Abstract
There is increasing evidence for functional crosstalk between inflammatory and thrombotic pathways in inflammatory vascular diseases such as atherosclerosis and vasculitis. Thus, complement activation on the endothelial cell (EC) surface during inflammation may generate thrombin via the synthesis of tissue factor. We explored the hypothesis that thrombin induces EC expression of the complement-regulatory proteins decay-accelerating factor (DAF), membrane cofactor protein (MCP), and CD59 and that this maintains vascular integrity during coagulation associated with complement activation. Thrombin increased DAF expression on the surface of ECs by 4-fold in a dose- and time-dependent manner as measured by flow cytometry. DAF up-regulation was first detectable at 6 hours and maximal 24 hours poststimulation, whereas no up-regulation of CD59 or MCP was seen. Thrombin-induced expression required increased DAF messenger RNA and de novo protein synthesis. The response depended on activation of protease-activated receptor 1 (PAR1) and was inhibited by pharmacologic antagonists of protein kinase C (PKC), p38 and p42/44 mitogen-activated protein kinase, and nuclear factor-κB. The increased DAF expression was functionally relevant because it significantly reduced C3 deposition and complement-mediated EC lysis. Thus, thrombin—generated at inflammatory sites in response to complement activation—is a physiologic agonist for the PKC-dependent pathway of DAF regulation, thereby providing a negative feedback loop protecting against thrombosis in inflammation.
Introduction
The complement system consists of a large group of plasma proteins that plays a central role in the defense against infections and in the modulation of inflammatory responses.1 However, by the nature of its cytolytic effects, complement has the potential to inflict injury on bystander host tissues. As a consequence, a variety of innate membrane-bound and soluble protective mechanisms have evolved. The membrane-bound proteins decay-accelerating factor (DAF, CD55), protectin (CD59), and membrane cofactor protein (MCP, CD46) are expressed on many cell types and provide protection against the constant low-level activation of the alternative pathway.2,3 These factors utilize distinct mechanisms for complement regulation and function cooperatively to facilitate cytoprotection. DAF prevents the formation and accelerates the decay of C3 and C5 convertases,2 while MCP binds C3b and C4b and allows their degradation by factor I.4 In contrast, CD59 operates at the distal end of the complement cascade, binding to C9 and preventing its incorporation into the C5b-9 complex, thereby inhibiting the function of the membrane attack complex (MAC).5
Thrombin is a multifunctional serine protease that is produced at the surface of endothelial cells (ECs) during the coagulation cascade and induces clot formation by catalyzing the conversion of fibrinogen to fibrin. In addition, thrombin plays an important role in both acute and chronic inflammation through its effects on leukocytes and ECs. Thus, thrombin is chemotactic for both neutrophils and monocytes and can also induce leukocyte adhesion to the vessel wall via its actions on ECs.6-9 Indeed, numerous functional effects have been demonstrated to occur as a consequence of the stimulation of ECs by thrombin. These include an increase in endothelial permeability to proteins10 and the release of soluble mediators, including platelet-activating factor and prostacyclin,6 the chemokines interleukin-8 and MCP-1,11,12 growth factors,13,14 and matrix metalloproteinases.15 In addition, thrombin may induce the expression of cellular adhesion molecules on ECs, including E- and P-selectin, vascular cell adhesion molecule-1, and intercellular adhesion molecule (ICAM)-1.12,13,16-18 Thrombin exerts its effects via a family of G-protein–coupled protease-activated receptors (PAR).19 Although both PAR1 and PAR3 are receptive to thrombin and expressed on ECs, it appears that the effects of thrombin on EC function are mediated predominantly through the classical thrombin receptor PAR1.20-24 The binding of thrombin to PAR1 results in receptor cleavage and exposure of a tethered ligand that is capable of activating the receptor and inducing intracellular signaling.25
Although DAF, MCP, and CD59 are all constitutively expressed on vascular endothelium,26 their specific roles during inflammation, when the risk of complement-mediated injury may be increased,27 remain to be determined. In our previous work, we have demonstrated that DAF, but not MCP or CD59, is up-regulated on ECs by the proinflammatory mediators tumor necrosis factor (TNF)-α and interferon (IFN)-γ and also by the MAC, resulting in enhanced cytoprotection by reducing the binding of complement factor C3 to the EC surface.28 However, while the effects of these mediators on DAF expression were not affected by a specific antagonist of protein kinase C (PKC),28 the PKC agonist phorbol 12, 13-dibutyrate (PBu) is known to increase DAF expression.29 Thus, we investigated the effect of thrombin, which is known to utilize PKC for stimulating EC permeability and microtubule formation.30 31 In this study, we demonstrate that thrombin does indeed up-regulate DAF expression through a PKC-dependent pathway and that this leads to enhanced cytoprotection by reducing the binding of C3 to the EC surface. Furthermore, thrombin-induced expression of DAF is dependent on gene transcription and was inhibited by pharmacologic antagonists of p38 and p42/44 mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-κB activation. These data therefore provide evidence linking the coagulation pathway to the induction of endothelial cytoprotection during inflammatory responses involving complement activation.
Materials and methods
Monoclonal antibodies and other reagents
The monoclonal antibodies (MoAbs) used included 6.5B5 (immunoglobulin [Ig] G1) against ICAM-1 32; BRIC229 (IgG1) against CD59 (International Blood Group Reference Laboraratory, Bristol, United Kingdom); and anti-DAF MoAb 1H4 (IgG1) and anti-MCP MoAb TRA-2-10 (IgG1), which were kind gifts from Dr D.M. Lublin and Professor J.P. Atkinson (Washington University School of Medicine, St Louis, MO). The antiendoglin (CD105) MoAb RMAC8 (IgG2a) was a kind gift from Dr A. d'Apice (St Vincent's Hospital, Victoria, Australia). The MoAb EN4 (IgG1) was obtained from Sanbio (Uden, Holland). Human plasma thrombin, hirudin, the PKC agonist PBu, and cycloheximide (CHX) were purchased from the Sigma Chemical Company (Poole, United Kingdom). The thrombin receptor peptide TRAP-6 (SFLLRN)33 was purchased from Bachem (Saffron Walden, United Kingdom) and was solubilized to 3 mmol/L in 0.1% trifluoroacetic acid (TFA) (Sigma). Recombinant human vascular endothelial growth factor (VEGF)-165 was purchased from Pepro Tech EC Ltd (London, United Kingdom). The PKC antagonists RO31-8220 and GF 109203X; the selective MEK-1 inhibitor PD98059,34 which inhibits the activation of p42/44 MAPK; the p38 MAPK inhibitor SB20219035; and the NF-κB inhibitor proteasome inhibitor-1 (PSI)36 were purchased from Calbiochem (Nottingham, United Kingdom). Normal human serum (NHS) was obtained from samples of blood taken from healthy volunteers and collected under sterile conditions into glass tubes and allowed to clot at 37°C prior to further incubation at 4°C for 2 hours to induce clot retraction. Following centrifugation at 1800g, the NHS was collected, pooled, and stored at −70°C.
Cell isolation and culture
Human umbilical vein endothelial cells (HUVECs) were isolated from umbilical cords by digestion with collagenase type II (Boehringer Mannheim, Lewes, United Kingdom) as described previously32and cultured in 1% gelatin-coated tissue culture flasks (Costar, Cambridge, MA) in medium 199 (M199) supplemented with 20% fetal bovine serum (FBS), 100-IU/mL penicillin, 0.1-mg/mL streptomycin, 2-mmol/L L-glutamine (all from Gibco BRL Life Technologies, Paisley, United Kingdom), 10-U/mL heparin (Leo Laboratories, Prince Risborough, United Kingdom), and 30-μg/mL EC growth supplement (ECGS) (Sigma). Human dermal microvascular ECs (DMECs) were isolated from human foreskins and cultured in fibronectin-coated flasks in HUVEC medium supplemented with 10% AB+ human serum (Sigma) as described previously.37ECs were passaged with trypsin/ethylenediaminetetraacetic acid (EDTA) (ICN Biomedicals Inc, Costa Mesa, CA), and each experiment was conducted with ECs at passage 3 to 6. For each experiment, ECs were plated out in M199 containing 10% FBS for 24 hours, and the medium was then replaced with M199 with 5% FBS for the duration of the experiment. In experiments involving hirudin, CHX, RO318220, SB202190, PD98059, and PSI, the inhibitors were added 30 to 60 minutes prior to the addition of the thrombin.
Flow cytometry
Monolayers of ECs were harvested by exposure to trypsin/EDTA and stained with the appropriate primary MoAb for 30 minutes at 4°C. After washing, ECs were stained with fluorescein isothiocynate (FITC)-labeled rabbit antimouse Ig (DAKO, Glostrup, Denmark) for 30 minutes at 4°C, followed by washing and fixation in 1% paraformaldehyde. Samples were analyzed on an Epics XL-MCL flow cytometer (Coulter, Hialeah, FL) by counting 10 000 cells per sample. In some experiments, the results are expressed as the relative fluorescent intensity (RFI), which represents the mean fluorescent intensity (MFI) with the test MoAb, divided by the MFI using an isotype-matched irrelevant MoAb. In experiments involving RO318220, SB202190, PD98059, TRAP-6, hirudin, and PSI, the RFI is expressed as a percentage of the RFI for thrombin-induced DAF expression.
Northern blot analysis
Northern analysis was performed as previously described.28 Briefly, confluent ECs in 75-cm2tissue culture flasks were incubated with thrombin (10 U/mL), VEGF (25 ng/mL), or plain medium alone for up to 24 hours at 37°C. In some experiments, the ECs were pretreated for 30 minutes with CHX (1 μg/mL). At the end of the stimulation, the cells were lysed in guanidium isothiocyanate (Sigma), and RNA was extracted as described.38 The probe for DAF was released from the plasmid vector39 by incubation for 2 hours at 37°C with the SalI and XbaI restriction enzymes. Purified RNA was run on 1% formaldehyde agarose gels and blotted onto GeneScreen membrane (DuPont, Letchworth, United Kingdom). Membranes were hybridized to appropriate 32P-deoxycytidine triphosphate–labeled complementary DNA probes overnight at 42°C, washed in solutions of 0.1% sodium dodecylsulfate (wt/vol) containing successively lower concentrations of standard sodium citate buffer (0.15-mol/L NaCl, 0.015-mol/L sodium citrate, pH 7), and specific hybridization was detected by autoradiography following exposure to Kodak XOMat film. For quantification, Northern blots were scanned using an Appligene Image Analysis System (Appligene, Durham, United Kingdom), and densitometry was performed using the National Institutes of Health (Bethesda, MD) Image program 1.52 software. Values were corrected with respect to ethidium bromide–stained ribosomal RNA loading patterns, and an arbitary value of 1 was assigned to unstimulated ECs.
C3-binding assay
ECs were cultured overnight at 37°C prior to the addition of thrombin or plain medium alone for 24 hours. Following harvesting with trypsin/EDTA, ECs were pelleted in 96-well v-bottom plates (Costar) and incubated with the antiendoglin MoAb RMAC8 for 30 minutes at 4°C. After washing with Hank balanced salt solution (HBSS)/1% bovine serum albumin (BSA), ECs were incubated with 100 μL of 5% NHS in M199 for 2 hours at 37°C prior to washing with HBSS/1% BSA and addition of FITC-conjugated rabbit antihuman C3c (DAKO) at 1:40 dilution for 30 minutes at 4°C. After washing twice with HBSS/1% BSA, the presence of C3c was estimated using flow cytometric analysis as described above. Control samples included the replacement of NHS with heat-inactivated human serum (HIHS) and the addition of 10-mmol/L EDTA to the NHS to inhibit complement activation. In the inhibition studies, the blocking MoAbs were added to the assay with the RMAC8 MoAb to achieve a final concentration of 50 μg/mL.
Cell lysis assay
ECs were cultured overnight in 24-well plates at 37°C prior to the addition of thrombin or plain medium alone for 24 hours. ECs were then incubated for 30 minutes at 37°C in M199 with 10% FBS containing 7-μmol/L calcein acetoxymethyl ester (Molecular Probes, Leiden, The Netherlands). Following washing in M199/1% BSA, EC monolayers were opsonized by incubating with 250 μL of MoAb RMAC8 for 30 minutes at 37°C. After washing with HBSS/1% BSA, ECs were incubated with 250 μL of 5%-to-20% baby rabbit complement (Serotec, Oxford, United Kingdom) in M199 for 30 minutes at 37°C. The supernatant from each well was then transferred to a 96-well microtiter plate (Costar) and, after washing in M199/1% BSA, the calcein remaining in the cells was released by incubation with 250-μL M199/1% BSA/0.1% Triton X-100 (Sigma). The lysate was then transferred to a 96-well plate, and the calcein released by complement and detergent was estimated using a CytoFluor 2300 fluorescence plate reader (Millipore, Bedford, MA). Percent specific lysis in triplicate wells was calculated as [(complement-mediated release − spontaneous release)/maximal release − spontaneous release)] × 100%, where maximal release = complement-mediated release + detergent-mediated release. Spontaneous release was less than 20% in all experiments.
Statistics
Differences between the results of experimental treatments were evaluated by the Mann-Whitney U test. Differences were considered significant at P < .05.
Results
Thrombin induces DAF expression on HUVECs and DMECs
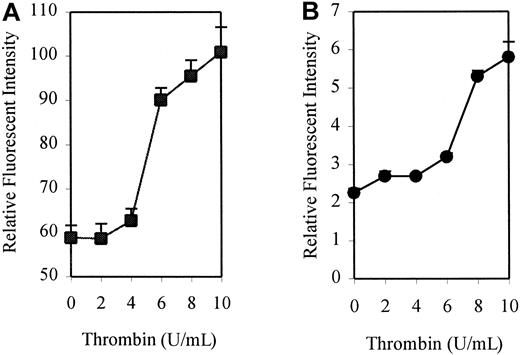
To investigate the effect of thrombin on the expression of DAF, monolayers of HUVECs were treated with human thrombin at a concentration of 10 U/mL for up to 72 hours, and surface expression of DAF was measured by flow cytometry. A 4-fold increase above the constitutive expression of DAF was observed following stimulation with thrombin, reaching a maximum level at 24 hours. An increase was first detectable at 6 hours, with levels returning to baseline by 72 hours poststimulation (Figure1). This up-regulation was dose-responsive, with increasing expression seen at concentrations of thrombin up to 10 U/mL (Figure 2A). Higher concentrations (15 and 20 U/mL) showed no significant increase in expression above that observed with 10 U/mL (data not shown). Parallel control experiments also demonstrated a similar dose-response for up-regulation of ICAM-1 expression (Figure2B). All further experiments were therefore conducted using stimulation with 10 U/mL thrombin for 24 hours.
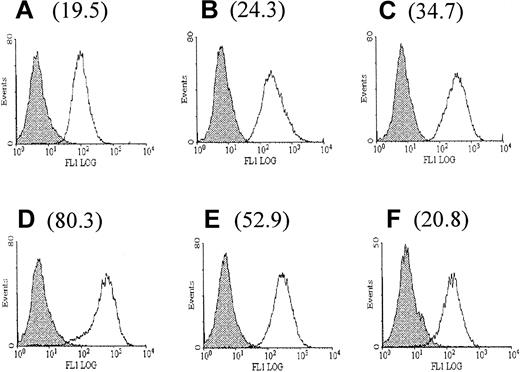
Up-regulation of DAF expression on HUVECs following stimulation with thrombin.
The expression of DAF on resting and thrombin-stimulated HUVECs was assessed by flow cytometry using MoAb 1H4. The figure shows background fluorescence (FITC-labeled rabbit antimouse antibody alone) (shaded histograms) and DAF expression (open histograms) on unstimulated HUVECs (A) and HUVECs stimulated with thrombin (10 U/mL) for 6 hours (B), 12 hours (C), 24 hours (D), 48 hours (E), and 72 hours (F). The RFI for DAF expression at each time point is shown in brackets. The RFI represents the mean MFI with the test MoAb divided by the MFI using an isotype-matched irrelevant MoAb. The results are representative of 3 experiments on separate EC cultures.
Up-regulation of DAF expression on HUVECs following stimulation with thrombin.
The expression of DAF on resting and thrombin-stimulated HUVECs was assessed by flow cytometry using MoAb 1H4. The figure shows background fluorescence (FITC-labeled rabbit antimouse antibody alone) (shaded histograms) and DAF expression (open histograms) on unstimulated HUVECs (A) and HUVECs stimulated with thrombin (10 U/mL) for 6 hours (B), 12 hours (C), 24 hours (D), 48 hours (E), and 72 hours (F). The RFI for DAF expression at each time point is shown in brackets. The RFI represents the mean MFI with the test MoAb divided by the MFI using an isotype-matched irrelevant MoAb. The results are representative of 3 experiments on separate EC cultures.
Dose response for thrombin-induced DAF and ICAM-1 expression.
HUVEC monolayers were incubated for 24 hours in the presence or absence of increasing concentrations of thrombin before harvesting and analysis by flow cytometry. DAF and ICAM-1 were detected using MoAbs 1H4 and 6.5B5, respectively. The results are expressed as mean ± SEM for RFI DAF (A) and ICAM-1 (B). The results are representative of 3 separate experiments performed in triplicate wells using different EC lines.
Dose response for thrombin-induced DAF and ICAM-1 expression.
HUVEC monolayers were incubated for 24 hours in the presence or absence of increasing concentrations of thrombin before harvesting and analysis by flow cytometry. DAF and ICAM-1 were detected using MoAbs 1H4 and 6.5B5, respectively. The results are expressed as mean ± SEM for RFI DAF (A) and ICAM-1 (B). The results are representative of 3 separate experiments performed in triplicate wells using different EC lines.
In contrast to DAF, no up-regulation in expression of either MCP or CD59 was observed following treatment of HUVECs with 10-U/mL thrombin for up to 48 hours. Failure to demonstrate an increase in expression of MCP and CD59 was not due to the proteolytic effects of trypsin because identical results were obtained when DAF, MCP, and CD59 were measured on ECs harvested with a nonenzymatic cell dissociation solution (not shown). It is possible that HUVECs, as large-vessel ECs of fetal origin, may not accurately represent those ECs of the microvasculature most affected during inflammatory responses. Therefore, DMECs were isolated from human skin and stimulated with thrombin for 24 hours. A significant induction of both DAF and ICAM-1 expression was observed with no up-regulation of MCP or CD59 (data not shown). Thus, in this model at least, ECs derived from large and small vessels appeared to behave similarly, and the remainder of the experiments were therefore performed with HUVECs.
Thrombin up-regulates DAF via activation of PAR1
The leech product hirudin, which binds thrombin and inhibits its proteolytic effects, was used to demonstrate specificity of thrombin-induced DAF expression. The up-regulation of DAF expression by thrombin was completely blocked by preincubating ECs with hirudin while the baseline DAF expression remained unaffected (Figure3A). Specific use of the thrombin receptor was further demonstrated using the PAR1-activating peptide TRAP-6, a short peptide containing the tethered ligand sequence of the thrombin receptor, which directly activates the thrombin receptor PAR1. As seen in Figure 3B, TRAP-6 dose-dependently increased expression of DAF, although to a lesser extent than using thrombin, a feature characteristic of the TRAP peptides.40
Effect of hirudin on thrombin-induced DAF expression and comparison of TRAP-6 to thrombin for induction of DAF.
(A) HUVEC monolayers were treated with hirudin (30 U/mL) or plain medium alone for 30 minutes prior to the addition of thrombin (10 U/mL) and cultured for a further 24 hours. Following harvesting, ECs were analyzed by flow cytometry for the expression of DAF using MoAb 1H4. The data are expressed as a percentage of the DAF expression (RFI) on thrombin-stimulated ECs and are presented as the mean ± SEM. (B) HUVEC monolayers were treated with increasing concentrations of TRAP-6 or vehicle alone for 24 hours and then harvested, stained with MoAb to DAF (1H4), and analyzed by flow cytometry. The data are expressed as a percentage of the DAF expression on thrombin-treated cells and are presented as the mean ± SEM of 3 experiments performed on separate HUVEC cultures.
Effect of hirudin on thrombin-induced DAF expression and comparison of TRAP-6 to thrombin for induction of DAF.
(A) HUVEC monolayers were treated with hirudin (30 U/mL) or plain medium alone for 30 minutes prior to the addition of thrombin (10 U/mL) and cultured for a further 24 hours. Following harvesting, ECs were analyzed by flow cytometry for the expression of DAF using MoAb 1H4. The data are expressed as a percentage of the DAF expression (RFI) on thrombin-stimulated ECs and are presented as the mean ± SEM. (B) HUVEC monolayers were treated with increasing concentrations of TRAP-6 or vehicle alone for 24 hours and then harvested, stained with MoAb to DAF (1H4), and analyzed by flow cytometry. The data are expressed as a percentage of the DAF expression on thrombin-treated cells and are presented as the mean ± SEM of 3 experiments performed on separate HUVEC cultures.
Role of PKC, p38 and p42/44 MAPK, and NF-κB in thrombin-induced DAF up-regulation
The signaling pathways for the up-regulation of DAF on ECs remain poorly understood with, to date, both PKC-dependent and -independent pathways described.28,29,41 Thrombin is known to exert its effects through a variety of downstream signaling mechanisms, which may involve PKC, p38 MAPK, p42/44 MAPK, and NF-κB.18,42-44To investigate the role of these in DAF induction, we used cell-permeable pharmacologic inhibitors of PKC (RO31-8220 and GF 109203X), MEK-1 (PD98059), p38 MAPK (SB202190), and NF-κB (PSI). Initial dose-response experiments identified the optimal concentrations for each of the inhibitors. HUVECs were preincubated with the relevant inhibitors for 1 hour prior to the addition of the thrombin. The role of PKC in the up-regulation of DAF expression by thrombin was demonstrated by complete inhibition of the response by RO31-8220 (Figure 4A) and GF 109203X (not shown). Furthermore, preincubation of ECs with the p38 inhibitor SB202190 prevented thrombin-induced DAF up-regulation (Figure 4B). In addition, as seen in Figure 4C, the involvement of p42/44 MAPK in the signaling pathway was suggested by inhibition of the response by PD98059. The effects of the MAPK inhibitors observed were dose-responsive, with inhibition first detectable with 2.5 μmol/L SB202190 and maximal at 25 μmol/L and with 5 μmol/L PD98059 maximal at 100 μmol/L.34 45 Finally, a role for NF-κB was suggested by the inhibition of DAF induction by PSI (Figure 4D). These experiments also demonstrated that the constitutive expression of DAF was reduced to some extent by RO31-8220, SB202190, and PD98059, suggesting a role for these pathways in the maintenance of DAF expression on resting ECs. The antagonists studied, and particularly SB202190, induced EC shape change but did not lead to any demonstrable cytotoxicity at the concentrations used, as assessed by cell counting and trypan blue exclusion on the EC populations and also by flow cytometric analysis of CD31 expression (data not shown).
Effect of pharmacologic inhibitors of PKC, p38 and p42/44 MAPK, and NF-κB on thrombin-induced DAF.
HUVEC monolayers were preincubated with (A) the PKC inhibitor RO31-8220 (1 μmol/L), (B) the p38 inhibitor SB202190 (25 μmol/L), (C) the MEK-1 inhibitor of p42/44 MAPK phosphorylation PD98059 (50 μmol/L), or (D) the NF-κB inhibitor PSI (10 μmol/L) for 1 hour prior to the addition of 10 U/mL thrombin for 24 hours. The ECs were then harvested and stained with MoAb to DAF (1H4) for analysis by flow cytometry. The data are expressed as a percentage of the DAF expression on thrombin-treated cells and are presented as the mean ± SEM of 3 experiments performed on separate HUVEC cultures.
Effect of pharmacologic inhibitors of PKC, p38 and p42/44 MAPK, and NF-κB on thrombin-induced DAF.
HUVEC monolayers were preincubated with (A) the PKC inhibitor RO31-8220 (1 μmol/L), (B) the p38 inhibitor SB202190 (25 μmol/L), (C) the MEK-1 inhibitor of p42/44 MAPK phosphorylation PD98059 (50 μmol/L), or (D) the NF-κB inhibitor PSI (10 μmol/L) for 1 hour prior to the addition of 10 U/mL thrombin for 24 hours. The ECs were then harvested and stained with MoAb to DAF (1H4) for analysis by flow cytometry. The data are expressed as a percentage of the DAF expression on thrombin-treated cells and are presented as the mean ± SEM of 3 experiments performed on separate HUVEC cultures.
Thrombin-induced DAF on ECs is protein synthesis–dependent
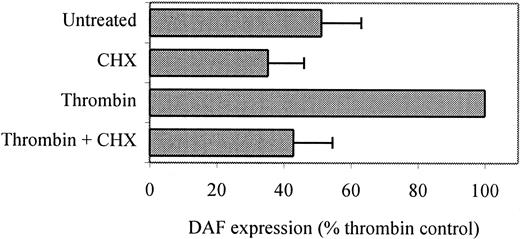
The protein synthesis inhibitor CHX (1 μg/mL) was added to ECs for 30 minutes prior to the addition of thrombin and remained throughout the 24 hours of stimulation. As seen in Figure5, the presence of CHX reduced DAF expression on resting ECs, probably due to inhibition of the normal turnover of constitutive DAF, as previously described.29In addition, CHX inhibited thrombin-induced DAF expression, confirming a dependence on de novo protein expression. Northern blot analysis was performed using messenger RNA (mRNA) from unstimulated HUVECs and cells stimulated with thrombin in the presence and absence of CHX for up to 24 hours. As shown in Figure6A, DAF mRNA at low levels was detectable in resting HUVECs, and following thrombin stimulation an increase was observed that was maximal at 2 to 6 hours and, although falling, remained 50% above baseline 24 hours poststimulation. Two DAF mRNA transcripts were detected at 2.4 and 1.8 kilobases, as previously reported.39,46 Quantification of mRNA levels using densitometric scanning of the 2.4-kilobase band demonstrated a 2.7-fold increase at 2 hours (Figure 6B). Moreover, preincubation of ECs with CHX prior to the addition of thrombin failed to inhibit the increase in DAF mRNA in response to thrombin (Figure 6A, lanes 7-8), while CHX alone increased DAF mRNA somewhat, an observation that has been made for other NF-κB–dependent genes, including ICAM-1.18 Comparative experiments were performed with VEGF, which also induces DAF expression on ECs.47 Relative to the effect of thrombin, the increase in DAF mRNA seen following treatment with VEGF was delayed, with an increase first detectable at 3 hours and peaking at 6 to 9 hours poststimulation. In addition, the VEGF-induced increase in DAF mRNA was completely inhibited by preincubation with CHX (Figure 6C-D). These data suggest that the induction of DAF by thrombin is a direct effect on gene transcription while that in response to VEGF is indirect and dependent on the synthesis of one or more intermediary proteins.
Thrombin-induced surface expression of DAF requires de novo protein synthesis.
HUVECS were plated at confluence (5 × 105 cells/well) in 6-well dishes and cultured overnight at 37°C. They were then pretreated with CHX (1 μg/mL) for 30 minutes prior to addition of thrombin (10 U/mL) for a further 24 hours. Following harvesting, DAF expression was measured by flow cytometry using MoAb 1H4. CHX at these concentrations was not toxic to ECs, as assessed by examination of the monolayers prior to staining using phase-contrast microscopy, cell counting, and estimation of trypan blue exclusion. The data are expressed as the RFI ± SEM from 2 similar experiments performed on separate HUVEC cultures.
Thrombin-induced surface expression of DAF requires de novo protein synthesis.
HUVECS were plated at confluence (5 × 105 cells/well) in 6-well dishes and cultured overnight at 37°C. They were then pretreated with CHX (1 μg/mL) for 30 minutes prior to addition of thrombin (10 U/mL) for a further 24 hours. Following harvesting, DAF expression was measured by flow cytometry using MoAb 1H4. CHX at these concentrations was not toxic to ECs, as assessed by examination of the monolayers prior to staining using phase-contrast microscopy, cell counting, and estimation of trypan blue exclusion. The data are expressed as the RFI ± SEM from 2 similar experiments performed on separate HUVEC cultures.
Thrombin-induced DAF gene transcription is independent of de novo protein synthesis.
(A) HUVECs in 75-cm2 flasks were pretreated with CHX (1 μg/mL) or plain medium alone for 30 minutes prior to the addition of thrombin (10 U/mL) and culture for up to 24 hours. Total RNA was isolated, and Northern blots were prepared. The figure shows unstimulated HUVEC (lane 1) thrombin treatment for 2 hours (lane 2), 4 hours (lane 3), 6 hours (lane 4), and 24 hours (lane 5); CHX alone for 4 hours (lane 6); and CHX and thrombin for 2 hours (lane 7) and 4 hours (lane 8). The ethidium bromide–stained gels confirmed equal loading of RNA in each lane. (B) Quantification of mRNA levels in resting and thrombin-stimulated ECs using densitometric scanning. (C) HUVECs in 75-cm2 flasks were pretreated with CHX (1 μg/mL) or plain medium alone for 30 minutes prior to the addition of VEGF (25 ng/mL) and culture for up to 24 hours. Total RNA was isolated, and Northern blots were prepared. The figure shows unstimulated HUVECs (lane 1); VEGF treatment for 3 hours (lane 2), 6 hours (lane 3), and 9 hours (lane 4); CHX alone for 6 hours (lane 5); CHX and VEGF for 6 hours (lane 6); and CHX and VEGF for 9 hours (lane 7). The ethidium bromide–stained gels confirmed equal loading of RNA in each lane. (D) Quantification of mRNA levels in resting and VEGF-stimulated ECs using densitometric scanning.
Thrombin-induced DAF gene transcription is independent of de novo protein synthesis.
(A) HUVECs in 75-cm2 flasks were pretreated with CHX (1 μg/mL) or plain medium alone for 30 minutes prior to the addition of thrombin (10 U/mL) and culture for up to 24 hours. Total RNA was isolated, and Northern blots were prepared. The figure shows unstimulated HUVEC (lane 1) thrombin treatment for 2 hours (lane 2), 4 hours (lane 3), 6 hours (lane 4), and 24 hours (lane 5); CHX alone for 4 hours (lane 6); and CHX and thrombin for 2 hours (lane 7) and 4 hours (lane 8). The ethidium bromide–stained gels confirmed equal loading of RNA in each lane. (B) Quantification of mRNA levels in resting and thrombin-stimulated ECs using densitometric scanning. (C) HUVECs in 75-cm2 flasks were pretreated with CHX (1 μg/mL) or plain medium alone for 30 minutes prior to the addition of VEGF (25 ng/mL) and culture for up to 24 hours. Total RNA was isolated, and Northern blots were prepared. The figure shows unstimulated HUVECs (lane 1); VEGF treatment for 3 hours (lane 2), 6 hours (lane 3), and 9 hours (lane 4); CHX alone for 6 hours (lane 5); CHX and VEGF for 6 hours (lane 6); and CHX and VEGF for 9 hours (lane 7). The ethidium bromide–stained gels confirmed equal loading of RNA in each lane. (D) Quantification of mRNA levels in resting and VEGF-stimulated ECs using densitometric scanning.
Thrombin-induced DAF reduces complement-mediated injury to HUVECs
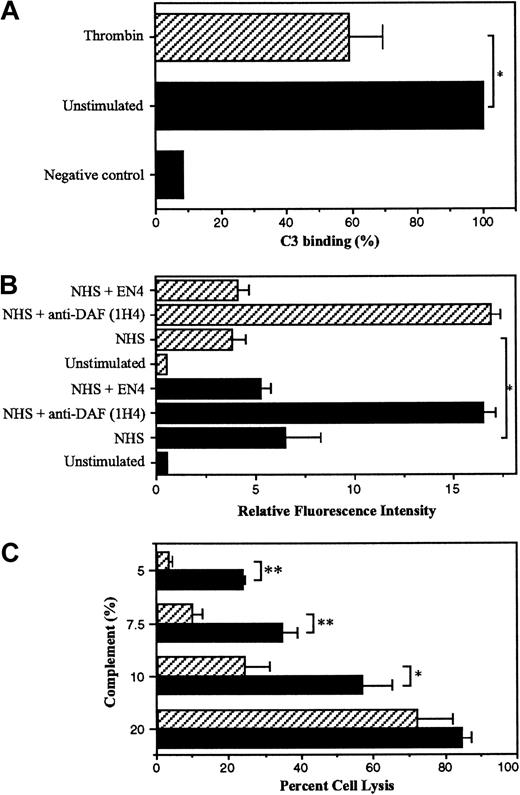
To investigate the potential functional role of thrombin-induced DAF, a flow cytometric assay measuring the binding of complement factor C3 to the EC surface was used as previously described.28ECs that were unstimulated and HUVECs treated with thrombin for 24 hours were opsonized with RMAC8, an IgG2a complement-fixing antibody directed against endoglin, followed by incubation with 5% NHS for 3 hours as a source of complement proteins. C3 binding to the cell surface was then quantified using an FITC-labeled anti-C3c antibody. IgG2a is the optimal murine isotype for complement fixation, and endoglin was chosen as the target antigen because it is highly expressed on resting ECs and is not known to be inducible.48 Parallel flow cytometric analysis confirmed that the EC expression of endoglin was not altered significantly by thrombin stimulation for 24 hours (endoglin RFI ± SEM: unstimulated ECs 155.7 ± 29.0, thrombin-treated ECs 186.6 ± 53.5). As seen in Figure 7A, stimulation with thrombin reduced the deposition of C3 on the EC surface by 40% compared with the unstimulated cells (P < .05). To confirm the role of DAF in the reduction of C3 binding following thrombin stimulation, the inhibitory anti-DAF MoAb 1H4 (noncomplement fixing) was included in the assay (Figure 7B). Following exposure to 5% NHS, opsonized ECs prestimulated with thrombin demonstrated low levels of C3 binding. Moreover, the reduction in C3 binding seen in response to thrombin treatment was reversed by the presence of MoAb 1H4, with levels of C3 deposited on the cell surface becoming equivalent to those observed on unstimulated ECs in the presence of MoAb 1H4 (Figure 7B). The noncomplement-fixing antibody EN4 (anti-CD31) was used as a negative control and had no effect on the level of C3 binding.
Thrombin-induced DAF expression protects ECs against complement-mediated injury.
HUVECs were plated at confluence (5 × 105 cells/well) in 6-well dishes and cultured overnight at 37°C prior to stimulation with 10 U/mL thrombin or plain medium alone (unstimulated) for 24 hours. Following harvesting, ECs were incubated with the antiendoglin MoAb RMAC8 or plain medium alone for 30 minutes at 4°C. The ECs were then washed in HBSS/1% BSA prior to addition of up to 5% NHS for 3 hours at 37°C. Binding of C3 was detected by flow cytometry using FITC-conjugated rabbit antihuman C3. (A) Percent C3 binding ± SD (n = 3) to unstimulated (black bars) and thrombin-stimulated HUVECs (hatched bars), with binding to unstimulated ECs (RFI ± SD = 8.1 ± 2.7) shown as 100%. The negative control represents C3 binding in the presence of HIHS but without preincubation with MoAb RMAC8. (B) C3 binding (RFI ± SD, n = 3) on unstimulated (black bars) and thrombin-stimulated HUVECs (hatched bars) in the presence of MoAbs 1H4 (anti-DAF) and EN4 (anti-CD31), both at 50 μg/mL. (C) HUVECs plated at confluence (1.5 × 105 cells/well) in 24-well plates were cultured overnight prior to stimulation with 10 U/mL thrombin or plain medium alone for 24 hours. Following loading with calcein acetoxymethyl ester, ECs were incubated with MoAb RMAC8 for 30 minutes at 37°C. The ECs were then washed in HBSS/1% BSA prior to the addition of baby rabbit complement or HIHS for 45 minutes at 37°C. Calcein release was measured and percent cell lysis calculated as outlined in “Materials and methods.” The data are presented as percent cell lysis ± SD, n = 3. The figure is representative of 3 similar experiments performed on different EC cultures. * P < .05, ** P < .001.
Thrombin-induced DAF expression protects ECs against complement-mediated injury.
HUVECs were plated at confluence (5 × 105 cells/well) in 6-well dishes and cultured overnight at 37°C prior to stimulation with 10 U/mL thrombin or plain medium alone (unstimulated) for 24 hours. Following harvesting, ECs were incubated with the antiendoglin MoAb RMAC8 or plain medium alone for 30 minutes at 4°C. The ECs were then washed in HBSS/1% BSA prior to addition of up to 5% NHS for 3 hours at 37°C. Binding of C3 was detected by flow cytometry using FITC-conjugated rabbit antihuman C3. (A) Percent C3 binding ± SD (n = 3) to unstimulated (black bars) and thrombin-stimulated HUVECs (hatched bars), with binding to unstimulated ECs (RFI ± SD = 8.1 ± 2.7) shown as 100%. The negative control represents C3 binding in the presence of HIHS but without preincubation with MoAb RMAC8. (B) C3 binding (RFI ± SD, n = 3) on unstimulated (black bars) and thrombin-stimulated HUVECs (hatched bars) in the presence of MoAbs 1H4 (anti-DAF) and EN4 (anti-CD31), both at 50 μg/mL. (C) HUVECs plated at confluence (1.5 × 105 cells/well) in 24-well plates were cultured overnight prior to stimulation with 10 U/mL thrombin or plain medium alone for 24 hours. Following loading with calcein acetoxymethyl ester, ECs were incubated with MoAb RMAC8 for 30 minutes at 37°C. The ECs were then washed in HBSS/1% BSA prior to the addition of baby rabbit complement or HIHS for 45 minutes at 37°C. Calcein release was measured and percent cell lysis calculated as outlined in “Materials and methods.” The data are presented as percent cell lysis ± SD, n = 3. The figure is representative of 3 similar experiments performed on different EC cultures. * P < .05, ** P < .001.
To further assess the physiologic relevance of these findings, we determined whether thrombin can protect HUVECs against complement-mediated lysis initiated by opsonization with the antiendoglin MoAb RMAC8 and addition of baby rabbit complement. Pretreatment of ECs with thrombin for 24 hours was cytoprotective, significantly reducing cell lysis following the addition of 5%, 7.5%, and 10% baby rabbit complement (Figure 7C). At serum concentrations of 20% or above (not shown), the cytoprotective response of thrombin was overcome. These observations suggest that the increased levels of EC surface DAF induced by exposure to thrombin provide additional protection to ECs against complement-mediated injury or activation.
Discussion
Although the study of thrombin has principally focused on its role in the coagulation cascade, it has also been demonstrated to have a significant potential role in inflammation.49 In particular, its effects on the vascular endothelium include an increase in vascular permeability,10 the up-regulation of cellular adhesion molecules,12,13,16-18 and the induction of angiogenesis.31 Situated at the blood-tissue interface, the endothelium is continuously exposed to the risk of complement-mediated injury,50,51 which may be significantly enhanced during inflammation following activation of the alternative or classical pathways.27 We have previously demonstrated that the proinflammatory mediators TNF-α, IFN-γ, and C5b-9 can induce EC DAF expression through a PKC-independent pathway and therefore enhance cytoprotection against activated complement.28 More recently we have shown that VEGF is a physiologic agonist for the previously described phorbol ester–inducible pathway of DAF up-regulation.47 In this report we provide evidence that thrombin also induces DAF expression by a mechanism that requires PKC but is in other ways dissimilar to that utilized by VEGF. Thus, while thrombin-mediated DAF up-regulation appears to be mediated by a direct activation of DAF mRNA transcription, one or more intermediary proteins is required for VEGF-mediated DAF expression.
Initial experiments demonstrated that exposure of HUVECs to thrombin increased the expression of DAF by up to 4-fold above the basal level on resting cells. In contrast to DAF, the basal levels of MCP and CD59 were not increased by thrombin up to 72 hours poststimulation. Previous work has demonstrated heterogeneity in responsiveness to thrombin between ECs from different vascular beds.52 However, a similar pattern of DAF up-regulation was also seen when DMECs were studied parallel with HUVECs. Dose-response studies confirmed that the optimal concentration of thrombin was the same as that previously described for ICAM-1 up-regulation.17 The levels of thrombin required for DAF and adhesion molecule expression used in this and other studies are relatively high and may exceed physiologically relevant levels. However, it has previously been shown that thrombin may bind subendothelial extracellular matrix, where it is relatively protected from circulating inhibitors and remains active.53 Thus, it has been proposed that thrombin may be concentrated in this way and subsequently released and made available to the endothelium.49 It is also possible that a cofactor is required for optimal signaling via EC PAR1, analogous to the thrombin-sparing effect of PAR3 on PAR4-mediated responses recently reported for murine platelets.54 Such a cofactor might be present in vivo but absent in in vitro assay systems; hence the need for higher concentrations of thrombin. The observation that prolonged exposure to thrombin for more than 6 hours is required to induce DAF expression (data not shown) and the findings of the lesser effect of the TRAP-6 peptide suggest that binding to PAR1 may not be the sole stimulatory mechanism.
To investigate the role of PKC in the up-regulation of DAF by thrombin, we preincubated ECs with the PKC antagonists RO31-8220 and GF 109203X, which resulted in complete inhibition of thrombin-induced DAF expression. Thus, these data confirm the presence of 2 physiologic pathways for DAF regulation in ECs: one PKC-dependent, the other PKC-independent and utilized by TNF-α, IFN-γ, and C5b-9.28 The presence of agonist-specific pathways for DAF induction may have important functional implications and be capable of mediating a specific distribution pattern for the molecule within tissues. This hypothesis is supported by the recent observation that activation of the PKC pathway by phorbol esters results in deposition of DAF in the extracellular matrix of EC, an effect not seen following cytokine stimulation.41
Further investigation using pharmacologic antagonists of p38 MAPK (SB202190) and p42/44 MAPK (PD98059) revealed inhibition of thrombin-induced DAF expression by both SB202190 and PD98059 in a dose-responsive manner. Furthermore, preincubation with the NF-κB inhibitor PSI, which prevents the activation of NF-κB through the inhibition of IκBα degradation,36 also abrogated the effect of thrombin on DAF expression. These findings are consistent with previously reported activation of p42/44 MAPK by thrombin in ECs55 and the requirement for NF-κB activation in thrombin-induced ICAM-1 expression in HUVECs.18 Moreover, the inhibition of the response by inhibitors of both p38 and p42/44 MAPK supports emerging evidence of crosstalk between MAPK signaling pathways, which is stimulus and cell-type specific.44,56,57 This may occur at the level of upstream regulatory kinases, as suggested in a recent report in which blockade of p38 MAPK in thrombin-stimulated HUVECs suppressed p42 MAPK activation.44 Alternatively, activation of both the p38 and p42/44 pathways may be required for gene transcription, as observed for p38 and Jun N-terminal kinase (JNK) MAPK in a model using NIH-3T3 cells.56 However, endothelial MAPK signaling pathways remain to be fully elucidated, and caution is required in interpreting results obtained using pharmacologic inhibitors. PD98059 is the most specific inhibitor of MEK-1 available and has been extensively tested against 18 different kinases to confirm its specificity.34 Although the specificity of the pyrindinyl imidazole inhibitors of p38 MAPK (SB202190 and SB203580 35) has varied between studies, they have been shown to inhibit other kinases, including JNKβ1 and cRaf-1 in addition to p38 MAPK.56 58-60 Therefore, a role for both JNKβ1 and cRaf-1 in the thrombin-induced up-regulation of DAF cannot be excluded.
The up-regulation of DAF on the EC surface by thrombin was dependent on increased steady-state mRNA and de novo protein synthesis and was first detectable 6 hours and maximal 24 hours poststimulation. The increased mRNA expression was maximal at 2 to 6 hours, falling to 1.5-fold above baseline levels after 24-hour stimulation. Moreover, experiments using CHX demonstrated that the effect of thrombin on the DAF gene was direct and independent of protein synthesis. This was in contrast to the increase in DAF mRNA seen in response to VEGF, which was maximal 6 to 9 hours poststimulation and was inhibited by the presence of CHX. In addition, VEGF-induced up-regulation of DAF on the EC surface is also delayed compared with thrombin.47,74 The demonstration of direct and indirect intracellular signaling pathways for DAF up-regulation may have important functional implications. Thus, the more rapid direct response may be important in regulating DAF expression following vascular injury,61 while the more prolonged indirect response to VEGF may have a predominant role in EC cytoprotection against complement-mediated injury during angiogenesis.
To establish whether thrombin-induced DAF was functionally important in the face of complement activation, we initially used a flow cytometric assay to measure levels of surface-bound C3. Stimulation with thrombin for 24 hours significantly reduced C3 binding, and inclusion of an inhibitory anti-DAF MoAb (1H4) reversed this effect. This suggests that up-regulation of DAF on the EC surface to the levels seen following stimulation with thrombin is protective against bystander complement-mediated injury. This inhibition of C3 binding is particularly important in view of the relative inefficiency of C5 activation, which is dependent on an absolute excess of C3 activation.62 This observation was further supported by the demonstration that thrombin treatment resulted in a significant inhibition of complement-mediated EC lysis. Furthermore, during inflammation, this cytoprotective response may be amplified by a synergistic effect between thrombin and proinflammatory cytokines63 and by other inducible protective mechanisms, including synthesis of factors H and I by ECs.64 65
In chronic inflammatory disease states, including atherosclerosis, systemic lupus erythematosus, and rheumatoid arthritis, endothelium is continuously exposed to low levels of complement activation but without significant EC lysis.66-68 Thrombin has been identified in both atherosclerotic plaques and in the synovial fluid in rheumatoid arthritis.69,70 The data presented demonstrate that, in addition to its known roles in coagulation and inflammation, thrombin may be important in protection against complement-mediated injury—so linking inflammation, coagulation, and the complement cascade. This association results in feedback loops of vascular protection mediated by the up-regulation of DAF and dependent on the actions of proinflammatory mediators. Thus, binding of C5b-9 to ECs will directly induce DAF expression28 and stimulate tissue factor synthesis,71,72 resulting in the local generation of thrombin. This will further increase DAF expression, thereby reducing the formation of C5b-9. These responses would be amplified by the presence of proinflammatory cytokines such as TNF, which, in addition to inducing DAF expression directly, will also enhance the effects of both C5b-9 28,73 and thrombin.63Furthermore, following vascular injury, thrombin may induce DAF deposition into the EC basement membrane via its action on the PKC-dependent pathway, thereby reducing complement activation on the exposed subendothelial extracellular matrix.41 61
In summary, we have demonstrated that thrombin, in addition to its role in coagulation and as a proinflammatory mediator, can induce the expression of the complement regulatory protein DAF on the EC surface. Thrombin is a physiologic agonist for the PKC-dependent pathway of DAF up-regulation, which may also require p38 and p42/44 MAPK activation, and NF-κB binding. The increased level of DAF seen following thrombin treatment of ECs enhances cytoprotection against complement-mediated injury. Thus, these observations reveal an important link between the coagulation cascade, inflammation, and the complement system, which may play a role in maintenance of vascular integrity during subacute and chronic inflammatory responses.
Acknowledgments
We are grateful for the help of P. Kiely, P. Singh, Dr L. Lovat, and M. McNamara for collection of foreskins and to the staff of the Maternity Unit of Hammersmith Hospital for the provision of umbilical cords. We would like to thank Doug Lublin for the gift of MoAb 1H4 and for reviewing the manuscript. We also thank Mark Walport, Kevin Davies, Bernie Morley, Marina Botto, Jeremy Pearson, Paul Morgan, Tony d'Apice, Teizo Fujita, and John Atkinson for their help with this study.
Supported by a British Heart Foundation Professorial Award (D.O.H.).
J.C.M. is an Arthritis Research Campaign Clinician Scientist Fellow.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
J. C. Mason, BHF Cardiovascular Medicine Unit, National Heart and Lung Institute, Imperial College School of Technology and Medicine, Hammersmith Hospital, Du Cane Rd, London W12 ONN, England; e-mail: justin.mason@ic.ac.uk.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal