Abstract
Progression through the mammalian cell cycle is regulated by cyclins, cyclin- dependent kinases (CDKs), and cyclin-dependent kinase inhibitors (CKIs). The function of these proteins in the irreversible growth arrest associated with terminally differentiated cells is largely unknown. The function of Cip/Kip proteins p21Cip1and p27Kip1 during erythropoietin-induced terminal differentiation of primary erythroblasts isolated from the spleens of mice infected with the anemia-inducing strain of Friend virus was investigated. Both p21Cip1 and p27Kip1 proteins were induced during erythroid differentiation, but only p27Kip1 associated with the principal G1CDKs—cdk4, cdk6, and cdk2. The kinetics of binding of p27Kip1 to CDK complexes was distinct in that p27Kip1 associated primarily with cdk4 (and, to a lesser extent, cdk6) early in differentiation, followed by subsequent association with cdk2. Binding of p27Kip1 to cdk4 had no apparent inhibitory effect on cdk4 kinase activity, whereas inhibition of cdk2 kinase activity was associated with p27Kip1binding, accumulation of hypo-phosphorylated retinoblastoma protein, and G1 growth arrest. Inhibition of cdk4 kinase activity late in differentiation resulted from events other than p27Kip1 binding or loss of cyclin D from the complex. The data demonstrate that p27Kip1 differentially regulates the activity of cdk4 and cdk2 during terminal erythroid differentiation and suggests a switching mechanism whereby cdk4 functions to sequester p27Kip1 until a specified time in differentiation when cdk2 kinase activity is targeted by p27Kip1 to elicit G1 growth arrest. Further, the data imply that p21Cip1 may have a function independent of growth arrest during erythroid differentiation.
Introduction
Cell differentiation imparts unique identity through a coordinated tissue-specific gene expression program. This program is tightly coordinated with cell cycle exit. Commitment to cell division is regulated by a family of G1 cyclin-dependent protein kinases (CDKs) (reviewed in Morgan1 and Pavletich2). CDKs are regulated by activating proteins, cyclins, (reviewed in Roberts3) and cyclin-dependent kinase inhibitors (CKIs), which either block or enhance the activity of the cyclin-CDK complexes (reviewed in Sherr and Roberts4 and Nakayama and Nambe5). The precise role these proteins play in establishment and maintenance of the nonproliferative state associated with terminal differentiation has not been determined.
Two families of CKIs regulate the function of CDKs and are so designated based on their structure and CDK targets (reviewed in Sherr and Roberts4). The INK4 family consists of 4 proteins (p16INK4a, p15INK4b, p18INK4c, and p19INK4d) that specifically inhibit the catalytic subunits of cyclin D-dependent kinases (cdk4 and cdk6)6. The Cip/Kip family consists of 3 members (p21Cip1, p27Kip1, and p57Kip2). Mixing experiments in vitro and overexpression studies suggested that Cip/Kip proteins ubiquitously inhibit the activities of cyclin D-, E-, and A-dependent kinases, but more physiological models challenge this conclusion. Cip/Kip specificity for cyclin D and E complexes during the irreversible growth arrest associated with terminal differentiation has not been described.
Murine erythroid progenitor cells infected with the anemia-inducing strain of Friend virus represent a model system in which to study the molecular events associated with terminal erythroid differentiation in response to erythropoietin (EPO). Infection of mice with a retroviral complex consisting of replication-defective spleen focus-forming virus and replication-competent Friend murine leukemia virus leads to polyclonal expansion of proerythroblasts in the spleens of susceptible mice due to expression of the unique spleen focus-forming virus protein gp55 (reviewed in Ben-David and Bernstein7). Gp55 binds to and partially activates the EPO receptor,8,9 resulting in the expansion of nontransformed proerythroblasts (FVA erythroblasts), dependent on EPO for growth in vivo and in vitro. FVA erythroblasts cultured in vitro with EPO for 48 hours undergo a transient proliferative burst followed by withdrawal from the cell cycle and accumulation into the G1 phase.10,11Coincident with cell cycle arrest, FVA erythroblasts undergo a program of terminal differentiation characterized by expression of β-globin mRNA, hemoglobin production, nuclear condensation, and enucleation.12-14 The molecular mechanisms associated with FVA erythroblast terminal differentiation and cell cycle exit are unknown.
We examined the regulation and function of members of the Cip/Kip and INK4 families of CKIs to determine their role in growth arrest of terminally differentiated erythroid cells. In the Cip/Kip family we limited our study to p21Cip1 (p21) and p27Kip1(p27). Expression of p57Kip2 was not evaluated because p57Kip2 protein is restricted to a limited number of tissues, primarily during development,15,16 and was not detected in mouse spleens.15 Here we report that p19INK4d (p19), p21, and p27 proteins are induced during terminal erythroid differentiation and exhibit differential specificities for G1 CDKs, leading to distinct functions. The data provide a model for the roles of p27, cdk4, and cdk2 in cell cycle exit during terminal differentiation.
Materials and methods
Cell isolation and culture
Nucleated erythroblasts were isolated from spleens of 8- to 12-week-old Balb-c mice infected 2 weeks previously with 104 spleen focus-forming units of the anemia-inducing strain of Friend virus (FVA), as described.17,18 Briefly, splenocytes were separated by velocity sedimentation at unit gravity on a continuous 1% to 2% bovine serum albumin (BSA) gradient. Purified erythroblasts were cultured in Iscove modified Dulbecco medium (IMDM) containing 1% BSA, 30% fetal bovine serum (FBS) (HyClone Laboratories, Logan, UT), 100 U/100 g per milliliter penicillin/streptomycin (Gibco BRL, Gaithersburg, MD), and, where indicated, 1 U/mL pure recombinant human erythropoietin (EPO) (Ortho Pharmaceutical, Raritan, NJ). EPO-independent murine erythroleukemia cells (MEL)19 were maintained in IMDM supplemented with 10% FBS and 100 U/100 g per milliliter penicillin/streptomycin. The EPO-dependent HCD57 cell line was a gift of Dr David Hankins and was maintained in IMDM containing 30% FBS, 100 U/100 g per milliliter penicillin/streptomycin, and 1 U/mL EPO. A retinoblastoma (Rb)+ T-lymphocyte cell line, Molt 4,20 was maintained in RPMI supplemented with 10% FBS. An Rb− human breast carcinoma cell line, MDA MB436,21 was the gift of Dr Maria Frexes (Vanderbilt University, Nashville, TN) and was maintained in McCoy medium supplemented with 10% FBS. NIH-3T3 cells (ATCC, Rockville, MD) were maintained in Dulbecco modified Eagle medium with 4.5 g/L glucose supplemented with 10% FBS.
Morphologic analyses
Cytocentrifuge (Shandon, Pittsburgh, PA) slide preparations of cells were stained with 3,3′-dimethoxybenzidine, counterstained with hematoxylin and photographed using oil immersion optics.
Flow cytometric DNA analysis
Single parameter analysis of DNA content was performed on cells freshly removed from culture, fixed in methanol, and resuspended in a staining solution of 500 μL RNase (200 U/mL) (Sigma, St Louis, MO) and 500 μL propidium iodide (PI) (50 g/mL) (Molecular Probes, Eugene, OR). Linear fluorescence signals of PI (area and width) were assessed on a Becton Dickinson FACScan flow cytometer with dye excitation by 15 mW 488 nm laser light. Data were stored as list mode files of at least 50 000 single cell events for subsequent off-line analysis using Modfit and WinList software (Verity Software, Topsham, ME). DNA cell cycle analysis was accomplished using the DIP_N2 and DIP_N3 algorithms in Modfit.
Northern blot analysis
Northern blot analysis was performed as previously described.10 Briefly, total RNA was isolated from cells by a single-step guanidinium thiocyanate–phenol method using Ultraspec RNA (Biotex Laboratories, Houston, TX) and electrophoretically separated in 1% agarose gels containing 0.66 mol/L formaldehyde. After electrophoresis, the ethidium bromide-stained gels were photographed and blotted onto nylon membranes (Bio-Rad Laboratories, Hercules, CA). Prehybridization and hybridization were performed in 50% formamide, 6 × SSC (1.5 mol/L NaCl, 150 mmol/L sodium citrate), 1 × Denhardt solution (0.02% Ficoll, 0.02% polyvinylpyrrolidone, 0.02% BSA), and 0.5% sodium dodecyl sulfate (SDS). All probes were radiolabeled by random priming22 using [-32P]dCTP (800 Ci/mmol). Hybridizations were performed at 42°C for 16 hours using 106 cpm of the labeled probe per milliliter hybridization buffer. Blots were washed twice with 2 × SSC, 0.5% SDS at room temperature for 10 minutes each and twice with 0.1 × SSC, 0.1% SDS at 55°C for 40 minutes each. Full-length Mo-MLV DNA23 was used to probe for the detection of murine retroviral sequences. The mouse β-globin probe was a PstI fragment containing the first 2 exons of the β-major globin gene.24 The cdk2 probe was a full-length human EcoRI fragment (Dr James Whitlock, Vanderbilt University). The cdk4 probe was a full-length human PstI/ BamHI fragment (Dr Steven Hanks, Vanderbilt University). The p21 probe was a XhoI fragment containing the entire murine cDNA (Dr David Beach, Cold Spring Harbor, NY). The cyclin D3 probe was an EcoRI fragment corresponding to the carboxyl terminal portion of the coding sequences. The p18 and p19 probes were BamHI/EcoRI fragments, and the p15 and p16 probes were EcoRI fragments containing the entire coding sequences. Dr Charles Sherr (St. Jude's Research Hospital, Memphis, TN) provided the cyclin D3, p18, p19, p15, and p16 cDNA.
Immunoblot analysis of CDK and CKI proteins
Cells were cultured under the indicated conditions and washed in PBS, and cell pellets were prepared. Pellets were snap frozen in an ethanol–dry ice slurry and stored at −70°C until further analysis. Cell lysates were prepared by resuspending cell pellets in boiling 2× sample buffer containing 83 mmol/L Tris-HCl (pH 6.8), 20% glycerol, 10% SDS, 1.28 mol/L mercaptoethanol, and 0.01% bromophenol blue. Lysis was facilitated by sonication. Approximately 150 μg of each whole cell lysate was electrophoretically separated on a 12% polyacrylamide gel. Proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad) and blocked overnight at 4°C in blocking buffer containing 20 mmol/L Tris (pH 7.5), 137 mmol/L NaCl, and 0.1% Tween 20 (TTBS) containing 5% nonfat dry milk (NFDM). Immunoblotting was performed using either 1 μg (p21, p27, p18, p19) or 0.1 μg (cdk2, cdk4, cdk6, cyclin D3) antibody per milliliter TTBS with 5% NFDM for 2 hours at room temperature. All antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Secondary antibodies included a 1:10 000 dilution in TTBS containing 5% NFDM of antimouse IgG (NA931; Amersham Life Science, Buckinghamshire, UK) (p21) or antirabbit IgG (A-6154; Sigma) (p27, cdk2, cdk4, cdk6, cyclin D3, p18, p19) conjugated to horseradish peroxidase (HRP) and allowed to incubate for 1 hour at room temperature. Bound antibodies were detected by enhanced chemiluminescence (Amersham).
Immunoblot analysis of Rb proteins
Freshly isolated cells were washed in PBS and resuspended in lysis buffer A containing 50 mmol/L HEPES (pH 7.0), 250 mmol/L NaCl, 0.1% NP40, 25 mol/L NaF, 200 mol/L Na3VO4, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), and 5 μg/mL aprotinin for 30 minutes on ice. Lysis was facilitated by vortexing at 10-minute intervals. Lysates were cleared by centrifugation, and 50 μg protein was electrophoretically separated on 7% polyacrylamide gels and transferred to nitrocellulose membranes. Membranes were blocked for 1 hour at room temperature in TTBS containing 5% NFDM. Immunoblotting was performed with 1 μg anti-Rb (PMG3-245; PharMingen, San Diego, CA) per milliliter TTBS blocking buffer at 37°C for 2 hours, followed by a 1:2000 dilution of antimouse IgG conjugated to HRP (A-4416; Sigma) for 1 hour at room temperature. Where indicated, gp55 protein was detected with a 1:500 dilution of rat monoclonal antibody 7C10 (Dr Sandra Ruscetti, National Cancer Institute, Frederick, MD) per milliliter blocking buffer for 1 hour at 37°C, followed by a 1:5000 dilution of goat antirat HRP (A-9037; Sigma). Bound antibody was detected using enhanced chemiluminescence.
Immunoprecipitation of CDK and CKI proteins
Cells were removed from culture at the indicated times, washed in PBS, and centrifuged at 1500 rpm for 5 minutes at 4°C. Cell pellets were immediately snap frozen in an ethanol–dry ice slurry and stored at −70°C until further analysis. Frozen cell pellets were resuspended in 1.2 mL lysis buffer B containing 50 mmol/L Tris (pH 7.4), 300 mmol/L NaCl, 2 mmol/L EDTA, 50 mmol/L sodium fluoride, 0.5% NP-40, 1 mmol/L sodium vanadate, 1 mmol/L PMSF, and 10 μg/mL each of leupeptin, aprotinin, trypsin inhibitor, and pepstatin A. Whole cell lysates were incubated for 30 minutes on ice, and lysis was facilitated by three 10-second cycles of sonication. Lysates were centrifuged at 10 000 rpm for 5 minutes at 4°C. Cell supernatants were removed and stored on ice. Remaining nuclear pellets were resuspended in 200 μL lysis buffer B and were incubated on ice for 15 minutes. Nuclear lysates were centrifuged as above, and the supernatant was combined with cellular supernatant. Pooled supernatants were centrifuged at 14 000 rpm for 20 minutes at 4°C. Lysates containing 350 μg protein were incubated with 2 μg of the indicated antibodies at 4°C for 2 hours. Protein A agarose (30 μL) was added to each sample and allowed to rotate for 2 hours at 4°C. Mixtures were centrifuged at 11 000 rpm for 5 minutes at 4°C. Agarose pellets were washed 4 times with buffer B and resuspended in 25 μL boiling 2× sample buffer, snap frozen, and stored at −70°C until further analysis. Frozen pellets were boiled for 5 minutes and electrophoretically separated on 12% polyacrylamide gels. Proteins were transferred and analyzed by immunoblot analysis as described above.
Rb kinase assay
Rb kinase assays were performed as described by Matsushime et al.25 Briefly, cell pellets were resuspended in lysis buffer C containing 50 mmol/L HEPES (pH 7.5), 150 mmol/L NaCl, 2.5 mmol/L EGTA, 1 mmol/L EDTA, and 0.1% Tween 20 containing 10% glycerol, 1 mmol/L dithiothreitol (DTT), 0.1 mmol/L PMSF, 0.2 U/mL aprotinin, 10 mmol/L glycerophosphate, 0.1 mmol/L sodium vanadate, 1 mmol/L sodium fluoride, and 10 μg leupeptin. Whole cell lysates were sonicated at 4°C 3 times for 10 seconds each, followed by centrifugation at 10 000g for 5 minutes. Immunoprecipitations were performed by incubating lysates containing 1 mg protein with 4 μg antibodies (cdk2, cdk4, cdk6) for 4 hours at 4°C followed by a 2-hour incubation with protein A-Sepharose. Immunoprecipitates were washed 4 times with 1 mL buffer C and 2 additional times with kinase buffer D containing 50 mmol/L HEPES (pH 7.5), 10 mmol/L MgCl2, and 1 mmol/L DTT. The beads were suspended in 30 μL reaction buffer D containing 2.5 mmol/L EGTA, 1 mmol/L DTT, 20 mol/L adenosine triphosphate (ATP), 10 mmol/L glycerophosphate, 0.1 mmol/L sodium vanadate, 1 mmol/L sodium fluoride, 2 μg Rb substrate freshly prepared from an Rb GST fusion protein,25 and 10 μCi (γ-32P) ATP (6000 Ci/mmol; NEN Life Science, Boston, MA) at 30°C for 30 minutes. The reaction was terminated by adding 2.5× SDS sample buffer. Samples were boiled for 5 minutes and electrophoretically separated on 12% polyacrylamide gels. Dried gels were exposed to x-ray film and examined by autoradiography.
Results
FVA erythroblasts undergo G1 growth arrest during EPO-mediated terminal differentiation
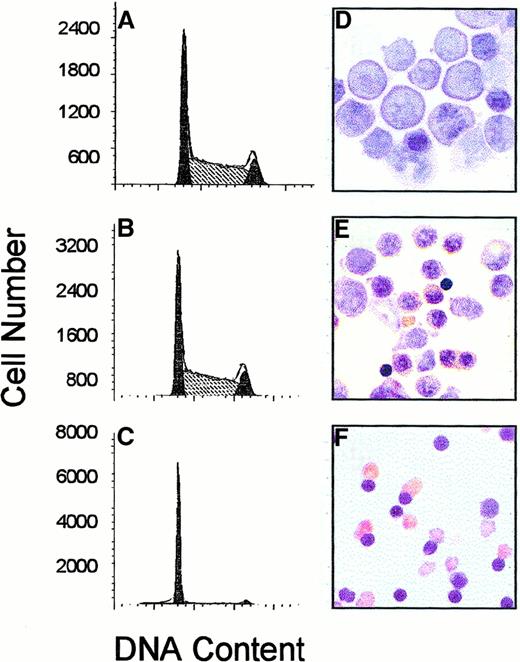
Freshly isolated FVA erythroblasts were cultured in the presence of EPO and analyzed for DNA content and differentiation status (Figure1). Approximately 32% of freshly isolated erythroblasts were in G1 phase at the initiation of culture. After culture in the presence of EPO, the percentage of cells in G1 phase increased to 49% and 84%, respectively, at 24 and 48 hours. Benzidine stains were prepared on cytospin slides at the same time points and demonstrated that cells were developmentally immature at the initiation of culture, as determined by the lack of hemoglobin production. By 24 hours cells had initiated hemoglobin production and nuclear condensation, and by 48 hours most cells had enucleated.
DNA cell cycle kinetics and morphologic changes during terminal erythroid differentiation.
FVA erythroblasts were cultured for 0 (A, D), 24 (B, E), or 48 (C, F) hours with EPO. Cells were removed from culture and analyzed for DNA cell cycle analysis of PI-stained cells by flow cytometry (A, B, C) or benzidine staining (D, E, F). Percentages of cells in G1 at 0, 24, and 48 hours of culture were 32%, 49%, and 84%, respectively.
DNA cell cycle kinetics and morphologic changes during terminal erythroid differentiation.
FVA erythroblasts were cultured for 0 (A, D), 24 (B, E), or 48 (C, F) hours with EPO. Cells were removed from culture and analyzed for DNA cell cycle analysis of PI-stained cells by flow cytometry (A, B, C) or benzidine staining (D, E, F). Percentages of cells in G1 at 0, 24, and 48 hours of culture were 32%, 49%, and 84%, respectively.
Multiple cell cycle–associated mRNAs are transcriptionally regulated during erythroid differentiation
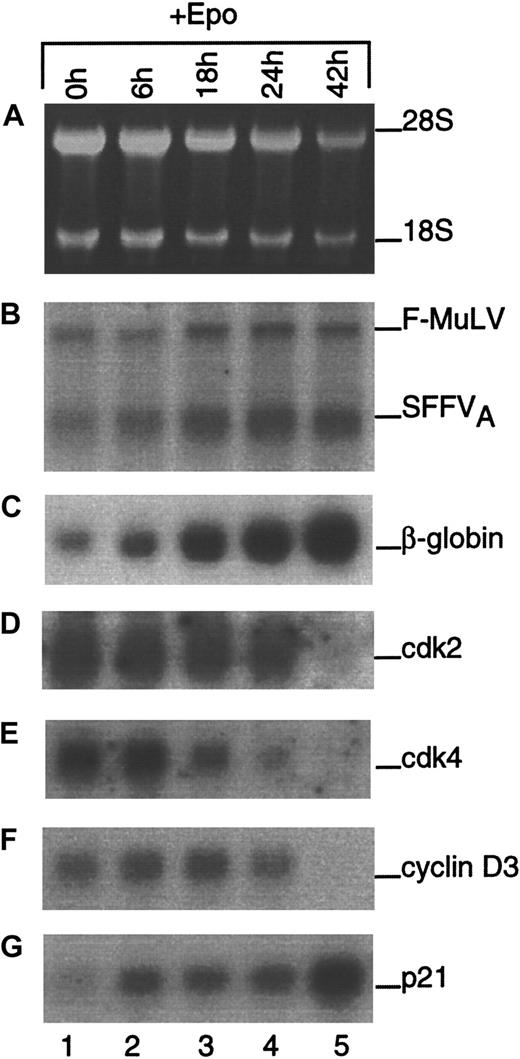
Initially, experiments were performed to determine which of the principle G1 CDK, cyclin, and CKI regulators were present in differentiating erythroblasts and to correlate their expression with an erythroid-specific differentiation marker, β-globin. Northern blot analyses of total RNA from cells cultured in the presence of EPO are shown in Figure 2. Ethidium bromide–stained ribosomal RNA and expression of viral mRNAs are shown as loading and blotting controls, respectively. Globin mRNA expression increased during the time course and served as evidence that the cells were differentiating. Levels of cdk2, cdk4, and cyclin D3 mRNA decreased during differentiation, albeit with different kinetics. Cyclin D1 and D2 mRNA were not expressed in differentiating erythroblasts at any time point (data not shown). As previously shown,11 the expression of p21 mRNA increased significantly from 0 to 42 hours of culture. These data suggested that cell cycle arrest might occur from the down-regulation of positive regulators of G1 as well as the up-regulation of G1 inhibitors. However, because the regulation of many cell cycle–associated gene products is known to occur at the posttranscriptional level, we shifted our focus to the study of protein expression.
Expression of cell cycle–associated mRNAs during terminal erythroid differentiation.
Total RNA was isolated from FVA erythroblasts at the indicated times during differentiation. Gel electrophoresis was performed, and ethidium bromide-stained rRNA was photographed as a control for loading (A). Northern blot analysis was performed using radiolabeled cDNA probes to Friend retroviral components (B), β-globin (C), cdk2 (D), cdk4 (E), cyclin D3 (F), and p21 (G).
Expression of cell cycle–associated mRNAs during terminal erythroid differentiation.
Total RNA was isolated from FVA erythroblasts at the indicated times during differentiation. Gel electrophoresis was performed, and ethidium bromide-stained rRNA was photographed as a control for loading (A). Northern blot analysis was performed using radiolabeled cDNA probes to Friend retroviral components (B), β-globin (C), cdk2 (D), cdk4 (E), cyclin D3 (F), and p21 (G).
Cip/Kip proteins are induced during erythroid differentiation
Steady state levels of proteins isolated from erythroblasts cultured with EPO are shown in Figure 3. Erythroblasts lacking p21 were included as a negative control for p21, and p21 wild-type erythroblasts, treated with the DNA damaging agent actinomycin D (ActD)—which activates wild-type p53 leading to enhanced production of p21 protein10,11—served as a positive control for p21. Levels of p21 protein increased during differentiation, peaking at 24 hours and declining by 48 hours. No p21 protein was detected in p21 null erythroblasts, whereas abundant p21 was detected in p21 wild-type erythroblasts treated with ActD. Levels of p27 protein also increased during differentiation, albeit with different kinetics; p27 protein levels continued to increase, peaking at 48 rather than 24 hours. Contrary to the previous observation that cdk2, cdk4, and cyclin D3 mRNA levels declined during differentiation, their protein levels remained constant and were unaffected by the absence of p21 or treatment with ActD. Levels of cdk6 and cyclin E proteins, however, decreased during differentiation. Interestingly, levels of cyclin E protein were greatly reduced in p21 null erythroblasts and in p21 wild-type cells treated with ActD. Because cyclin E protein levels are known to be positively regulated by E2F1,26 it was not surprising that cyclin E protein levels were reduced under experimental conditions in which ActD resulted in the activation of p53 and the induction of p21. We have previously shown that ActD-treated cells accumulate in G1,11 most likely leading to the sequestration of functional E2F and the decreased transcription of cyclin E. However, decreased cyclin E protein in untreated p21 null erythroblasts was unexpected and remains to be investigated further. Induction of p21 and p27 proteins during erythroid differentiation suggested they might function in growth arrest by binding to and inhibiting one or more G1 CDKs.
Expression of cell cycle–associated proteins during terminal erythroid differentiation.
Total cellular protein was isolated from FVA erythroblasts cultured for 0, 24, or 48 hours with EPO (lanes 1-3), p21 null erythroblasts (lane 4), or p21+/+ erythroblasts exposed to the DNA-damaging agent ActD for 6 hours (lane 5). Proteins were electrophoretically separated in 12% polyacrylamide gels and probed with antibodies to p21, p27, cdk2, cdk4, cdk6, cyclin D3, or cyclin E. gp55 protein expression is shown as a control for protein loading.
Expression of cell cycle–associated proteins during terminal erythroid differentiation.
Total cellular protein was isolated from FVA erythroblasts cultured for 0, 24, or 48 hours with EPO (lanes 1-3), p21 null erythroblasts (lane 4), or p21+/+ erythroblasts exposed to the DNA-damaging agent ActD for 6 hours (lane 5). Proteins were electrophoretically separated in 12% polyacrylamide gels and probed with antibodies to p21, p27, cdk2, cdk4, cdk6, cyclin D3, or cyclin E. gp55 protein expression is shown as a control for protein loading.
Endogenous Rb accumulates in a hypophosphorylated form during erythroid differentiation
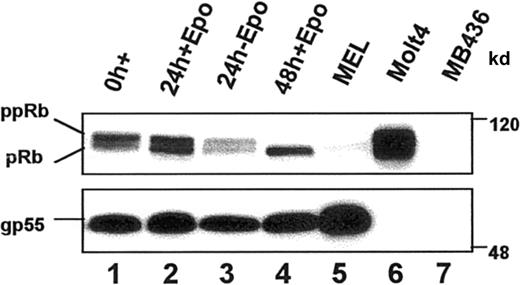
Inhibition of G1 CDK function in vivo should prevent phosphorylation of their common substrate, the retinoblastoma (Rb) protein (reviewed in Mulligan and Jacks27). We examined the phosphorylation status of endogenous Rb by observing the migration pattern of Rb proteins using standard gel electrophoresis and Western blot analysis (Figure 4). Rb migrated as 2 species in undifferentiated erythroblasts, a predominant hyperphosphorylated (ppRb) species of approximately 117 kd and a less abundant hypophosphorylated (pRb) form of approximately 110 kd. After 24 hours in culture with EPO, pRb and ppRb were present in approximately equal amounts. After 48 hours of culture, when cells were maximally growth arrested into G1 (Figure 1C), all the Rb protein had accumulated in the hypo-phosphorylated (pRb) form. Hypophosphorylated Rb did not accumulate in cells cultured in the absence of EPO, indicating that differentiation was required for pRb accumulation. Known Rb+ (Molt4) and Rb− (MB436) nonerythroid cell lines were included as controls. Interestingly, the transformed counterpart of FVA erythroblasts, MEL cells, had significantly reduced levels of Rb compared to FVA erythroblasts, suggesting that the loss of Rb protein may be associated with Friend virus-induced erythroleukemic transformation. Expression of the viral protein, gp55, was evaluated as a control for erythroid cell protein loading. The data are consistent with a G1 cell cycle arrest accompanied by an accumulation of hypo-phosphorylated Rb during terminal erythroid differentiation.
Endogenous Rb protein expression during terminal erythroid differentiation.
Steady state levels and the phosphorylation status of Rb protein were determined by immunoblot analysis of whole cell lysates from FVA erythroblasts cultured with or without EPO for the indicated times. Known Rb− (MB436) and Rb+ (Molt 4) cell lines were included as controls. Equal amounts of protein were electrophoretically separated in a 7% polyacrylamide gel, transferred to nitrocellulose, and immunoblotted with anti-Rb. Positions of hyperphosphorylated (ppRb) and hypophosphorylated (pRb) Rb are indicated. gp55 protein expression is shown as a control for protein loading.
Endogenous Rb protein expression during terminal erythroid differentiation.
Steady state levels and the phosphorylation status of Rb protein were determined by immunoblot analysis of whole cell lysates from FVA erythroblasts cultured with or without EPO for the indicated times. Known Rb− (MB436) and Rb+ (Molt 4) cell lines were included as controls. Equal amounts of protein were electrophoretically separated in a 7% polyacrylamide gel, transferred to nitrocellulose, and immunoblotted with anti-Rb. Positions of hyperphosphorylated (ppRb) and hypophosphorylated (pRb) Rb are indicated. gp55 protein expression is shown as a control for protein loading.
Temporal regulation of G1 CDK kinase activity during terminal erythroid differentiation
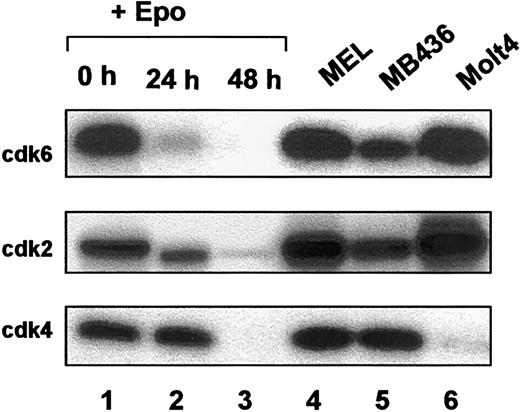
To determine which of the G1 CDKs may be associated with loss of Rb phosphorylation in vivo, we performed in vitro Rb kinase assays using immunoprecipitated CDK proteins (Figure5). At the initiation of culture, cdk6 kinase activity was abundant but was extinguished very early in the time course. Cdk2 kinase activity decreased gradually and was characterized by a marked decrease by 24 hours and again by 48 hours. Cdk4 kinase activity, on the other hand, remained constant in the first 24 hours of culture and was abruptly extinguished late in the differentiation course. The same cell lines as shown previously (Figure4) were included as controls. Although the amount of endogenous Rb protein was significantly reduced in MEL cells than in their nontransformed counterparts, FVA cells (Figure 4), the Rb kinase activity of all 3 G1 kinases tested in MEL cells was equivalent to or higher than that observed in FVA cells. These data suggest that any deregulation of Rb in MEL cells occurs downstream of G1 CDK kinase activity. The kinetics of loss of CDK kinase activity demonstrated that G1 CDK kinase function is temporally regulated during terminal erythroid differentiation.
Rb kinase activity of CDK immunoprecipitates during terminal erythroid differentiation.
Whole cell lysates from FVA erythroblasts (0, 24, 48 hours) (lanes 1-3), murine erythroleukemia (MEL) (lane 4), MB436 (lane 5), and Molt 4 (lane 6) cells were immunoprecipitated with antibodies to cdk6, cdk2, or cdk4. Immunoprecipitates were subjected to in vitro kinase assays using Rb as a substrate.
Rb kinase activity of CDK immunoprecipitates during terminal erythroid differentiation.
Whole cell lysates from FVA erythroblasts (0, 24, 48 hours) (lanes 1-3), murine erythroleukemia (MEL) (lane 4), MB436 (lane 5), and Molt 4 (lane 6) cells were immunoprecipitated with antibodies to cdk6, cdk2, or cdk4. Immunoprecipitates were subjected to in vitro kinase assays using Rb as a substrate.
p27, but not p21, associates with cdk4, cdk6, and cdk2 immune complexes during terminal erythroid differentiation
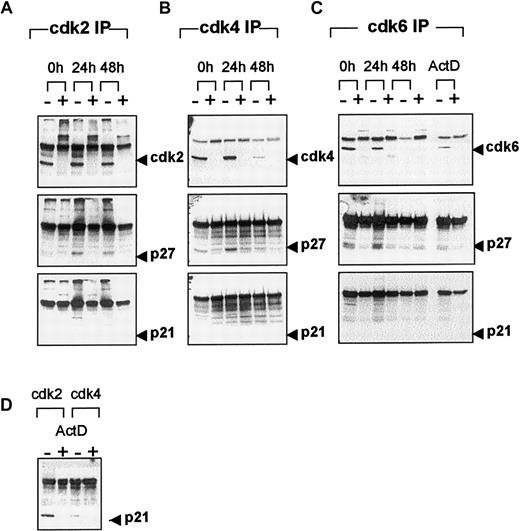
To determine whether CDK kinase activity was differentially regulated by p21, p27, or both during erythroid differentiation, we performed CDK immunoprecipitations and examined the complexes for the presence of the Cip/Kip inhibitors. Western blot analysis with anti-cdk2 (Figure 6A) demonstrated that levels of cdk2 protein remained constant during differentiation. This finding was consistent with the previous observation that steady state levels of cdk2, as determined by Western blot analysis (Figure 3C), remained unchanged. At the initiation of culture, p27 protein was not detected in cdk2 immune complexes, but then appeared by 24 hours and persisted at 48 hours. No p21 protein was detected in the cdk2 immune complex at any time. When cdk4 immunoprecipitations were examined for the presence of cdk4 protein (Figure 6B), an unexpected result was observed. Levels of immunoprecipitatable cdk4 protein decreased significantly during the differentiation time course, despite the fact that steady state levels of cdk4, as determined by Western blot analysis (Figure 3D), remained unchanged. Unlike the observation in cdk2 immune complexes, p27 protein was detected in the cdk4 immune complex at the initiation of culture, persisted at 24 hours, and was undetectable at 48 hours. The absence of p27 in the complex at 48 hours was consistent with the inability to immunoprecipitate cdk4 at that time. As observed in cdk2 immunoprecipitations, no p21 protein was detected in cdk4 complexes at any time. In a separate experiment p21 was readily detected in cdk2 and, to a lesser extent, in cdk4 immune complexes after the treatment of cells with ActD (Figure 6D). The levels of cdk6 protein in cdk6 immune complexes (Figure 6C) decreased during differentiation, consistent with the observed decrease in steady state levels of cdk6 (Figure 3E). At 0 and 24 hours, p27 protein was associated with cdk6 immune complexes. No p21 was detected in cdk6 immune complexes during differentiation or in cells treated with ActD. The data demonstrate that p27, but not p21, binds to cdk4 and cdk6 early in differentiation and to cdk2 later in differentiation and that p27 may be responsible for the observed loss of Rb kinase activity in vivo and in vitro. The reason for the inability to immunoprecipitate cdk4 at 48 hours was unclear until experimental conditions were reversed and the p27 pool of protein was immunoprecipitated first, followed by Western blot analysis for CDKs.
CDK-immune complex formation during terminal erythroid differentiation.
Whole cell lysates from FVA erythroblasts cultured with EPO for 0, 24, or 48 hours were immunoprecipitated with anti-cdk2 (A), -cdk4 (B), or -cdk6 (C) in the absence (−) or presence (+) of relevant blocking peptide. FVA erythroblasts were cultured with EPO for 6 hours in the presence of ActD and immunoprecipitated with anti-cdk2 or -cdk4 (D) or -cdk6 (C) in the absence (−) or presence (+) of relevant blocking peptide. Immune complexes were electrophoretically separated in 12% polyacrylamide gels and transferred to PVDF membranes. Western blot analysis was performed with antibodies to cdk2, cdk4, cdk6, p27, or p21.
CDK-immune complex formation during terminal erythroid differentiation.
Whole cell lysates from FVA erythroblasts cultured with EPO for 0, 24, or 48 hours were immunoprecipitated with anti-cdk2 (A), -cdk4 (B), or -cdk6 (C) in the absence (−) or presence (+) of relevant blocking peptide. FVA erythroblasts were cultured with EPO for 6 hours in the presence of ActD and immunoprecipitated with anti-cdk2 or -cdk4 (D) or -cdk6 (C) in the absence (−) or presence (+) of relevant blocking peptide. Immune complexes were electrophoretically separated in 12% polyacrylamide gels and transferred to PVDF membranes. Western blot analysis was performed with antibodies to cdk2, cdk4, cdk6, p27, or p21.
p27 association with cdk4 and cdk2 is temporally distinct during terminal erythroid differentiation
To further investigate the kinetics of p27/CDK associations during differentiation, p27 immunoprecipitations were performed and evaluated for the presence of cdk2, cdk4, cdk6, or cyclin D3 (Figure7). At the initiation of culture, the small amount of p27 protein available was associated exclusively with cdk4. By 24 hours, when levels of p27 protein were significantly increased, the amount of cdk4 associated with p27 increased proportionally. Despite the fact that a larger percentage of the total cdk4 protein pool was now associated with p27, the in vitro cdk4 kinase activity remained unchanged (Figure 5, lanes 1 and 2). No cdk2–p27 association was detected at 0 hours, but cdk2 protein was detected in the p27 complex by 24 hours, when a significant decrease in in vitro cdk2 kinase activity was observed (Figure 5, lanes 1 and 2). After 48 hours of culture, abundant cdk4 protein was detected in p27 immune complexes despite the fact that a cdk4–p27 association was not observed when the reciprocal experiment was performed (Figure 6B). Cdk2 and cyclin D3 proteins were also observed in p27 immune complexes at 48 h, when no cdk2 or cdk4 in vitro kinase activity was apparent (Figure 5, lane 3). Cdk6 protein was not detectable in p27 immune complexes at any time (data not shown), suggesting that most of the p27 pool associates with cdk4 rather than with cdk6.
p27 immune complex formation during terminal erythroid differentiation.
Whole cell lysates from FVA erythroblasts (0, 24, or 48 hours) were immunoprecipitated with anti-p27 in the absence (−) or presence (+) of blocking peptide. Immune complexes were electrophoretically separated in 12% polyacrylamide gels and transferred to PVDF membranes. Western blot analysis was performed with antibodies to p27, cdk2, cdk4, or cyclin D3.
p27 immune complex formation during terminal erythroid differentiation.
Whole cell lysates from FVA erythroblasts (0, 24, or 48 hours) were immunoprecipitated with anti-p27 in the absence (−) or presence (+) of blocking peptide. Immune complexes were electrophoretically separated in 12% polyacrylamide gels and transferred to PVDF membranes. Western blot analysis was performed with antibodies to p27, cdk2, cdk4, or cyclin D3.
INK4 expression during terminal erythroid differentiation
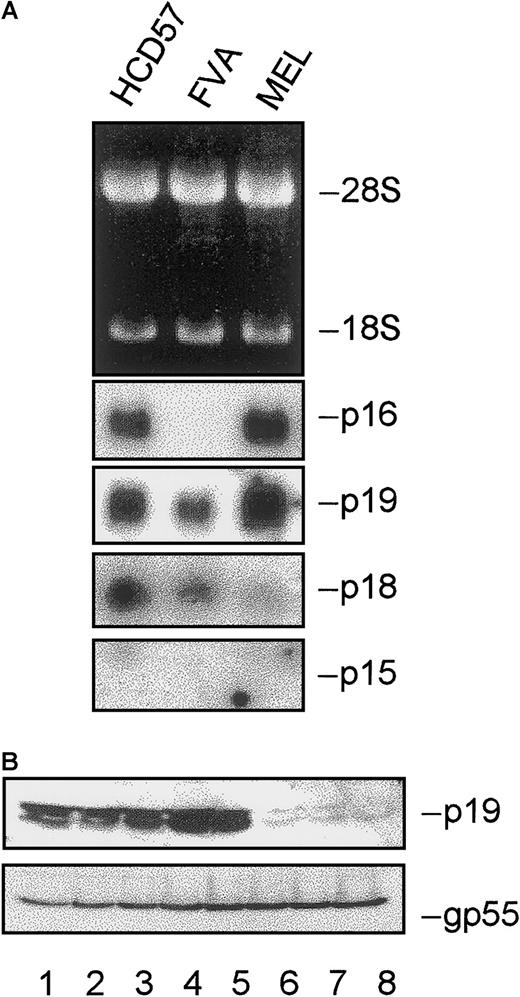
To provide a complete understanding of the regulatory proteins associated with cell cycle exit of differentiating erythroblasts, we examined expression of members of the INK4 family of CKIs. Figure8A represents total RNA isolated from 2 established erythroid cell lines, HCD57 (EPO-dependent) and MEL cells (EPO-independent), as well as undifferentiated FVA erythroblasts. p16 mRNA was present in both cell lines but absent in FVA erythroblasts. p19 and p18 mRNAs were present in all cells examined, whereas a 1.3-kb mRNA species representing p15 (previously reported in certain mouse tissues, excluding spleen28) was not detected in any of the cells tested. Figure 8B represents expression of p19 protein during FVA erythroblast differentiation. p19 protein levels increased during differentiation until 26 hours of culture and then decreased markedly. We were unable to detect p18 protein at any time during differentiation (data not shown).
INK4 expression during terminal erythroid differentiation.
(A) Total RNA was isolated from HCD57, FVA, or MEL erythroblasts. Gel electrophoresis was performed, and ethidium bromide–stained rRNA was photographed as a control for loading. Northern blot analysis was performed using radiolabeled cDNA probes to p16, p19, p18, and p15. (B) Total cellular protein was isolated from FVA erythroblasts cultured for 0, 6, 14, 20, 26, 37, 42, or 48 hours with EPO (lanes 1-8). Proteins were electrophoretically separated in 12% polyacrylamide gels and probed with antibodies to p19. gp55 protein expression is shown as a control for protein loading.
INK4 expression during terminal erythroid differentiation.
(A) Total RNA was isolated from HCD57, FVA, or MEL erythroblasts. Gel electrophoresis was performed, and ethidium bromide–stained rRNA was photographed as a control for loading. Northern blot analysis was performed using radiolabeled cDNA probes to p16, p19, p18, and p15. (B) Total cellular protein was isolated from FVA erythroblasts cultured for 0, 6, 14, 20, 26, 37, 42, or 48 hours with EPO (lanes 1-8). Proteins were electrophoretically separated in 12% polyacrylamide gels and probed with antibodies to p19. gp55 protein expression is shown as a control for protein loading.
Discussion
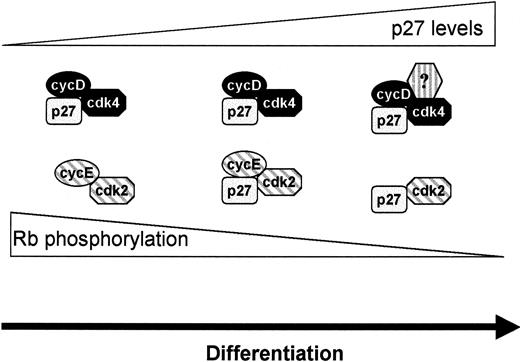
We used the FVA system as an experimental paradigm to study the stopping mechanism associated with cell cycle exit of terminally differentiating cells. In this model, primary erythroblasts differentiate in response to EPO. Regulation of G1 cell cycle–associated proteins during erythroid differentiation was primarily restricted to the induction of CKI family members p21, p27, and p19 and the reduction of cdk6 and cyclin E protein levels. Levels of the principal G1 CDK proteins, cdk4 and cdk2, and the only cyclin D expressed in FVA erythroblasts, D3, remained constant during differentiation. p27 protein accumulated initially in cdk4 (and, to a lesser extent, in cdk6) immune complexes and subsequently in cdk2 immune complexes. Although the association of p27 with cdk4 had no apparent inhibitory effect on cdk4 in vitro kinase activity, the kinetics of p27 accumulation in cdk2 immune complexes was consistent with the loss of cdk2 kinase activity and the growth arrest of cells into G1. A model that describes the role of cdk2, cdk4, and p27 in the growth arrest of differentiating cells is shown in Figure9. At initiation of the differentiation time course, low levels of p27 protein associate primarily with cdk4. As differentiation proceeds, the amount of p27 protein increases and p27/cdk4 complexes accumulate, with no apparent adverse effect on cdk4 kinase activity. When cdk4 complexes become saturated with p27, excess p27 protein is available to associate with cdk2, in which it has a profound inhibitory effect on cdk2 kinase activity. This model implies a switching mechanism whereby cdk4 (and possibly cdk6) functions to sequester p27 as it accumulates in the cell until the precise time that initiation of G1 arrest is indicated. Then excess p27 becomes available to inhibit cdk2/cyclin E complexes.
Model of p27 function in terminal erythroid differentiation.
p27 protein accumulates in cyclin D/cdk4 complexes during differentiation without inhibiting kinase activity. When cyclin D/cdk4 complexes become saturated with p27, p27 accumulates in cyclin E/cdk2 complexes and inhibits cdk2 kinase. Complete loss of cdk2 kinase activity requires loss of cyclin E from the complex. Loss of cyclin D/cdk4 kinase activity occurs by an unknown mechanism.
Model of p27 function in terminal erythroid differentiation.
p27 protein accumulates in cyclin D/cdk4 complexes during differentiation without inhibiting kinase activity. When cyclin D/cdk4 complexes become saturated with p27, p27 accumulates in cyclin E/cdk2 complexes and inhibits cdk2 kinase. Complete loss of cdk2 kinase activity requires loss of cyclin E from the complex. Loss of cyclin D/cdk4 kinase activity occurs by an unknown mechanism.
Complete loss of cdk2 kinase activity does not occur until late in differentiation and most likely results from additional events distinct from p27 binding; the levels of associated p27/cdk2 proteins remained comparable at 24 and 48 hours (Figure 7) despite marked differences in cdk2 kinase activity (Figure 5). Loss of cyclin E from cdk2 complexes likely represents a late event responsible for extinguishing cdk2 kinase activity in conjunction with p27 binding. At 48 hours of culture, when cdk2 kinase activity was largely extinguished (Figure 5), steady state cyclin E protein levels were significantly reduced (Figure3). Evidence supporting the hypothesis that p27 binds cdk2 in the absence of cyclin E is provided by studies in which in vitro translated CDK proteins bind p27 in the absence of cyclins.29 These observations do not exclude the possibility that other events, in addition to accumulation of p27 and loss of cyclin E proteins, are involved in regulating cdk2 kinase function during differentiation.
The difference in kinetics of inhibition of cdk6 and cdk4 kinase activity during differentiation suggests that these proteins are not functionally redundant. Unlike Cdk6, Cdk4 activity was not extinguished until very late in differentiation, presumably because of an event other than p27 binding. One possibility is that a new protein entered the complex and was responsible for the inhibition of cdk4 kinase activity. INK4 family members are known to specifically inhibit cyclin D–associated kinases. INK4 candidates responsible for the inhibition of cdk4 or cdk6, or both, in FVA erythroblasts include p19 and p18 proteins because p15 and p16 mRNA species were absent; p18 protein was undetectable in differentiating FVA erythroblasts. Two different mRNA species have been reported for p18 in mouse tissues, 2.2 and 1.1 kb.28 The 2.2-kb species, as detected in FVA erythroblasts, demonstrated limited expression in other mouse tissues and may generate a protein product for which the p18 antibody used does not react. Alternatively, p18 protein may not be translated in FVA erythroblasts, or it may be expressed at levels below our limit of detection. These possibilities require further investigation. p19 protein expression increased early in differentiation but was undetectable at later time points, making it an unlikely candidate for inhibiting cdk4 kinase, though p19 inhibition of cdk6 kinase remains a possibility. Alternatively, posttranslational modification of cdk4 or cdk6 or some other existing member of the complexes may be associated with the loss of kinase activity. Either of these mechanisms may be responsible for the inability of cdk4 antibodies to recognize the complex in immunoprecipitation experiments performed late in differentiation. Further experiments will be required to identify the precise mechanisms associated with inhibition of cdk4 and cdk6 kinases in terminal erythroid differentiation.
Our data demonstrating that p27 fails to inhibit the activity of cyclin D–associated kinase, but not cyclin E-associated kinase, is consistent with previously reported observations. First, all cyclin D-CDK Rb kinase activity in proliferating mammalian cells is found in complexes containing Cip/Kip proteins.30-36 Second, Soos et al30 demonstrated that immunoprecipitation of p27 complexes from a B-lymphocyte cell line contained kinase activity with substrate specificity for Rb but not histone H1, a hallmark of cyclin D–dependent kinase activity. In those studies, immunodepletion of cdk6 removed most p27-associated kinase activity, implying that p27 resided in a kinase-active cyclin D-cdk6–p27 complex. These data suggest that under certain circumstances Cip/Kip proteins do not inhibit cyclin D-dependent kinase activity in vivo and that inhibition in vitro may depend on stoichiometry. Evidence supporting the latter comes from the observation that cyclin D-cdk6–p27/p21 complexes that exist in a 1:1:1 ratio remain kinase active,31,32,37 whereas complexes containing a higher ratio of p21 or p27 proteins are kinase inactive.38,39 On the contrary, equimolar concentrations of p21 in cyclin E–cdk2 complexes were found to be inhibitory.40 Taken together, these studies demonstrate that Cip/Kip proteins are functionally heterogeneous with regard to their ability to regulate the activity of cyclin/CDK complexes.
Several reports have suggested a role for p27 in terminal differentiation of other cell types, particularly oligodendrocytes.41-45 Durand et al44demonstrated that p27-deficient oligodendrocytes proceeded through additional cell divisions before differentiation when compared to wild-type cells, suggesting that p27 is part of a timing mechanism that determines when precursors stop dividing and differentiate. These investigators45 also demonstrated enhanced sensitivity of p27-null oligodendrocytes to various mitogens.44 A role for p27 in erythroid differentiation is supported by the observation that p27-deficient mice exhibit hyperplasia of multiple organs, including the spleen,46-48 and have increased numbers of early and late erythroid progenitor cells (BFU-E and CFU-E) in the spleen and bone marrow.48 This observation suggests that the absence of p27 may allow for continued cell proliferation, at the expense of differentiation, of early erythroid progenitor cells.
p21 has been implicated in terminal differentiation of various tissues. MyoD-induced expression of p21 in terminally differentiating muscle cells49 and forced overexpression of p21 in myoblasts resulted in terminal differentiation,50 suggesting that p21 plays a crucial role in muscle development. Further, examination of p21-deficient keratinocytes showed a more rapid S phase and reduced expression of a subset of differentiation markers.51However, mice lacking p21 did not exhibit any developmental abnormalities, including muscle or skin,52 suggesting that other proteins (perhaps p27) may substitute for p21 function. We did not detect a role for p21 in the growth arrest associated with terminal erythroid differentiation. Unlike p27, no p21 protein was found associated with cdk2, cdk4, or cdk6 immune complexes. One possibility is that p21 is not present in the same cellular compartment as G1 CDK during erythroid differentiation. Alternatively, p21 may associate with proteins other than G1 CDK during differentiation. Finally, it remains possible that low levels of p21 did associate with one or all G1 CDK complexes but were below our limits of detection. Our data suggest that p21 may have a function other than growth arrest during terminal erythroid differentiation. In a previous study we reported that p21 protein levels were induced during differentiation primarily through a p53-dependent mechanism.11 In that study we were unable to detect a differentiation advantage in p53 wild-type cells expressing abundant levels of p21 versus p53 null cells expressing low levels of p21. Similarly, no differences were observed in the ability of cells to accumulate into G1, regardless of the p53 genotype and p21 protein levels. We speculated that p53-dependent induction of p21 during erythroid differentiation may represent a novel tumor suppressor function intended to monitor normal differentiation in the event that differentiation is blocked by activation of an oncogene or some other unforeseen event. Ultimately, the use of erythroblasts derived from transgenic animals lacking p21, p27, or both will be necessary to differentiate the roles these gene products play in erythroid cell growth arrest and terminal differentiation.
It remains to be determined how the growth arrest pathway is linked to EPO signaling. Carroll et al53 have reported that the length of G1 phase is altered in a murine EPO-dependent cell line, Ba/F3, by culturing the cells in different concentrations of EPO, leading to conditions that favored proliferation versus differentiation.53 Investigation of cell cycle regulators under these conditions should provide insight into the molecular mechanisms of growth versus differentiation.
Acknowledgment
We thank the Utah Regional Cancer Center for their core grant, NCI 5P30CA42014, allowing us the use of the flow cytometry facility.
Supported by the American Cancer Society, The Primary Children's Medical Center Research Foundation, National Institutes of Health grants P50DK49219 (L.L.K., from the Center of Excellence in Molecular Hematology), T32DK07115 (L.A.B.), and CA163687 (A.I.S), and Utah Regional Cancer Center core grant NCI 5P30CA42014.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Linda L. Kelley, Department of Medicine, University of Utah School of Medicine AR159, 50 North Medical Dr, Salt Lake City, UT 84132; e-mail: linda.kelley@hsc.utah.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal