Abstract
The variety of patients with thalassemia in Thailand offers an opportunity to fully characterize the kinetic causes of the anemia and to study apoptosis of marrow erythroid precursors as a possible factor contributing to its severity. Kinetic studies showed that in hemoglobin H (HbH) disease, the extent of hemolysis, as well as the minimally ineffective erythropoiesis, usually falls within the compensatory capacity of normal erythropoiesis; therefore, anemia in patients with HbH partly represents a failure to expand erythropoiesis adequately. Hemoglobin Constant Spring (HbCS), a common variant of α thalassemia in Bangkok, causes more severe hemolysis and a distinct increase in ineffective erythropoiesis. Ineffective erythropoiesis plays a much more prominent role in β thalassemia/hemoglobin E (β-thal/HbE) disease, in which the variability of the anemia is puzzling. We compared mild and severe cases and found that patients with severe disease had a maximal marrow erythropoietic response that failed to compensate for very short survival of red blood cells and a marked quantitative increase in ineffective erythropoiesis. Analysis of apoptosis of marrow erythroid precursors done both on shipped samples and in Bangkok showed a moderate increase in HbH disease, consistent with the small increase in ineffective erythropoiesis. In patients with homozygous HbCS, there was a further increase in apoptosis, consistent with the additional increase in ineffective erythropoiesis. Patients with β-thal/HbE disease had the most ineffective erythropoiesis and the most erythroid apoptosis. Thus, it appears that α-chain deposition in erythroid precursors, either αA or αcs, leads to accelerated apoptosis and ineffective erythropoiesis.
Introduction
As a consequence of globalization and decreases in neonatal and childhood mortality, the severe α and β thalassemias have become worldwide clinical problems.1-4 Better understanding of the molecular biologic aspects of the thalassemias5,6 has led to improvements in population screening1,7 and prenatal diagnosis7,8 which, in turn, have led to dramatic reductions in the number of births of children with β thalassemia major (Cooley anemia) in the Mediterranean littoral.1,7 However, in much of the world, but particularly in Asia, the severe forms of α thalassemia and β thalassemia/hemoglobin E (β-thal/HbE) remain increasingly important clinical problems.1 Better understanding of the pathophysiologic features of the disease could result in measures that might raise hemoglobin values in patients with this disease from 60 g/L to 80 g/L, thereby yielding a much better quality of life.
The primary kinetic lesion in HbH disease, a moderately severe form of α thalassemia, is hemolysis.9-14 One ferrokinetic study indicated that there is a component of ineffective erythropoiesis in HbH.13 Studies assessing plasma levels of soluble transferrin receptor also suggested that there is a component of ineffective erythropoiesis in HbH disease.15-17Furthermore, morphologic data indicate that excess β chains do precipitate in marrow erythroid precursors,18 19 where they might cause some intramedullary cell death.
An α-thalassemia variant, HbH/hemoglobin Constant Spring (HbH/HbCS; α0-thal/HbCS), causes anemia similar to that in classic HbH (α0-thal/α+-thal).20-23The CS gene has a frequency of 6% to 8% in Bangkok and 3% to 5% in the remainder of Thailand.21 However, the cellular biologic characteristics of HbH with HbCS variants is considerably different, with hyperhydration of red blood cells (RBCs) producing an almost normal mean corpuscular volume (MCV) and much greater hypochromia.20 In contrast to classic HbH, there are many more RBC inclusions,21 the RBCs are even more rigid, and the RBC membranes are hyperstable.22 This extreme lack of deformability could lead to an increased rate of hemolysis in HbCS containing variants; however, studies of this issue are not available.
In both HbH and HbH/CS, the pathophysiologic mechanism is determined partly by the accumulation of excess, partly oxidized β-globin chains on the RBC membrane skeleton.22,24 Additionally, in the HbCS variants, there is deposition of αCS globin on the RBC membrane skeleton.22 Presumably, αCSaccumulation during marrow erythropoiesis could lead to ineffective erythropoiesis, as does αA accumulation in severe forms of β thalassemia.25,26 However, we do not know of any kinetic studies that systematically explored the differences between HbH and HbH/CS. Nor are there studies of hemoglobin constant spring/constant spring (HbCS/CS) homozygotes who, surprisingly, have a mild hemolytic anemia.27
The β-thal/HbE syndrome is puzzling because hemoglobin values vary from 26 g/L to 135 g/L.28,29 The presence of the β+ gene, α-thalassemia genes, or homozygosity for theXmnI cleavage site in the γG-globin gene all modify the severity of the anemia; however, the extreme variation remains unexplained.29 Because increased ineffective erythropoiesis, increased hemolysis, decreased production, or combinations of these defects could possibly account for the differences, ferrokinetic measurements30 with radiochromium RBC survival studies were undertaken.
We previously reported that accelerated apoptosis occurs in marrow erythroid precursors in patients with β thalassemia major25 and proposed that apoptosis could contribute importantly to the ineffective erythropoiesis.31 Thus, we predicted that accelerated apoptosis occurred in β-thal/HbE erythroid precursors and wondered whether variations in apoptosis might provide a clue to the puzzling heterogeneity. To test the idea that the consequences of excess α-globin chain accumulation in marrow erythroid precursors of patients with severe β thalassemia are ineffective erythropoiesis and accelerated apoptosis,31 we needed to do a parallel study of the consequences of β-globin accumulation in erythroid precursors of patients with severe α thalassemia. Therefore, we studied apoptosis of marrow erythroid precursors in patients with HbH, HbH/CS, and HbCS/CS.9,12 20-22 In Thailand, there are many patients with these severe forms of α and β thalassemia who have been well characterized clinically and genotypically and only undergo transfusion when an intercurrent event causes a further sharp drop in hemoglobin level. Patients in each of these disease categories underwent erythrokinetic studies so that we could correlate apoptosis of marrow erythroid precursors with kinetic variables.
Patients, materials, and methods
Kinetic studies
Patients.
Twenty-seven patients ranging in age from 16 to 46 years, with approximately equal numbers of patients of each sex with the genotypes of interest, were studied at the Thalassemia Research Center and Division of Hematology, Department of Medicine, Siriraj Hospital. Study protocols were approved by the Ethical Clearance Committee on Human Rights Related to Research Involving Human Subjects Research, Mahidol University, Bangkok, Thailand. There were 4 patients with classic HbH disease (α0-thal/α+-thal), 6 patients with HbH/CS (α0-thal/HbCS), 6 patients with HbCS homozygosity, and 11 patients with β0-thal/HbE disease. All diagnoses were based on standard hematologic criteria, hemoglobin analyses, globin-synthesis ratios, and DNA analyses. All patients were in stable condition, without any complications, and none had received blood transfusions within the 3 months before the kinetic analyses.
Hematologic investigations. Venous blood was drawn with the usual precautions after the patient had been lying quietly for at least 20 minutes. Blood cells were counted with a Coulter counter (model ZF 6), Sysmex K 800, and Sysmex NE 1500. Reticulocytes were stained with 1% methylene blue, and the percentage of reticulocytes was calculated after counting 1000 RBCs. HbH, HbCS, HbE, and hemoglobin F (HbF) were demonstrated by starch gel electrophoresis in alkaline (pH 8.6) Tris-EDTA borate buffer.32 Hemoglobin fractions were quantitated with cellulose electrophoresis.33 HbF was also assessed by alkaline denaturation.34 Erythrocyte inclusion bodies found in the α thalassemia variants were stained and induced by incubating RBCs with 1% new methylene blue, and the percentage was calculated on the basis of counting 1000 RBCs. Measurements of serum iron and total iron-binding capacity were done as recommended by the International Committee for Standardization in Hematology (ICSH).35 36 The transferrin iron saturation was then calculated.
Erythrokinetic studies. The RBC mass and RBC survival were determined by using autologous RBCs labeled with chromium 51 (51Cr) as recommended by the ICSH. 37 Twenty milliliters of a patient's whole blood in citrate phosphate buffer solution was incubated with 3.7 MBq Na251CrO4 (Radiochemical Centre, Amersham, England) for 30 minutes at room temperature. Fifty milligrams of ascorbic acid was added to stop the reaction. An accurate volume of intravenously injected labeled blood was calculated by weighing the syringe before and after the injection. Blood samples were drawn 20 to 30 minutes after injection and known volumes of whole blood and plasma were counted in a well-type gamma counter (LKB1222). The RBC mass was calculated according to the isotope dilution technique. The predicted blood volume was calculated on the basis of body weight and height. To determine the 51Cr RBC terminal half-life (T½), blood samples were drawn according to schedule during a period of 3 to 4 weeks and the RBC 51Cr activity was plotted semilogarithmically against time. A survival curve was drawn by using the least squares method.37
Measurement of plasma volume and the ferrokinetic study were performed on the second day of the study.38 Donor plasma that was free of hepatitis virus and human immunodeficiency virus was tagged in vitro with high-specific-activity iron sulfate 59 (Fe59; New England Nuclear, Boston, MA), and carrier cold iron was added to saturate the labeled plasma to the same saturation as the patient's plasma iron saturation. Two milliliters of 59Fe-labeled plasma (0.074 MBq/mL) was injected intravenously. The disappearance of59Fe radioactivity from plasma was measured during a period of 100 to 120 minutes, and the T½ of disappearance was determined by semilogarithmic plotting.30 The plasma radioiron activity at 0 minutes was determined by back extrapolation of the curve to zero time. Known volumes of whole blood samples were drawn at 2- to 4-day intervals for 3 to 4 weeks, and the samples were counted in a well-type gamma counter. The 51Cr and 59Fe cross-count factor was used for correction of 51Cr activity.
The plasma volume, plasma iron turnover rate (PITR; expressed as milligrams of iron per liter of whole blood per day ), and standard erythroid iron turnover (SEIT) were then calculated.30 Erythrocyte iron utilization was calculated by plotting the 59Fe activity in RBCs during the 2-week period after the injection of 59Fe-labeled plasma. The percentage of RBC iron utilization was the maximum radioiron activity appearing in the circulating erythrocytes at 10 to 14 days.30
Studies of apoptosis of marrow erythroid precursors. All patients were adults aged 20 to 40 years, and approximately equal numbers of male and female patients were studied at the Thalassemia Center at Siriraj Hospital. Complete blood counts (CBCs) were done at regular visits, along with serum ferritin measurements and other relevant chemical studies. The diagnoses were based on hemoglobin analysis by either electrophoresis or high-performance liquid chromatography (Variant; Bio-Rad, Hercules, CA) and globin gene analysis. None of the patients had undergone transfusion for 3 months before samples were obtained. Whether patients had undergone splenectomy was noted.
After informed consent was obtained according to the research protocol, patients underwent routine bone marrow aspiration under local anesthesia. There were no adverse events. Approximately 10 mL of marrow aspirate was added to 2.5-mL citrate-phosphate-dextrose anticoagulant, 1.5 mL fetal-calf serum, and 1.0 mL TC-199. Standard peripheral blood smears and bone marrow smears were performed for morphologic study after staining with Wright stain.
Twenty-three patients were analyzed in group I—4 with HbH, 5 with HbH/CS, 4 with HbCS/CS, and 10 with β-thal/HbE. Marrow samples were shipped on ice to Stanford University, Stanford, CA, and arrived 36 to 48 hours later. Initially, patient samples were coded and the genotype, diagnosis, and CBC results were provided only after measurements of apoptosis were completed. These samples were concentrated by Ficoll separation (Isoprep 1077; Robbins Scientific Corp, Sunnyvale, CA), which also removed most RBCs. The nucleated cells (2 × 107 in 80 μL) were then incubated with 20 μL mouse monoclonal antihuman CD45 antibody linked to magnetic beads (Miltenyi Biotech, Auburn, CA). The suspension was then passed through magnetic columns that bound the CD45-positive cells and allowed the erythroid precursors, which are negative for CD45, to pass through unimpeded. Cytospin preparations showed that more than 95% of cells were erythroid precursors representing all 4 stages. Magnetic-bead separation provided a better yield in cells and in morphologic features than separation using panning.25 The erythroid precursors were counted and assayed for the extent of apoptosis.
The results obtained in the patients in group I showed that there were potentially interesting differences in the extent of apoptosis in the several clinical categories. However, we were concerned about the possible effects that shipment and storage might have had on abnormal erythroid precursors, and we were particularly interested in repeating studies of samples that seemed to be discrepant or to have a shipping artifact. Therefore, 2 of us (L.M. and S.L.S.) traveled to Bangkok, where 14 patients (group II) were studied—2 with HbH, 3 with HbH/CS, 2 with HbCS/CS, 6 with β-thal/HbE, and 1 with HbEE. The procedures for bone marrow aspiration and sample preparation were identical to those described above except that the marrow samples were processed within minutes and the entire experiment, including fluorescence-activated cell sorter (FACS) analysis, was completed the same day. Five patients in group I were also in group II so that results from fresh and shipped samples could be compared directly.
Two patients had values that seemed likely to be erroneous. Patient 25 (with β0-thal/HbE) had a very high value for dead cells (8%) on propidium iodide (PI) assay, and therefore we suspected an artifact. On restudy in Bangkok, this patient had 0.6% PI-positive cells. Patient 5 (with HbH/CS) was restudied because the value on the annexin V assay was 12.4%, which was twice as high as that of the 4 other patients with the same diagnosis. On restudy in Bangkok, the annexin V value was 4.9%. Three other patients were available for restudy: patient 4 (with HbH/CS), whose annexin V value was 5.3% at Stanford and 4.0% in Bangkok; patient 18 (with HbH), whose annexin V value was 7.5% at Stanford and 5.3% in Bangkok; and patient 8 (with HbCS/CS), whose annexin V value was 12.7% at Stanford and 12.8% in Bangkok. The 2 apparently aberrant values (patient 5 and patient 25) were censored.
Control samples consisted of erythroid precursors harvested from allogeneic bone marrow transplantation donors at Stanford and collected with institutional review board approval, as described previously.25
Measurement of apoptosis
In the patients in group I, 2 methods based on flow cytometry were used to assess apoptosis. During the apoptotic process, phosphatidylserine (PS) moves from the inner leaflet to the outer leaflet of the plasma membrane phospholipid bilayer.39Externally oriented PS can be detected by annexin V labeled with fluorescein isothiocyanate–conjugated (FITC).39,40 Three microliters of annexin V–FITC (Annexin V Kit; R&D Systems, Minneapolis, MN) was added to 106 erythroid precursors suspended in 0.5 mL binding buffer and incubated for 10 minutes at room temperature. The samples were immediately placed on ice, and just before the flow cytometry, 10 μL PI (R&D Systems) was added. The addition of PI allowed us to determine the number of live (PI excluded) and dead (PI fluorescent) cells.39 At Stanford, we used a FACSTAR (Becton Dickinson, San Jose, CA) to determine the number of annexin V–positive erythroid precursors in 20 000 cells counted.
To confirm our results using a different method, we used the Hoechst 33342 dye technique,41 which kinetically labels the nuclei of cells undergoing apoptosis. We further standardized this method on human erythroid precursors induced to undergo apoptosis by incubating them in medium devoid of erythropoietin (data not shown). To a 0.5-mL erythroid precursor suspension, we added 10 μL Hoechst 33342 (10 μg/mL; Molecular Probes, Eugene, OR) and incubated the mixture for exactly 4 minutes at 37°C, after which the mixture was immediately placed on ice. PI was added just before FACS analysis. The results were analyzed by using Flow-Jo software (Treestar, San Carlos, CA).
Group II patients were studied at the Siriraj Hospital, where the FACS scanner did not have ultraviolet capabilities; therefore, only the annexin V–FITC method and PI were used to identify apoptotic and dead cells, respectively. Otherwise, the cell separation and incubation techniques were identical.
Statistical analyses were performed by using the Student ttest (Microsoft Excel statistical function; Microsoft Corp, Redmond, WA).
Results
Erythrokinetics
Comparison between HbH disease, HbH/CS, and HbCS/CS.
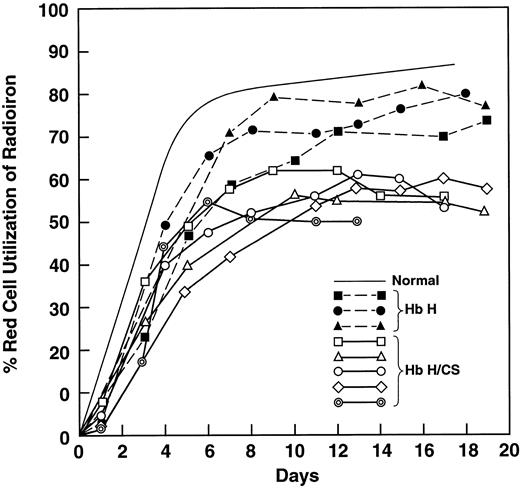
We studied 4 patients with HbH disease and compared them with 6 patients with HbH/CS (Table 1). The MCV in HbH/CS disease is almost normal because of the well-described hyperhydration20,22 and the RBCs contain many inclusions.21 Values for iron-binding capacity and transferrin saturation were not different; however, the serum iron level in the patients with HbH/CS was 60% higher. Even though the 2 groups of patients were comparably anemic, the PITR, an index of erythropoiesis, was 3 times the control value in patients with HbH and 5 times the control value in those with HbH/CS (P < .02). Patients with HbH had a minimal reduction in utilization of radioiron, whereas patients with HbH/CS had a mean value of 59% (P < .01; Table 1 and Figure1), indicating considerable ineffective erythropoeisis.30 38 The radiochromium RBC survival was much shorter in patients with HbH/CS (P < .05; Table 1).
Variables in patients with hemoglobin H (HbH) and HbH/constant spring (HbH/CS)
| Patient no. . | Hgb (g/L) . | MCV (fl) . | SI (μmol/L) . | TIBC (μmol/L) . | Tf sat (%) . | PITR* . | RBC 59Fe use (%) . | 51Cr RBC T½ (days) . |
|---|---|---|---|---|---|---|---|---|
| HbH | ||||||||
| 12 | 89 | 62 | 18 | 42 | 44.1 | 1.4 | 71.9 | 21.3 |
| 13 | 95 | 78 | 22 | 34 | 64.7 | 2.3 | 72.7 | 16.0 |
| 14 | 90 | 79 | 17 | 39 | 43.1 | 1.7 | 79.8 | 15.8 |
| 15 | 78 | 63 | 24 | 40 | 90.4 | 2.7 | — | 9.7 |
| Mean ± SD | 88 ± 7 | 70.5 ± 9.3 | 20.3 ± 3.3 | 38.8 ± 3.4 | 60.6 ± 22.2 | 2 ± 0.6 | 74.8 ± 4.3 | 15.7 ± 4.7 |
| HbH/CS | ||||||||
| 16 | 91 | 78 | 30 | 40 | 75.0 | 3.5 | 62.0 | 6.8 |
| 17 | 113 | 79 | 27 | 51 | 53.0 | 3.4 | 56.6 | 12.0 |
| 18 | 82 | 90 | 32 | 41 | 77.6 | 4.2 | 61.6 | 9.3 |
| 19 | 127 | 89 | 42 | 34 | 100.0 | 2.4 | — | 6.8 |
| 20 | 95 | 79 | 35 | 41 | 94.1 | 5.7 | 60.9 | 6.2 |
| 21 | 85 | 77 | 29 | 45 | 64.1 | 3.0 | 54.4 | 8.7 |
| Mean ± SD | 99 ± 18 | 82 ± 5.9 | 32.5 ± 5.4 | 42 ± 5.7 | 77.3 ± 17.7 | 3.7 ± 1.1 | 59.1 ± 3.4 | 8.3 ± 2.2 |
| Pvalue | .22 | .08 | <.001 | .29 | .26 | .02 | 0.01 | 0.05 |
| Normal | — | 84.9 ± 8.1 | 20.6 ± 5.8 | 55.9 ± 7.2 | 35.8 ± 8.9 | 0.72 | 80 | 25.7 ± 0.9 |
| Patient no. . | Hgb (g/L) . | MCV (fl) . | SI (μmol/L) . | TIBC (μmol/L) . | Tf sat (%) . | PITR* . | RBC 59Fe use (%) . | 51Cr RBC T½ (days) . |
|---|---|---|---|---|---|---|---|---|
| HbH | ||||||||
| 12 | 89 | 62 | 18 | 42 | 44.1 | 1.4 | 71.9 | 21.3 |
| 13 | 95 | 78 | 22 | 34 | 64.7 | 2.3 | 72.7 | 16.0 |
| 14 | 90 | 79 | 17 | 39 | 43.1 | 1.7 | 79.8 | 15.8 |
| 15 | 78 | 63 | 24 | 40 | 90.4 | 2.7 | — | 9.7 |
| Mean ± SD | 88 ± 7 | 70.5 ± 9.3 | 20.3 ± 3.3 | 38.8 ± 3.4 | 60.6 ± 22.2 | 2 ± 0.6 | 74.8 ± 4.3 | 15.7 ± 4.7 |
| HbH/CS | ||||||||
| 16 | 91 | 78 | 30 | 40 | 75.0 | 3.5 | 62.0 | 6.8 |
| 17 | 113 | 79 | 27 | 51 | 53.0 | 3.4 | 56.6 | 12.0 |
| 18 | 82 | 90 | 32 | 41 | 77.6 | 4.2 | 61.6 | 9.3 |
| 19 | 127 | 89 | 42 | 34 | 100.0 | 2.4 | — | 6.8 |
| 20 | 95 | 79 | 35 | 41 | 94.1 | 5.7 | 60.9 | 6.2 |
| 21 | 85 | 77 | 29 | 45 | 64.1 | 3.0 | 54.4 | 8.7 |
| Mean ± SD | 99 ± 18 | 82 ± 5.9 | 32.5 ± 5.4 | 42 ± 5.7 | 77.3 ± 17.7 | 3.7 ± 1.1 | 59.1 ± 3.4 | 8.3 ± 2.2 |
| Pvalue | .22 | .08 | <.001 | .29 | .26 | .02 | 0.01 | 0.05 |
| Normal | — | 84.9 ± 8.1 | 20.6 ± 5.8 | 55.9 ± 7.2 | 35.8 ± 8.9 | 0.72 | 80 | 25.7 ± 0.9 |
Hgb indicates hemoglobin level; MCV, mean corpuscular volume; SI, serum iron; TIBC, total iron-binding capacity; Tf sat, transferrin iron saturation; PITR, plasma iron turnover rate; RBC, red blood cell;59Fe, iron 59; 51Cr, chromium 51; and T½, terminal half-life.
Expressed as mg Fe/L whole blood/day.
Ferrokinetic analyses showing red blood cell utilization of iron 59 in 3 patients with hemoglobin H (HbH) disease and 5 patients with HbH constant spring (HbH/CS).
Solid symbols and dotted lines represent results in patients with HbH disease; open symbols and heavy solid lines, results in patients with HbH/CS; and the thin solid line, the normal value. Values in patients with HbH disease were slightly below normal, whereas comparable values in patients with HbH/CS were reduced to a mean of 59%.
Ferrokinetic analyses showing red blood cell utilization of iron 59 in 3 patients with hemoglobin H (HbH) disease and 5 patients with HbH constant spring (HbH/CS).
Solid symbols and dotted lines represent results in patients with HbH disease; open symbols and heavy solid lines, results in patients with HbH/CS; and the thin solid line, the normal value. Values in patients with HbH disease were slightly below normal, whereas comparable values in patients with HbH/CS were reduced to a mean of 59%.
These results suggested that the contribution of HbCS might be to increase both ineffective erythropoiesis and hemolysis. Accordingly, 6 patients with homozygous HbCS/CS were studied (Table2). There was a mild anemia with a normal MCV20 22 and a distinct reticulocytosis. RBCs were hypochromic, with basophilic stippling. Radiochromium RBC survival curves showed evidence of hemolysis (Table 2). The PITR was 3 times the normal level, whereas the mean RBC radioiron utilization value was low at 58%—similar to the 59% in patients with HbH/CS (Table 1 and Figure 1).
Variables in patients with hemoglobin constant spring/constant spring (HbCS/CS)
| Patient no. . | Hgb (g/L) . | MCV (fl) . | Retics (%) . | HbCS (%) . | SI (μmol/L) . | TIBC (μmol/L) . | Tf sat (%) . | 51Cr RBC T½ (days) . | PITR* . | RBC59Fe use (%) . |
|---|---|---|---|---|---|---|---|---|---|---|
| 22 | 114 | 93 | 4.7 | 6.74 | 18.0 | 36.2 | 49.7 | 12.25 | 2.2 | 56.1 |
| 23 | 116 | 96 | 4.0 | 5.63 | 29.2 | 35.6 | 82.0 | 14.5 | 2.15 | 73.8 |
| 24 | 107 | 100 | 5.6 | 3.17 | 17.4 | 36.2 | 48.1 | 15.0 | 2.29 | 43.5 |
| 25 | 117 | 90 | 6.2 | — | 9.0 | 59.3 | 15.2 | 13.75 | 1.96 | 58.0 |
| 26 | 95 | 90 | 7.6 | — | 19.8 | 39.5 | 30.0 | 13.75 | 2.68 | 58.5 |
| 27 | 102 | 90 | 8.9 | — | 26.8 | 41.5 | 64.6 | 12.75 | 2.59 | — |
| Mean ± SD | 109 ± 9 | 93.2 ± 4.1 | 6.2 ± 1.8 | 5.2 ± 1.8 | 20.0 ± 7.2 | 41.4 ± 9.1 | 48.3 ± 23.8 | 13.7 ± 1.0 | 2.3 ± 0.3 | 58.0 ± 10.8 |
| Normal | — | — | — | — | 20.6 ± 5.8 | 56.6 ± 7.7 | 35.8 ± 8.9 | 25.7 ± 0.9 | 0.72 | 80 |
| Patient no. . | Hgb (g/L) . | MCV (fl) . | Retics (%) . | HbCS (%) . | SI (μmol/L) . | TIBC (μmol/L) . | Tf sat (%) . | 51Cr RBC T½ (days) . | PITR* . | RBC59Fe use (%) . |
|---|---|---|---|---|---|---|---|---|---|---|
| 22 | 114 | 93 | 4.7 | 6.74 | 18.0 | 36.2 | 49.7 | 12.25 | 2.2 | 56.1 |
| 23 | 116 | 96 | 4.0 | 5.63 | 29.2 | 35.6 | 82.0 | 14.5 | 2.15 | 73.8 |
| 24 | 107 | 100 | 5.6 | 3.17 | 17.4 | 36.2 | 48.1 | 15.0 | 2.29 | 43.5 |
| 25 | 117 | 90 | 6.2 | — | 9.0 | 59.3 | 15.2 | 13.75 | 1.96 | 58.0 |
| 26 | 95 | 90 | 7.6 | — | 19.8 | 39.5 | 30.0 | 13.75 | 2.68 | 58.5 |
| 27 | 102 | 90 | 8.9 | — | 26.8 | 41.5 | 64.6 | 12.75 | 2.59 | — |
| Mean ± SD | 109 ± 9 | 93.2 ± 4.1 | 6.2 ± 1.8 | 5.2 ± 1.8 | 20.0 ± 7.2 | 41.4 ± 9.1 | 48.3 ± 23.8 | 13.7 ± 1.0 | 2.3 ± 0.3 | 58.0 ± 10.8 |
| Normal | — | — | — | — | 20.6 ± 5.8 | 56.6 ± 7.7 | 35.8 ± 8.9 | 25.7 ± 0.9 | 0.72 | 80 |
Retics indicates reticulocytes.
Expressed as in Table 1.
Results in patients with β0-thal/HbE. We studied these patients to provide contrast with patients with α thalassemia (Table 1 and 2) and to try to understand why only some of these patients are severely anemic. There were 6 patients with mild disease (mean hemoglobin value, 93 g/L) and 5 with severe disease (mean hemoglobin value, 64 g/L; Table 3). The MCV value was higher in the severe-disease group, probably reflecting the greater reticulocytosis (Table 3). The increase in transferrin saturation in the severe-disease group was significant. RBC utilization of radioiron was greatly but equally decreased, at about 35% for both mild and severe cases, and these values were much lower than comparable values in patients with variant forms of α thalassemia (Figure 1 and Tables 1-3). There were 2 striking differences between severe and mild cases: the PITR was 2 times higher in severe cases (P < .0001), with values 9 to 10 times those of controls, and the radiochromium survival assay showed a much increased rate of hemolysis in severe cases (P < .005; Table 3).
Variables in patients with β0thalassemia/hemoglobin E
| Patient no. . | Hgb (g/L) . | MCV (fl) . | Retics (%) . | SI (μmol/L) . | TIBC (μmol/L) . | Tf sat (%) . | HbE (%) . | HbF (%) . | 51Cr RBC T½ (days) . | PITR3-150 . | RBC59Fe use (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild cases | |||||||||||
| 1 | 80 | 66 | 4.2 | 21 | 45 | 46.2 | — | — | 10.5 | 2.5 | 49.0 |
| 2 | 103 | 69 | 1.2 | 15 | 29 | 51.0 | 50.44 | 37.68 | 19.5 | 2.8 | 30.0 |
| 3 | 85 | 62 | 1.1 | 33 | 35 | 93.7 | 75.80 | 13.67 | 20.7 | 2.0 | 43.0 |
| 4 | 94 | 68 | 2.0 | 30 | 32 | 93.6 | 61.75 | 22.76 | 13.5 | 3.7 | 37.2 |
| 5 | 72 | 69 | 1.4 | 15 | 26 | 56.5 | 45.45 | 27.06 | 16.8 | 4.5 | 27.8 |
| 6 | 123 | 73 | 0.8 | 20 | 32 | 67.4 | 33.94 | 34.50 | 21.0 | 3.3 | 27.0 |
| Mean ± SD | 93 ± 18 | 67.8 ± 3.7 | 1.8 ± 1.2 | 22.3 ± 7.6 | 33.2 ± 6.6 | 68.1 ± 21.0 | 53.5 ± 16.0 | 27.1 ± 9.6 | 17.0 ± 4.3 | 3.1 ± 0.9 | 35.7 ± 9.0 |
| Severe cases | |||||||||||
| 7 | 52 | 65 | 4.0 | 24 | 27 | 91.0 | 68.86 | 18.60 | 10.0 | 7.6 | — |
| 8 | 69 | 86 | 8.5 | 44 | 50 | 96.9 | 47.21 | 30.73 | 8.6 | 6.1 | 41.8 |
| 9 | 64 | 77 | 7.6 | 43 | 46 | 92.9 | 45.21 | 30.54 | 10.3 | 6.6 | 27.6 |
| 10 | 69 | 90 | 17.2 | 33 | 35 | 93.6 | 41.55 | 31.09 | 10.8 | 6.7 | 27.6 |
| 11 | 65 | 92 | 24.4 | 50 | 59 | 84.8 | 50.70 | 24.48 | 6.2 | 6.9 | 44.1 |
| Mean ± SD | 64 ± 7 | 82.0 ± 11.1 | 12.3 ± 8.3 | 38.8 ± 10.3 | 43.4 ± 12.6 | 91.8 ± 4.5 | 50.7 ± 10.7 | 27.1 ± 5.5 | 9.2 ± 1.9 | 6.8 ± 0.5 | 35.3 ± 8.9 |
| Pvalue | .01 | .04 | .05 | .02 | .15 | .04 | .76 | .99 | <.005 | <.0001 | .95 |
| Normal | — | 84.9 ± 8.1 | 1.7 ± 1.6 | 20.6 ± 5.8 | 55.9 ± 7.2 | 35.8 ± 8.9 | — | — | 25.7 ± 0.9 | 0.72 | 80 |
| Patient no. . | Hgb (g/L) . | MCV (fl) . | Retics (%) . | SI (μmol/L) . | TIBC (μmol/L) . | Tf sat (%) . | HbE (%) . | HbF (%) . | 51Cr RBC T½ (days) . | PITR3-150 . | RBC59Fe use (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild cases | |||||||||||
| 1 | 80 | 66 | 4.2 | 21 | 45 | 46.2 | — | — | 10.5 | 2.5 | 49.0 |
| 2 | 103 | 69 | 1.2 | 15 | 29 | 51.0 | 50.44 | 37.68 | 19.5 | 2.8 | 30.0 |
| 3 | 85 | 62 | 1.1 | 33 | 35 | 93.7 | 75.80 | 13.67 | 20.7 | 2.0 | 43.0 |
| 4 | 94 | 68 | 2.0 | 30 | 32 | 93.6 | 61.75 | 22.76 | 13.5 | 3.7 | 37.2 |
| 5 | 72 | 69 | 1.4 | 15 | 26 | 56.5 | 45.45 | 27.06 | 16.8 | 4.5 | 27.8 |
| 6 | 123 | 73 | 0.8 | 20 | 32 | 67.4 | 33.94 | 34.50 | 21.0 | 3.3 | 27.0 |
| Mean ± SD | 93 ± 18 | 67.8 ± 3.7 | 1.8 ± 1.2 | 22.3 ± 7.6 | 33.2 ± 6.6 | 68.1 ± 21.0 | 53.5 ± 16.0 | 27.1 ± 9.6 | 17.0 ± 4.3 | 3.1 ± 0.9 | 35.7 ± 9.0 |
| Severe cases | |||||||||||
| 7 | 52 | 65 | 4.0 | 24 | 27 | 91.0 | 68.86 | 18.60 | 10.0 | 7.6 | — |
| 8 | 69 | 86 | 8.5 | 44 | 50 | 96.9 | 47.21 | 30.73 | 8.6 | 6.1 | 41.8 |
| 9 | 64 | 77 | 7.6 | 43 | 46 | 92.9 | 45.21 | 30.54 | 10.3 | 6.6 | 27.6 |
| 10 | 69 | 90 | 17.2 | 33 | 35 | 93.6 | 41.55 | 31.09 | 10.8 | 6.7 | 27.6 |
| 11 | 65 | 92 | 24.4 | 50 | 59 | 84.8 | 50.70 | 24.48 | 6.2 | 6.9 | 44.1 |
| Mean ± SD | 64 ± 7 | 82.0 ± 11.1 | 12.3 ± 8.3 | 38.8 ± 10.3 | 43.4 ± 12.6 | 91.8 ± 4.5 | 50.7 ± 10.7 | 27.1 ± 5.5 | 9.2 ± 1.9 | 6.8 ± 0.5 | 35.3 ± 8.9 |
| Pvalue | .01 | .04 | .05 | .02 | .15 | .04 | .76 | .99 | <.005 | <.0001 | .95 |
| Normal | — | 84.9 ± 8.1 | 1.7 ± 1.6 | 20.6 ± 5.8 | 55.9 ± 7.2 | 35.8 ± 8.9 | — | — | 25.7 ± 0.9 | 0.72 | 80 |
Hb F indicates hemoglobin F.
Expressed as in Table 1.
Studies of apoptosis of erythroid precursors
Patients.
Thirty-seven analyses were carried out in 32 patients who were typical of the diagnostic categories. The 23 patients in group I underwent analysis of apoptosis of marrow erythroid precursors by 2 methods, the annexin V–FITC and the Hoechst 33342 techniques, which generally yielded values within 3% of each other (Figure2). Because of our concerns regarding possible shipping artifacts and to explore several interesting findings42 (such as the apparent increase in apoptosis in HbCS/CS), 14 additional analyses (group II) were done in Bangkok, including 5 in patients also in group I. Analysis of the 2 groups revealed no significant differences (data not shown). Therefore, the results obtained in the 2 groups by using the annexin V–FITC method were pooled (Table 4). The results of all assays using both methods are shown in Figure 2. A single patient with HbEE was studied (data not shown) and the values for apoptosis and cell death were entirely normal.
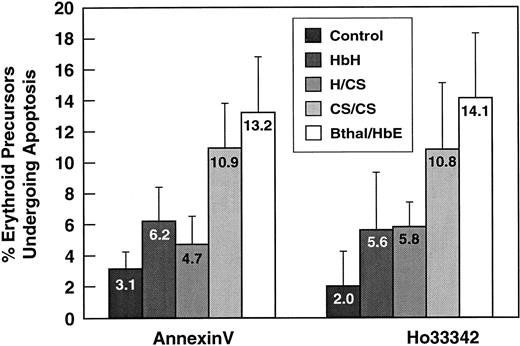
Apoptosis in thalassemia variants from Thailand.
Mean (± SD) values for the percentage of erythroid precursors undergoing apoptosis in 37 studies in 32 patients with the 4 clinical diagnoses of α and β thalassemia. The studies used both the annexin V and, where available, the Hoechst 33342 method. Results of statistical analyses based only on the Hoechst data from group 1 were as follows: control versus HbH/CS, P < .03; control versus hemoglobin constant spring/constant spring (HbCS/CS),P < .05; control versus β thalassemia/hemoglobin E (β-thal/HbE), P < .0001; β-thal/HbE versus HbH,P < .006; β-thal/HbE versus HbH/CS,P < .005; and β-thal/HbE versus HbCS/CS, not significant.
Apoptosis in thalassemia variants from Thailand.
Mean (± SD) values for the percentage of erythroid precursors undergoing apoptosis in 37 studies in 32 patients with the 4 clinical diagnoses of α and β thalassemia. The studies used both the annexin V and, where available, the Hoechst 33342 method. Results of statistical analyses based only on the Hoechst data from group 1 were as follows: control versus HbH/CS, P < .03; control versus hemoglobin constant spring/constant spring (HbCS/CS),P < .05; control versus β thalassemia/hemoglobin E (β-thal/HbE), P < .0001; β-thal/HbE versus HbH,P < .006; β-thal/HbE versus HbH/CS,P < .005; and β-thal/HbE versus HbCS/CS, not significant.
Apoptosis of erythroid precursors (annexin V assessment) in patients in group I and group II combined
| Diagnostic category . | No. of patients . | Erythroid precursors undergoing apoptosis (%) . |
|---|---|---|
| Control | 12 | 3.1 ± 1.1 |
| HbH | 6 | 6.2 ± 2.2 |
| HbH/CS | 7 | 4.7 ± 1.8 |
| HbCS/CS | 6 | 10.9 ± 2.9 |
| β-thal/HbE | 15 | 13.2 ± 3.6 |
| Diagnostic category . | No. of patients . | Erythroid precursors undergoing apoptosis (%) . |
|---|---|---|
| Control | 12 | 3.1 ± 1.1 |
| HbH | 6 | 6.2 ± 2.2 |
| HbH/CS | 7 | 4.7 ± 1.8 |
| HbCS/CS | 6 | 10.9 ± 2.9 |
| β-thal/HbE | 15 | 13.2 ± 3.6 |
β-thal/HbE indicates β thalassemia/hemoglobin E. Results of statistical analyses were as follows: control versus HbH,P < .02; control versus HbH/CS, not significant; control versus HbCS/CS, P < .001; control versus β-thal/HbE, P < .00001; β-thal/HbE versus HbH,P < .0001; β-thal/HbE versus HbH/CS,P < .00001; β- thal/HbE versus HbCS/CS, not significant; HbCS/CS versus HbH, P < .01; and HbCS/CS versus HbH/CS, P < .001.
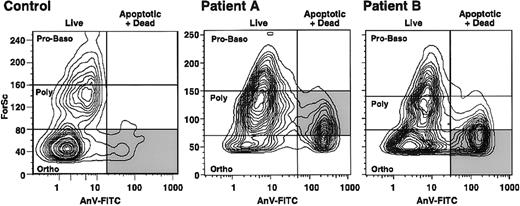
Stage of maturation at which β-thalassemic erythroid precursors undergo enhanced apoptosis. The stage of erythropoiesis at which apoptosis occurs can be determined by using flow cytometry to measure forward scatter and thereby identify the size of the cells undergoing apoptosis (cells positive on annexin V–FITC assessment). The larger erythroid precursors are the least mature forms.43In samples of normal marrow (Figure 3), most of the erythroid apoptosis occurred at the smallest, or late orthochromic normoblast, stage. We analyzed 2 patients with β0-thal/HbE in whom erythroid precursors showed substantial apoptosis and determined the stage at which apoptosis was detected (Figure 3). Most of the erythroid precursors in one patient were polychromatophilic erythroblasts, whereas orthochromic erythroblasts predominated in the other patient; however, in both patients, there was little apoptosis in proerythroblasts and basophilic erythroblasts and an increase in the extent of apoptosis with increasing erythroid maturity (Figure 3).
Flow cytometry results showing the stages of erythroid precursor maturation at which apoptosis occurred in a control subject at Stanford University and 2 patients (A and B) with β0-thal/HbE.
Forward scatter (ForSc), an index of cell size, is shown on the ordinate, whereas the number of dead and apoptotic erythroid precursors, as determined by annexin V reactivity (AnV-FITC), is plotted on the abscissa (vertical line). The horizontal lines indicate the sites of the stages of erythroid maturation. Proerythroblasts and basophilic erythroblasts (Pro-Baso) are the largest, polychromatophilic erythroblasts (Poly) are somewhat smaller, and orthochromic erythroblasts (Ortho) are the smallest. The shaded area indicates the major apoptotic erythroblast population in each subject.
Flow cytometry results showing the stages of erythroid precursor maturation at which apoptosis occurred in a control subject at Stanford University and 2 patients (A and B) with β0-thal/HbE.
Forward scatter (ForSc), an index of cell size, is shown on the ordinate, whereas the number of dead and apoptotic erythroid precursors, as determined by annexin V reactivity (AnV-FITC), is plotted on the abscissa (vertical line). The horizontal lines indicate the sites of the stages of erythroid maturation. Proerythroblasts and basophilic erythroblasts (Pro-Baso) are the largest, polychromatophilic erythroblasts (Poly) are somewhat smaller, and orthochromic erythroblasts (Ortho) are the smallest. The shaded area indicates the major apoptotic erythroblast population in each subject.
Discussion
Kinetic studies
In our patients with HbH disease, the mean radiochromium survival was about 16 days (Table 1); therefore, like others,9,12-14,44 we identified hemolysis as the major kinetic feature of the anemia. The PITR30 38 was increased about only 3 fold (Table 1), not the expected 5-fold increase in normal marrow. This erythropoiesis is only minimally ineffective, with radioiron utilization of about 75% (Table 1 and Figure 1). This lack of a full compensatory erythropoiesis in HbH has not previously been emphasized and we do not have an explanation for it.
We believe that these are the first studies showing that RBC survival times in patients with HbH/CS were much shorter than those in comparably anemic patients with HbH (Table 1). The PITRs in the patients with HbH/CS were increased to more than 5 times the control values, thereby achieving the anticipated level of erythroid compensation (not seen in HbH). Furthermore, this erythropoiesis was clearly ineffective, since the RBC radioiron utilization rate was less than 60% (Table 1 and Figure 1). Thus, to maintain the same hemoglobin levels as in HbH, erythropoiesis in patients with HbH/CS increased almost 2 fold (PITR = 3.7 versus 2.0) to overcome more ineffective erythropoiesis and more hemolysis.
Table 2 shows the important contribution of HbCS to this pathophysiologic mechanism. Mildly anemic patients with homozygous HbCS (HbCS/CS) had increased hemolysis, which is perhaps pathophysiologically related to the extreme RBC rigidity.22 The membrane accumulation of αCS, in addition to the excess β globin,22may have caused the ineffective erythropoiesis, as does αA accumulation in severe β thalassemia (Table3).25 26 The PITR, however, was increased to only 3 times the control value (Table 2; PITR = 2.3). Thus, the anemia in HbCS/CS is a composite of hemolysis, distinct ineffective erythropoiesis, and suboptimal erythropoietic compensation.
The patients with severe cases of β0-thal/HbE had a greatly increased rate of hemolysis compared with patients with mild cases (Table 3), and the attempted compensation is indicated by the reticulocytosis and by a PITR of 6.8, which is 9 to 10 times the normal value. This PITR is similar to that in Cooley anemia30 and contrasts with the PITR of 3.1 in the mild cases. There was no difference in the percentage of radioiron utilization in mild and severe cases; however, the calculation of radioiron utilization does not take into account the extent of erythropoiesis. Calculation of SEIT does provide such a measure.30 With a normal SEIT value of 0.52 mg iron/L of whole blood per day, patients with mild cases had a value of 2.8 ± 0.9, whereas those with severe cases had a value of 6.2 ± 0.6 (P < .004).30 A radioiron utilization rate of 35% in severe cases means that about 65% of an enormous erythroid turnover of 6.2 (SEIT) is dying in the marrow, in contrast to about 65% of an SEIT of 2.8 in mild cases. Thus, patients with severe disease have increased hemolysis and the erythropoietic compensation is virtually maximal but very ineffective.
Studies of apoptosis
In murine β thalassemia,31 the extent of ineffective erythropoiesis correlates directly with the extent of erythroid apoptosis. We hypothesized that there would be a similar correlation in human α and β thalassemia and studied this issue in both Stanford and Bangkok. The results were consistent, so the annexin V results were pooled (Table 4) and all results are shown in Figure 2.
Compared with healthy controls, patients with HbH and HbH/CS had perhaps a doubling in apoptosis detected by at least one of our methods, whereas patients with HbCS/CS had a further significant doubling in apoptosis to 4 times the control value (Figure 2 and Table4). The increased apoptosis in HbCS/CS suggests that the αCS accumulation22 may begin at the erythroid precursor stage and, like αA, cause increased apoptosis.25 26 Except for the fact that the level of apoptosis in patients with HbH and HbH/CS was similar (Table 4 and Figure 2), there was a parallel and step-wise increase in both ineffective erythropoiesis (Tables 1-3) and apoptosis from HbH to HbH/CS to HbCS/CS to β-thal/HbE (Table 4 and Figure 2). We thus propose that accumulations of α-globin chains (either the αA that occurs in β thalassemia or the αCS that occurs in HbCS variants) in erythroid precursors are particularly effective in causing apoptosis. However, β-globin accumulation may also cause the moderate increase in apoptosis observed in marrow erythroid precursors in HbH (Table 4 and Figure 2).
The β-thal/HbE problem. The excess of α chains expressed as the α/non-α ratio does correlate with the severity of anemia,24,45 with hemoglobin values ranging from 26 g/L to 135 g/L.28 29 However, the levels of HbF or HbE do not account for the variation (Tables 3 and 5). The percentage of β-thal/HbE erythroid precursors undergoing apoptosis was greatly increased, but the value did not correlate with the severity of the anemia (Table 5). However, at a given percentage of apoptosis, at least twice as many erythroid precursors were undergoing apoptosis in the more severe cases, since the SEIT value was 6.2 for severe cases and 2.8 for mild cases. Nevertheless, the pathophysiologic cause of this remarkable clinical heterogeneity remains obscure.
Clinical severity of β-thal/HbE
| Patient no. . | Hgb (g/L) . | HbF (%) . | HbE (%) . | Ferritin (μg/L) . | Erythroid precursors undergoing apoptosis (%)5-150 . | |
|---|---|---|---|---|---|---|
| Annexin V . | Hoechst . | |||||
| 009 β0 | 85 | 15.1 | 71.4 | 1645 | 14.4 | 17.1 |
| 012 β0 | 103 | 15.2 | 76.3 | 201 | 11.7 | 8.9 |
| 013 β0 | 113 | 25.7 | 62.5 | 324 | 11.9 | 14.1 |
| 014 β0 Splx | 46 | 19.9 | 67.2 | 4516 | 19.3 | 13.2 |
| 015 β0 | 54 | 17.5 | 67.4 | 2580 | 13.7 | 13.7 |
| 020 β0Splx | 56 | 37.1 | 59.1 | 5445 | 14.9 | 6.5 |
| 025 β+ | 83 | 20.4 | 56.4 | 2170 | 10.9 | — |
| 025-1 β+ | 75 | 20.4 | 56.4 | 3274 | 11.5 | — |
| 043 β | 83 | 26.9 | 68.9 | 318 | 15.7 | — |
| 044 β0Splx | 44 | 35.7 | 55.7 | 6660 | 19.6 | — |
| 046 β0 | 61 | 33.35-151 | 51.2 | 447 | 15.1 | — |
| 049 β0Splx | 44 | 51.55-151 | 48.0 | 978 | 6.9 | — |
| 051 β0 | 48 | 13.2 | 75.5 | 739 | 10.7 | — |
| 052 β0 | 98 | 3.6 | 95.1 | 244 | 12.7 | — |
| 054 β0 | 53 | 11.45-151 | 74.1 | 4765 | 6.9 | — |
| 055 β0 | 71 | 27.25-151 | 57.1 | 964 | 12.4 | — |
| Patient no. . | Hgb (g/L) . | HbF (%) . | HbE (%) . | Ferritin (μg/L) . | Erythroid precursors undergoing apoptosis (%)5-150 . | |
|---|---|---|---|---|---|---|
| Annexin V . | Hoechst . | |||||
| 009 β0 | 85 | 15.1 | 71.4 | 1645 | 14.4 | 17.1 |
| 012 β0 | 103 | 15.2 | 76.3 | 201 | 11.7 | 8.9 |
| 013 β0 | 113 | 25.7 | 62.5 | 324 | 11.9 | 14.1 |
| 014 β0 Splx | 46 | 19.9 | 67.2 | 4516 | 19.3 | 13.2 |
| 015 β0 | 54 | 17.5 | 67.4 | 2580 | 13.7 | 13.7 |
| 020 β0Splx | 56 | 37.1 | 59.1 | 5445 | 14.9 | 6.5 |
| 025 β+ | 83 | 20.4 | 56.4 | 2170 | 10.9 | — |
| 025-1 β+ | 75 | 20.4 | 56.4 | 3274 | 11.5 | — |
| 043 β | 83 | 26.9 | 68.9 | 318 | 15.7 | — |
| 044 β0Splx | 44 | 35.7 | 55.7 | 6660 | 19.6 | — |
| 046 β0 | 61 | 33.35-151 | 51.2 | 447 | 15.1 | — |
| 049 β0Splx | 44 | 51.55-151 | 48.0 | 978 | 6.9 | — |
| 051 β0 | 48 | 13.2 | 75.5 | 739 | 10.7 | — |
| 052 β0 | 98 | 3.6 | 95.1 | 244 | 12.7 | — |
| 054 β0 | 53 | 11.45-151 | 74.1 | 4765 | 6.9 | — |
| 055 β0 | 71 | 27.25-151 | 57.1 | 964 | 12.4 | — |
Splx indicates splenectomy.
As assessed by the annexin V and Hoechst 33342 methods.
Determined by alkaline denaturation.
Stage of erythropoiesis at which apoptosis occurs. The stage of erythropoiesis at which accelerated apoptosis occurred in severe β-thal/HbE was found to increase progressively with erythroid maturation (Figure 3), consistent with the concept that the pathophysiologic mechanism in β thalassemia is directly related to accumulation of excess, partly oxidized α-globin chains. With increasing cell maturation, there is increasing synthesis of globin chains, thus increasing the likelihood of more α-globin accumulation.
Measurements made to detect apoptosis in living human tissues (unlike studies in cell cultures) represent the extent of apoptosis at a single moment. In living tissues, cells undergoing apoptosis signal this state to macrophages, which rapidly attack and remove such cells.46 A reliable marker of apoptosis, cell-surface exposure of PS, is such a signal.39,40 Thus, the value for apoptosis in our studies represented a composite of cells entering apoptotic programs and their removal by macrophages. Macrophages in patients with β-thal/HbE are intensely activated47 and thus the value for apoptosis recorded may underrepresent the actual extent of apoptosis.
These studies further clarified critical differences in the kinetics of the anemia in α and β thalassemia, showed a correlation between ineffective erythropoiesis and erythroid apoptosis, and focused on the important pathophysiologic role played by α-chain deposition in erythroid precursors.
Acknowledgments
We thank the Faculty of Medical Technology for the blood chemistry analyses and the Division of Hematology, Department of Medicine, Faculty of Medicine, Siriraj Hospital, for the hematologic and hemoglobin analyses.
Supported by Seameo Tropmed, National Research Council of Thailand, and US Public Health Service grants HL ROI-34408 and RO1-DK13682.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stanley L Schrier, Division of Hematology, Department of Medicine, Stanford University School of Medicine, 300 Pasteur Dr, Room S-161, Stanford, CA, 94305.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal