; wt, wild type; mt, mutant; none, untransfected. Arrowheads indicate shifted oligonucleotide. The bars above the gels indicate the transfected construct contained in the KMH2 cells used in the experiment.
Constitutive expression of the N-terminal deletion mutant of TRAF3 blocks NF-κB activation following CD40 ligation.
(A) KMH2 transfectants expressing the full-length TRAF 3 construct (F-TRAF3) or the selectable marker alone (pSV2) were stimulated for 1 hour with CD40L. Nuclear extracts were analyzed for NF-κB DNA-binding activity EMSA. (B) KMH2 transfectants expressing the deletion mutant of TRAF3 (δ300TRAF3) or the selectable marker alone (pSV2neo) were stimulated for 1 hour with CD40L and nuclear extracts were analyzed for NF-κB DNA-binding activity. (C) Mean ± SE of densitometric results from 3 independent experiments as performed in panels A and B. Comp indicates competitor; ns, nonspecific; wt, wild type; mt, mutant; none, untransfected. Arrowheads indicate shifted oligonucleotide. The bars above the gels indicate the transfected construct contained in the KMH2 cells used in the experiment.
We next sought to determine a molecular mechanism for the inhibitory properties of TRAF 3 in the CD40 pathway. Figure 4 demonstrates that a deletion mutant of TRAF3 (δ300TRAF3) missing the N-terminal 300 aa, but containing both the TRAF-N and the TRAF-C domains, is not degraded on CD40 ligation. In addition, expression of this mutant blocks CD40 signaling in KMH2 cells (Figure 5). We began by describing the interaction of TRAF 3 and the mutant δ300TRAF3 with TANK, a cytoplasmic component in the pathway toward NF-κB activation. We next asked whether δ300TRAF3 mutant interacts with TANK and what effect CD40 stimulation might have on this interaction.
Sustained interaction between TANK and a nondegradable TRAF 3 deletion mutant (δ300TRAF3) but not with endogenous TRAF3 following CD40 ligation
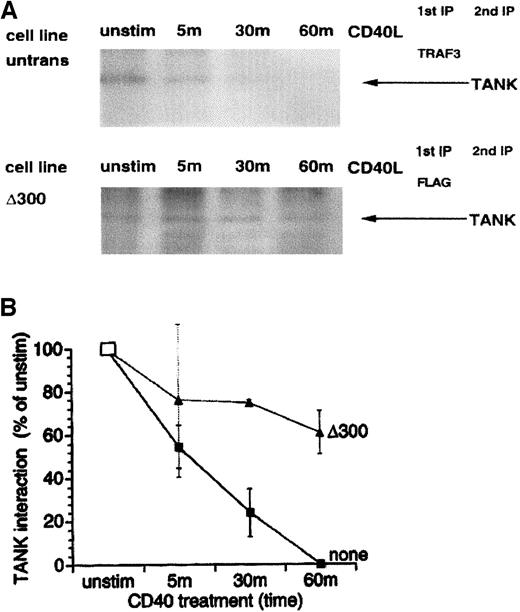
The association of endogenous TRAF3 and TANK in unstimulated KMH2 cells was established by sequential immunoprecipitations of TRAF3 and TANK in metabolically labeled cellular proteins (Figure6). On stimulation of CD40 with its ligand, the amount of TANK protein detectable in TRAF3 immunoprecipitates decreased to approximately 50% of the control within 5 minutes and disappeared by 1 hour of ligation (Figure 6A, upper panel; Figure 6B). Figure 1 shows that TRAF3 was degraded in KMH2 cells in response to CD40 ligation and this degradation preceded NF-κB activation. Thus, lack of detection of TANK in TRAF3 immunoprecipitates can be attributed to the unavailability of TRAF3 in CD40-stimulated cells. To rule out the possibility that the TANK level may have decreased in response to CD40 stimulation, we measured the expression of TANK in KMH2 cells at various times after ligation. The total level of TANK remained constant for at least up to 60 minutes after CD40 stimulation of KMH2 cells (data not shown). δ300TRAF3 was found to associate with TANK, as demonstrated by immunoprecipitation of the N-terminal FLAG epitope tag on δ300TRAF3, followed by immunoprecipitation of TANK (Figure 6A). The association between δ300TRAF3 and TANK decreased to a much lesser extent than occurred with the endogenous TRAF3 throughout 1 hour of CD40 ligation. Seventy percent of TANK bound in the unstimulated state remained bound to δ300TRAF3 after 1 hour of CD40 ligation (Figure 6B). As a control, interaction between TANK and F-TRAF3 was found to parallel that with endogenous TRAF3. That is, TANK interaction with F-TRAF3 declined with CD40 stimulation so that only one tenth of the original amount of TANK remained bound to F-TRAF3 by 1 hour of CD40 ligation (data not shown). Taken together, these data imply that physical sequestration of TANK by TRAF3 serves as a regulatory mechanism in resting cells and degradation of TRAF3 following CD40 stimulation may be critical to the role of TANK as a key mediator of NF-κB activity.
Sustained interaction of TANK with a nondegradable TRAF3 deletion mutant but not endogenous TRAF3 following CD40 ligation.
KMH2 cells and δ300TRAF3 transfectants were metabolically radiolabeled in culture and stimulated with CD40L for the indicated times prior to lysis. TRAF3 was immunoprecipitated from untransfected and transfected KMH2 cell extracts using antihuman TRAF3 antibody and anti-FLAG antibody, respectively. This was followed by TANK immunoprecipitation from either the TRAF3 immunoprecipitates (A) or FLAG immunoprecipitates (B), using anti-human TANK antibody. The position of TANK is indicated by the arrow.
Sustained interaction of TANK with a nondegradable TRAF3 deletion mutant but not endogenous TRAF3 following CD40 ligation.
KMH2 cells and δ300TRAF3 transfectants were metabolically radiolabeled in culture and stimulated with CD40L for the indicated times prior to lysis. TRAF3 was immunoprecipitated from untransfected and transfected KMH2 cell extracts using antihuman TRAF3 antibody and anti-FLAG antibody, respectively. This was followed by TANK immunoprecipitation from either the TRAF3 immunoprecipitates (A) or FLAG immunoprecipitates (B), using anti-human TANK antibody. The position of TANK is indicated by the arrow.
Nondegradable deletion mutant (δ300TRAF3) blocks CD40-mediatedIL-6 gene expression
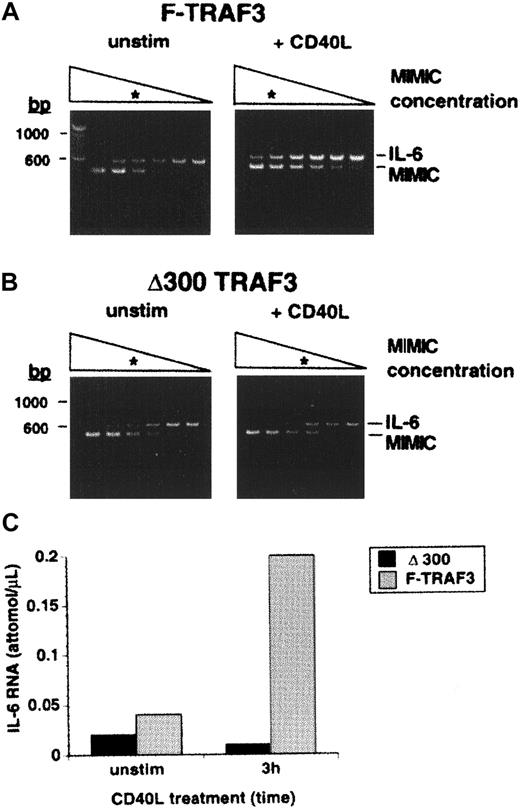
To establish a functional consequence of TANK retention by TRAF3, we examined the effect of CD40 ligation on expression of theIL-6 gene in δ300TRAF3 transfectants. An NF-κB binding site exists in the promotor of the IL-6 gene29and IL-6 is known to be up-regulated in response to CD40 ligation in KMH2 cells.28 We quantified the magnitude of CD40-mediated regulation of this gene in the F-TRAF3 and δ300TRAF3 transfectants by RT-PCR. The quantitative RT-PCR method uses a known titration of a size-modified IL-6 competitor DNA to measure the amount of IL-6 cDNA in a sample. By this method, it was evident that the IL-6 gene transcription was up-regulated by approximately 5-fold in vector and F-TRAF3 transfectants after 3 hours of CD40 ligation (Figure7A,C and data not shown), whereas IL-6 RNA levels remained unchanged in δ300TRAF3 transfectants in the same time period (Figure 7B,C). Therefore, the nondegradable δ300TRAF3 mutant inhibited CD40-mediated up-regulation of the IL-6 gene, while maintaining interaction with the TANK protein.
CD40-mediated up-regulation of
IL-6 gene expression is abrogated in δ300TRAF3, but not F-TRAF3 KMH2 transfectants. F-TRAF (A) or the δ300TRAF3 (B) transfectants were stimulated for 3 hours with CD40L. IL-6gene expression was monitored using the RT-PCR MIMIC system (Colette, Palo Alto, CA). In panels A and B, the triangles are schematic representations of the concentrations of MIMIC competitor and the asterisks denote the concentrations where sanple and competitor are equivalent. (C) Representative quantification data of 3 independently performed experiments are shown. The attomolar concentration was extrapolated for the PCR results.
CD40-mediated up-regulation of
IL-6 gene expression is abrogated in δ300TRAF3, but not F-TRAF3 KMH2 transfectants. F-TRAF (A) or the δ300TRAF3 (B) transfectants were stimulated for 3 hours with CD40L. IL-6gene expression was monitored using the RT-PCR MIMIC system (Colette, Palo Alto, CA). In panels A and B, the triangles are schematic representations of the concentrations of MIMIC competitor and the asterisks denote the concentrations where sanple and competitor are equivalent. (C) Representative quantification data of 3 independently performed experiments are shown. The attomolar concentration was extrapolated for the PCR results.
Discussion
The CD40-NF-κB pathway is a prominent feature of RS cells. Nearly all RS cells express abundant CD405 and the cells have fully functional NF-κB signaling pathways27,28 and characteristic production of cytokines.30 What controls CD40-dependent activation of NF-κB? In contrast to the relationship between CD40 and TRAF2 in NF-κB activation, very little is known regarding the role of TRAF3 in this signaling cascade. We studied the pathway in KMH2 cells, a Hodgkin-derived cell line of B lineage that expresses a transcriptional (mRNA)2 and protein phenotypic profile23 of primary RS cells, including expression of CD40 and NF-κB27,28 and a mutation in I-κBα.31 The finding of this mutation in KMH2 as well as in vivo suggests that this cell line is ideal to study because it closely resembles the diseased state with respect to this pathway.
Initial studies suggest that the mutation in I-κBα contributes to the constitutive NFκB activity in cultures and primary RS cells.31,32 We show here that CD40 stimulation can “super-activate” NF-κB nuclear translocation and cytokine gene transcription at levels exceeding constitutive NF-κB expression permitted by the mutated I-κBα. The “super-activation” of NF-κB by CD40 and the presence of CD40 ligand-expressing T cells surrounding RS cells in vivo, highly suggest that CD40 contributes to the activation of NF-κB in RS cells, including those with I-κBα mutations. The mutated I-κBα might retain some inhibitory capacity in these cells, or the pathway is further regulated by another isoform of I-κB, perhaps I-κBβ.33
One prior study failed to demonstrate an increase in NF-κB nuclear translocation following CD40 ligation,32 in contrast to the CD40-induced expression of NF-κB in Hodgkin cell lines demonstrated in the present study. This discrepancy is most likely due to technical reasons. For example, we have titrated the amount nuclear protein for analysis by electrophoretic mobility assay to avoid saturating the densitometric curve even at the zero time point. By contrast, the increases in NF-κB could not be observed (see reference 32) because the radiograph film is already saturated even for the untreated cells. For this reason, we used 5-fold less protein in each sample. With this modification, the assay was sensitive for detecting quantitative changes in NF-κB protein levels.
Significantly, we found that NF-κB levels and function can be markedly decreased by a protease inhibitor even in the presence of a mutated I-κBα. Thus, a pharmacologic agent, pepstatin A, can overcome both intense CD40 activation and a genomic mutation in a central regulatory gene.
We have found a novel role of TRAF3 in Hodgkin cells: the cytoplasmic pool of TRAF3 was eliminated prior to NF-κB activation by CD40 (Figure 1). TRAF3 bound TANK in the cytoplasm18 and removal of TRAF3 allowed the release or activation of TANK to propagate the signal from CD40 to NF-κB (Figure 8). When TRAF3 was not degraded, TANK might be retained in a state so it cannot activate downstream cascades. Consistent with this theme, the cytosolic component of TRAF3 could act as a negative regulator of CD40 signaling, perhaps by sequestering TANK or other protein complexes necessary for NF-κB activation. Indeed, the present data suggest that TANK sequestration by TRAF3 might be a potential regulatory mechanism in KMH2 cells and an additional potential target for intervention in the CD40-NF-κB pathway.
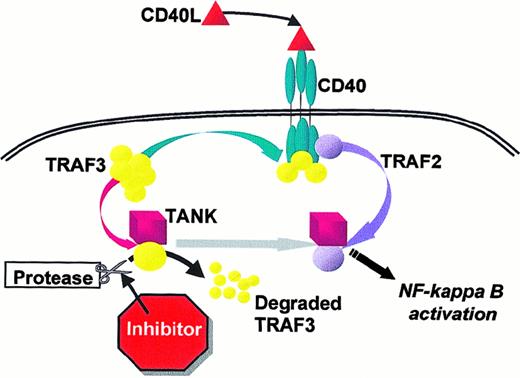
Blocking the CD40 pathway in Reed-Sternberg cells
. Shown is a schematic model of the roles for TRAF3 in CD40 signaling. TRAF3 has a membrane-associated role, interacting with the cytoplasmic tail of CD40.12-14 20 In the present study, we show a distinct role for TRAF3 in the cytoplasm, as an inhibitor of CD40 signaling in the resting state. A potential site for intervention to block CD40 signaling occurs in the cytoplasm at the point of proteolytic degradation of the TRAF3 molecule, which appears to be necessary for propagation of the signal from CD40 to NF-κB.
Blocking the CD40 pathway in Reed-Sternberg cells
. Shown is a schematic model of the roles for TRAF3 in CD40 signaling. TRAF3 has a membrane-associated role, interacting with the cytoplasmic tail of CD40.12-14 20 In the present study, we show a distinct role for TRAF3 in the cytoplasm, as an inhibitor of CD40 signaling in the resting state. A potential site for intervention to block CD40 signaling occurs in the cytoplasm at the point of proteolytic degradation of the TRAF3 molecule, which appears to be necessary for propagation of the signal from CD40 to NF-κB.
This action of TRAF3 in the cytoplasm is distinct from its role when associated with the membrane-bound CD40 cytoplasmic tail. CD40 is able to stimulate NF-κB and JNK activation without binding to TRAF2 or 3, most likely via TRAF6.20 We have shown here, however, that δ300TRAF3 plays an inhibitory role downstream in CD40 signaling. The mutated TRAF3 protein can block CD40-mediated NF-κB activation by maintaining the presence of the C-terminal portion of TRAF3 in the cytoplasm of KMH2.
Abrogation of both CD40-mediated TRAF3 degradation and NF-κB activation caused by the protease inhibitor, pepstatin A, has several implications. Although the immediate event in NF-κB activation also requires a proteolytic event, it is not likely that pepstatin A interferes with that step. The degradation of the inhibitor of NF-κB, I-κB, involves ubiquitination and breakdown in the proteasome.34,35 Pepstatin A is not known to affect proteasomal enzymes but rather acts as an inhibitor of acid/aspartate proteases.36 37 Conversely, a proteasome inhibitor, ALLN, which blocks I-κB breakdown, had no effect on TRAF3 degradation. Two other aspartate protease inhibitors, ritonavir and indinavir, both highly specific to the human immunodeficiency virus protease, also had no effect on TRAF3 degradation. Therefore, the effect of pepstatin A on CD40 signaling is quite specific to the CD40-TRAF3 juncture and not simply a consequence of general inhibition of other steps in the pathway, even in the presence of a mutated I-κBα.
We have demonstrated that transfection of the F-TRAF3 does not inhibit NF-κB activation via CD40 (Figures 4 and 5), in contrast to a previous report.12 The discrepancy may arise because in our studies, the F-TRAF3 was not expressed in KMH2 cells at the same level as in the report involving the 293 cell line.12Perhaps this lower expression level in KMH2 did not “overwhelm” the protease system and the transfected F-TRAF3 was sufficiently degraded on CD40 ligation to allow signal transduction to proceed. The deletion mutant of TRAF3, however, was resistant to CD40-triggered proteolysis (Figure 4). Because this deletion mutant retains the CD40- and TANK-binding domains, its sustained expression in the cells appears to be sufficient for interfering with CD40 signaling at the point of TRAF3 and blocking the pathway toward NF-κB activation (Figures 4 and 5). These data strongly support the importance of TRAF3 breakdown in CD40 signaling.
The next link in the pathway toward NF-κB activation may depend on TANK and we analyzed the dynamics of interaction between TRAF3 and TANK during CD40 ligation. The results presented here suggest that TRAF3 negatively regulates the CD40 pathway in resting cells by physical sequestration of TANK, and that degradation of TRAF3 following CD40 ligation facilitates the release of TANK for progression of CD40 signals such as NF-κB activation and subsequent gene transcription. Further support to this hypothesis was achieved by examination of the interaction of a nondegradable mutant of TRAF3, δ300TRAF3, with TANK. Truncation of a portion of the N-terminus and retention of the TRAF domain of TRAF3 in δ300TRAF3 deletion mutant led to the inhibition of the CD40-initiated signal transduction toward NF-κB activation and downstream transcription of the gene encoding the cytokine IL-6. We demonstrated that in KMH2 transfectants expressing δ300TRAF3, endogenous full length TRAF3 but not mutant δ300TRAF3 is susceptible to degradation on CD40 ligation (Figure 4). These results suggest that δ300TRAF3 is a dominant negative regulatory mutant of TRAF3, and emphasize the importance of TRAF3 in controlling CD40-mediated stimulation of the systemic immune response.
CD40-mediated NF-κB activation is a tightly controlled pathway in B cells that regulates mechanisms underlying the immune response: cell proliferation, protection from apoptosis, and transcription of cytokine genes.6,38 Results presented here indicate that TRAF3 is critical for regulation of NF-κB and IL-6 transcription through CD40 in the context of the neoplastic Hodgkin-derived cell, KMH2. When adequately controlled, the immunologic response is protective to the organism. However, when otherwise beneficial and critical signaling activities become dysregulated, they themselves may cause disease and contribute to the pathogenesis of B-cell neoplasia, the B symptoms of Hodgkin disease and inappropriate immune responses, such as autoimmunity.39-41 The ability to pharmacologically disrupt events upstream of NF-κB in the CD40 pathway, specifically by maintenance of the inhibitory adapter, TRAF3, provides a point of entry for intervention in diseases of B lymphocytes.
Acknowledgments
We thank Dr Hiroshi Kamesaki for the KMH2 cell line, Dr G. Mosialos for the expression vector containing epitope-tagged full-length TRAF3 cDNA (pSG5FLAGLAP1) and Immunex Corporation for the purified human CD40 ligand.
Supported by the American Cancer Society (grant no. DHP112 to J.C.) and the O. Benwood Hunter Endowment.
Correspondence:Jeffrey Cossman, Georgetown University Medical Center, NW 103 Medical-Dental Bldg, 3900 Reservoir Rd, NW, Washington, DC 20007; e-mail: cossmanj@gunet.georgetown.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal