Abstract
Because of its immunosuppressive properties, interleukin-10 (IL-10) is thought to play an important role in a number of human disease states, including inflammation, autoimmunity, and transplant rejection. In this study, we demonstrate that introduction of human or viral IL-10 genes into Burkitt's lymphoma cells markedly reduced their ability to grow as subcutaneous (sc) tumors in SCID mice. In vivo assays for angiogenesis revealed an inhibition of the angiogenic capacity of the IL-10–transfected lines. Recombinant human IL-10 abolished and viral IL-10 reduced vascular endothelial growth factor (VEGF)-165–induced neovascularization. Furthermore, IL-10 blocked the VEGF- and fibroblast growth factor (FGF)-2–induced proliferation of microvascular endothelial cells in vitro. The current observations suggest a direct role for IL-10 in the prevention of angiogenesis in human lymphoid malignancies.
Introduction
Interleukin-10 (IL-10) is thought to play a potential pathogenic or therapeutic role in a number of human disease states such as inflammation, autoimmunity, and transplant rejection.1-3 The immunomodulatory effects of IL-10 have yielded mixed results in various tumor systems. Because many tumors and tumor-infiltrating lymphocytes express IL-10, its role as a tumor escape mediator has been suggested. IL-10 inhibits the tumoricidal capacity of macrophages,4,5 the cytotoxicity and cytokine production by tumor specific T cells,6 and blocks the presentation of tumor antigens by antigen-presenting cells (APCs).7,8 Paradoxically, in vivo studies of tumor rejection responses have produced opposite results. Chinese hamster ovary (CHO) cells,9 mammary adenocarcinoma cells,10,11 and melanoma cells12,13transfected with IL-10 were less effective at establishing primary tumors and/or metastasis than untransfected cells in syngeneic and SCID mice. Additionally, systemic administration of IL-10 has inhibited tumor metastasis and stimulated antitumor immune responses in various murine models.13 14
The mechanisms behind these antitumor effects are poorly understood. A role for natural killer (NK) cells,11,13,15 T cells,11,14 macrophages,16 and nitric oxide (NO)17 has been implicated. The damage and decrease in neovascularization found in IL-10–secreting tumors has led to the suggestion that IL-10 exerts its antitumor and metastatic activity by inhibiting angiogenesis in vivo.9,12,16 Recent studies have revealed that the stimulation of tissue inhibitors of metalloproteases (TIMP) and inhibition of matrix metalloproteases (MMPs) secretion, induced by IL-10 in prostate tumor cells, affects induction of angiogenesis in vitro.18 19 However, its direct effect in the in vivo angiogenic assays has not been demonstrated.
In this report, we sought to analyze and compare the antitumor and angiogenic capacity of human IL-10 (hIL-10) and viral IL-10 (vIL-10) using a human B-cell Burkitt's lymphoma (BL) line constitutively expressing either cytokine. We show that IL-10 abolishes tumorigenicity in SCID mice. With the use of an in vivo assay that detects corneal vascularization in rabbits, we provide evidence that IL-10 exerts its antitumor activity by inhibiting angiogenesis.
Materials and methods
Mice
CB-17 SCID mice were bred and maintained in pathogen-limited conditions at the Microbiology and Tumor Biology Center (MTC), Karolinska Institutet. Four- to 6-week-old mice, usually littermates or otherwise age matched within 2 weeks, were used in all experiments.
Cells and transfections
DG75 is an Epstein-Barr virus (EBV) negative BL line.20 The hIL-10 and vIL-10 complementary DNAs (cDNAs) were excised from the pcDSrα 296–modified expression vector,21 and subcloned into the pcDNA3 Neo vector (Invitrogen). Plasmid control (pcDNA3 Neo) or plasmids containing the hIL-10 or vIL-10 cDNAs were introduced into tumor cells by electroporation using a Gene-Pulser (BioRad Laboratories, Richmond, CA) under the following conditions: 5 × 106 cells in 0.5 mL Dulbecco′s phosphate-buffered saline (PBS) (without Ca2+and Mg2+) were pulsed with 875 V/cm and 975 μF. Transfectants were selected in 750 μg/mL G418 (Gibco, BRL, Paisley, Scotland) for establishment of lines and clones. Cells were maintained in RPMI 1640 (Gibco, BRL) supplemented with 10% heat inactivated fetal calf serum (FCS), 100 mg/mL of streptomycin, 100 U/mL penicillin, and 750 μg/mL G418 (referred as complete medium). They were cultured at 37°C/5% CO2.
IL-10 production by the clones and sublines was determined by measuring the IL-10 released to the medium by specific enzyme-linked immunosorbent assay (ELISA) (capture clone JES3-9D7, biotinylated detection clone JES3-12G8, PharMingen, San Diego, CA). The sensitivity of the detection for IL-10 ELISA was 20 to 40 pg/mL. Results are expressed as units of IL-10 of 1 × 106 cells/mL per 36 hours.
Phenotypic analysis
Anti-HLA ABC, -DR (clone L243), -CD11a, -CD40, -CD54, -CD80, -CD86, and -CD95 phycoerythrin (PE)- 1 or fluorescein isothiocyanate (FITC)-conjugated antibodies were purchased from Becton Dickinson (Mountain View, CA). Cells (105) were labeled with FITC- or PE-conjugated antibodies or isotype matched controls for 30 minutes on ice. The cells were washed with cold PBS containing 1% FCS and 0.1% NaN3. The 104 cells were analyzed on a FACScan flow cytometer using CellQuest software from Becton Dickinson. Gates were established on viable cells using forward and side-scatter parameters.
Tumorigenicity in SCID mice
Otherwise indicated, tumor cells (107) were inoculated subcutaneously (sc) in the right flank in a 0.2 mL volume of PBS. Tumor size was determined twice weekly by palpation and measurements of the tumor. Mice were killed when the tumors reached a size of greater than or equal to 15 mm in diameter. Mice without any signs of tumor growth were kept under observation for at least 6 weeks after inoculation. Small groups of mice, never exceeding 5 mice per group, were tested in several independent experiments throughout the study to minimize random fluctuations in the quantity or quality of cells inoculated. Results from several independent tests were pooled.
In vivo natural killer cell depletion
One day before inoculation of tumor cells, and thereafter once a week, each mouse received an intravenous dose of rabbit antiasialoGM1 antibody (WAKO, Dallas, TX) (25 μL per mouse) in a total volume of 0.2 mL of PBS.
Proliferation assays
DG75 cells were seeded by 1:2 dilutions starting at a concentration of 4 × 106 per well in flat bottom microtiter plates in a volume of 200 μL and cultured for 48 hours at 37°C/5% CO2. Cultures were pulsed with 1 μCi of 3H-TdR (ARC, St Louis, MO) per well during the last 10 hours of the incubation period, harvested onto Fiberglas filters, and 3H-TdR incorporation determined by liquid scintillation counting. The results are expressed as mean cpm (counts per minute) of triplicate cultures.
Bovine coronary venular endothelial cells (CVECs) proliferation was quantified by total cell number. The 1 × 103 cells resuspended in 10% FCS were seeded in flat-bottom microtiter plates. After adherence (4 to 6 hours) the medium was replaced with 0.1% FCS or 1% FCS DMEM, containing test substances and, thereafter, the plates were incubated for 48 hours. Increasing concentrations of IL-10 (10 to 300 U/mL) were added together with growth factors vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF)-2 (20 ng/mL each). At the end of incubation, the supernatants were removed from the multiwell plates and cells were fixed with methanol and stained with Diff-Quik (Baxter Scientific Products, McGaw, IL). Cell numbers were obtained by counting in 7 random fields at a magnification of 100 × with the aid of an ocular grid (21 mm2). Data are reported as mean ± SEM of total cell number recovered in each well, as previously described.22
Rabbit cornea assay for angiogenesis
Corneal assays were performed in New Zealand white rabbits (Charles River, Calco, Como, Italy), as described previously,23 and in accordance with the guidelines of the European Economic Community for animal care and welfare (EEC Law No. 86/609). Briefly, slow release preparations and cell suspensions (in a volume of 5 μL) were implanted into surgically produced corneal micropockets. Slow release pellets were prepared by incorporating test substance in Elvax-40 (Du-Pont de Nemours, Wilmington, DE). The angiogenic response was measured daily with a slit-lamp stereomicroscope and capillary progression scored as previously reported.24 The number of samples inducing angiogenesis over the total number of implants performed was recorded during each observation. Implants not producing a neovascular growth within 10 days were considered negative. Implants showing an inflammatory reaction were evaluated and scored separately. The potency of angiogenic activity (angiogenic score) was calculated on the basis of the number and growth rate of newly formed capillaries. The angiogenic score was calculated as: (vessel density × distance from limbus).22 24 Corneas were removed at the end of the experiment, as well as at defined intervals after surgery, and fixed in formalin for histologic examination.
Endothelial cells
CVECs were obtained by a bead-perfusion technique through the coronary sinus as previously reported.25 Cells were maintained in culture in DMEM supplemented with penicillin and streptomycin and 10% FCS on gelatin coated dishes, and were used between the 15th and 20th passage.
Statistical analysis
Data on tumorigenicity and angiogenicity was compared by Student t test (2 sided).
Results
Interleukin-10 prevents tumor development of DG75 sublines
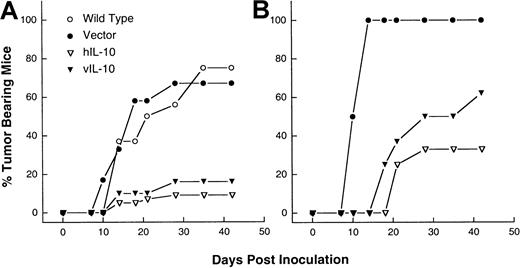
The genes encoding hIL-10 and vIL-10 were transduced into a EBV-negative BL line DG75. Endogenous IL-10 production from parental, referred as wild-type (WT), or mock-transfected DG75, referred as vector control lines, was not detected (Table1). Lines and clones expressing IL-10 activity ranging from 13 to 730 units IL-10/106 cells/mL per 36 hours were selected. In SCID mice, BL cells give rise to progressively growing sc tumors.26 On the basis of titration experiments performed with the WT cell line, a dose of 107 cells was chosen because 75% (12 of 16) of the mice had palpable tumors 2 weeks after sc inoculation. The tumorigenicity of the transfected sublines and clones was compared with that of WT and vector control cell lines. To avoid clonal variability, a subline and 3 different clones of either hIL-10 or vIL-10 transfectants with different IL-10 activities were tested. The vector control cells developed tumors with similar incidence (67%, 12 of 18 mice) and kinetics as the WT cells. The tumor incidence was markedly reduced for all the IL-10 expressing lines and clones. The proportion of animals in which tumors developed was 9% (4 of 42) and 16% (6 of 38) for the hIL-10 and vIL-10 transfectants, respectively (Figure1). When these tumors developed, they grew with similar kinetics as the WT or vector control sublines reaching a diameter of 15 mm within 12 to 15 days (data not shown).
Phenotype of DG75 wild-type, vector, hIL-10, and vIL-10 transfectants
| . | Wild-type . | Vector . | hIL-10 line . | hIL-10 cl-17 . | hIL-10 cl-30 . | hIL-10 cl-6 . | vIL-10 line . | vIL-10 cl-1 . | vIL-10 cl-9 . | vIL-10 cl-19 . |
|---|---|---|---|---|---|---|---|---|---|---|
| CD11a | 5 | 6 | 3 | 2 | 10 | 3 | 3 | 8 | 4 | 4 |
| CD40 | 34 | 32 | 35 | 47 | 36 | 40 | 32 | 40 | 29 | 50 |
| CD54 | 19 | 52 | 42 | 40 | 16 | 33 | 13 | 7 | 25 | 19 |
| CD80 | 41 | 47 | 54 | 75 | 20 | 53 | 25 | 11 | 16 | 35 |
| CD86 | 10 | 17 | 15 | 16 | 17 | 14 | 11 | 11 | 18 | 17 |
| HLA-ABC | 296 | 246 | 290 | 148 | 496 | 401 | 213 | 426 | 72 | 401 |
| HLA-DR | 357 | 331 | 340 | 131 | 485 | 216 | 352 | 444 | 215 | 200 |
| CD95 (Fas) | 18 | 21 | 24 | 21 | ND | 23 | 27 | ND | 21 | 19 |
| IL-10 (U/mL)* | <0.05 | <0.05 | 154 ± 30 | 678 ± 112 | 251 ± 54 | 68 ± 17 | 73 ± 12 | 658 ± 78 | 114 ± 24 | 726 ± 136 |
| . | Wild-type . | Vector . | hIL-10 line . | hIL-10 cl-17 . | hIL-10 cl-30 . | hIL-10 cl-6 . | vIL-10 line . | vIL-10 cl-1 . | vIL-10 cl-9 . | vIL-10 cl-19 . |
|---|---|---|---|---|---|---|---|---|---|---|
| CD11a | 5 | 6 | 3 | 2 | 10 | 3 | 3 | 8 | 4 | 4 |
| CD40 | 34 | 32 | 35 | 47 | 36 | 40 | 32 | 40 | 29 | 50 |
| CD54 | 19 | 52 | 42 | 40 | 16 | 33 | 13 | 7 | 25 | 19 |
| CD80 | 41 | 47 | 54 | 75 | 20 | 53 | 25 | 11 | 16 | 35 |
| CD86 | 10 | 17 | 15 | 16 | 17 | 14 | 11 | 11 | 18 | 17 |
| HLA-ABC | 296 | 246 | 290 | 148 | 496 | 401 | 213 | 426 | 72 | 401 |
| HLA-DR | 357 | 331 | 340 | 131 | 485 | 216 | 352 | 444 | 215 | 200 |
| CD95 (Fas) | 18 | 21 | 24 | 21 | ND | 23 | 27 | ND | 21 | 19 |
| IL-10 (U/mL)* | <0.05 | <0.05 | 154 ± 30 | 678 ± 112 | 251 ± 54 | 68 ± 17 | 73 ± 12 | 658 ± 78 | 114 ± 24 | 726 ± 136 |
Mean log fluorescence intensity as measured by FACS. Representative data from one of 5 independent experiments. ND: not determined.
Production of IL-10 by 106 cells/mL per 36 hours, as measured by enzyme-linked immunosorbent assay (ELISA). Mean ± SEM of 4 independent experiments.
IL-10 inhibits the tumorigenicity of DG75 sublines.
The 107 cells from wild-type (WT) as well as vector control, hIL-10–, and vIL-10–transfected sublines were injected sc into SCID mice (A), or antiasialoGM1-treated SCID mice (B). The combined results of 6 independent experiments are shown.
IL-10 inhibits the tumorigenicity of DG75 sublines.
The 107 cells from wild-type (WT) as well as vector control, hIL-10–, and vIL-10–transfected sublines were injected sc into SCID mice (A), or antiasialoGM1-treated SCID mice (B). The combined results of 6 independent experiments are shown.
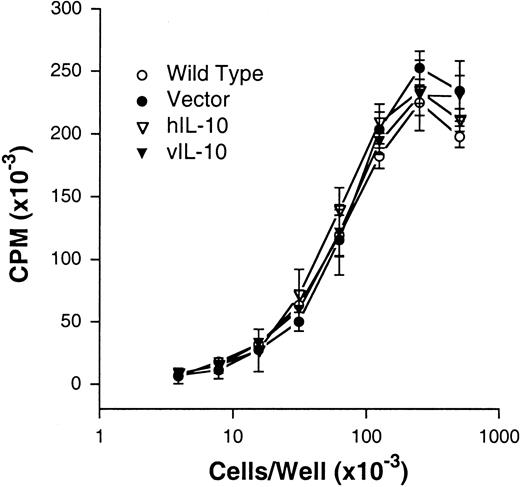
IL-10 is known to affect the proliferation and autonomous growth of human EBV-transformed B cells and melanoma cell lines.27-29 To determine whether the incapability of DG75 IL-10 transfectants to form tumors was related to a direct effect of IL-10 on cellular proliferation, we compared the growth and anchorage dependency (tested as agarose clonability) of all the sublines. No differences were found in the agarose clonability (data not shown) or proliferative capacity or of the WT, vector control, and IL-10 transfectants. Because the proliferative curves of all clones tested overlap, only the WT, vector, hIL-10, and vIL 10 curves are shown in Figure 2. Furthermore, exogenous IL-10 did not affect the proliferation of any of the sublines (data not shown).
IL-10 does not affect the proliferative capacity of DG75 sublines.
The 1:2 dilutions of cells ranging from 4 × 105 to 3 × 102 cell per well were performed in flat-bottomed microtiter plates in a final volume of 200 μL. Results are expressed as mean cpm ± SD from triplicate values of 3H-TdR incorporation during the last 8 hours of a 48-hour incubation period. One representative experiment of 3 is shown.
IL-10 does not affect the proliferative capacity of DG75 sublines.
The 1:2 dilutions of cells ranging from 4 × 105 to 3 × 102 cell per well were performed in flat-bottomed microtiter plates in a final volume of 200 μL. Results are expressed as mean cpm ± SD from triplicate values of 3H-TdR incorporation during the last 8 hours of a 48-hour incubation period. One representative experiment of 3 is shown.
The effect of interleukin-10 is local
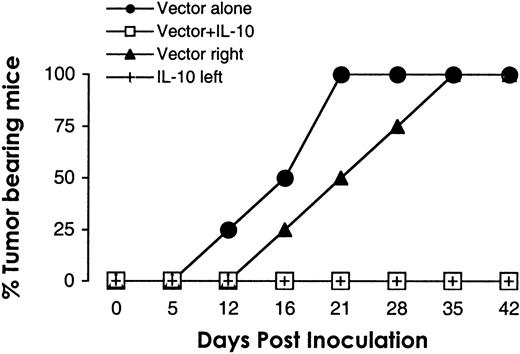
To address the question of whether the tumor suppressing effect of IL-10 was local or systemic, equal numbers of hIL-10 subline and vector control DG75 cells were simultaneously injected subcutaneously at the same site or contralaterally. Coinjection of both cell types at the same site resulted in overall suppression of tumor growth. The IL-10 subline delayed (by 1 to 2 weeks) but did not prevent the development of contralaterally injected vector control cells. In all mice (4 of 4), tumors developed at the site of DG75 vector control injection (Figure3).
IL-10 inhibits tumor growth locally.
The 107 cells from hIL-10 DG75 subline and vector control DG75 line were mixed before sc injection in the flank (■), or simultaneously injected sc in contralateral flanks in SCID mice (vector right flank, ▴; hIL-10 left flank, +). For comparison, the growth of 107 vector control cells (●) was assessed in the same experiment. Tumor growth was monitored twice a week for 42 days. n = 4. (Note: All 4 vectors coincide on days 0 and 5; the 3 noncontrol vectors coincide on day 12; and the control and right flank vectors coincide on days 35 and 42.)
IL-10 inhibits tumor growth locally.
The 107 cells from hIL-10 DG75 subline and vector control DG75 line were mixed before sc injection in the flank (■), or simultaneously injected sc in contralateral flanks in SCID mice (vector right flank, ▴; hIL-10 left flank, +). For comparison, the growth of 107 vector control cells (●) was assessed in the same experiment. Tumor growth was monitored twice a week for 42 days. n = 4. (Note: All 4 vectors coincide on days 0 and 5; the 3 noncontrol vectors coincide on day 12; and the control and right flank vectors coincide on days 35 and 42.)
The interleukin-10 effect is partially dependent on natural killer cell activity
In B cells and in various tumors, IL-10 down-regulates the expression of major histocompatibility complex (MHC) and costimulatory molecules.29-32 The decreased MHC class I expression has been correlated to an increase in NK sensitivity and a decrease in the metastasic potential.11,13 15 In this study, the expression of MHC class I/II and costimulatory molecules varied to a certain degree between different clones and lines, yet this did not correlate with the levels of IL-10 expression or with the tumorigenic capacity (Table 1). Therefore, we believe that in our system the effects of IL-10 are not dependent on changes in the expression of MHC or costimulatory molecules, or to enhanced expression of Fas molecules (Table 1). However, we observed that all IL-10 transfectants had a tendency to grow as single cells, whereas the WT line and the vector control line grew in small loose clumps.
Because NK cells have been shown to play a role in the antitumor effects of IL-10,11,13 15 we tested the tumorigenicity of the IL-10–transfected sublines in SCID mice pretreated with antiasialoGM1 antibody to suppress NK activity. When DG75 vector cells were injected into NK-depleted SCID mice, all mice (8 of 8) had tumors develop 2 weeks after inoculation. Although the incidence of tumor formation induced by IL-10 transfectants increased when compared with untreated mice, from 10% to 33% (4 of 12) for hIL-10, and from 16% to 62% (5 of 8) for vIL-10, the differences in tumorigenicity between the vector and IL-10 transfectants were still maintained (Figure 1B). This indicates that NK cells could not fully account for the observed effects. Moreover, the DG75 sublines were resistant to activated NK cells from SCID mice in vitro (data not shown).
Interleukin-10 inhibits angiogenesis in vivo
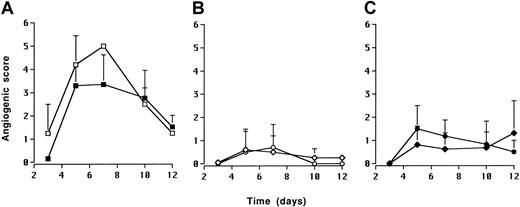
On the basis of the results that IL-10 transfectants were not able to form solid tumors, and that we could not ascribe this effect to any in vitro phenotypic or behavioral change or to an increase in NK sensitivity, we sought to look for the angiogenic capacity of the sublines. To this end, DG75 vector, hIL-10, and vIL-10 cell lines were implanted into rabbit corneal micropockets for evaluation of their angiogenic activity. Four days after inoculation of 4 × 105 and 2.5 × 105 DG75 vector cells, angiogenesis was observed in 2 of 2 and 4 of 5 implants respectively (Figure 4A). On the basis of these results, a dose of 2.5 × 105 cells was chosen for further experiments. Angiogenesis was not induced by either DG75 hIL-10 (0 of 5) or DG75 vIL-10 (0 of 7) implants when tested alone (Figure4B). Because the DG75 hIL-10 line inhibited the tumorigenicity of the DG75 vector control line when admixed in SCID mice (Figure 3A), we tested whether DG75 hIL-10 or DG75 vIL-10 lines could also suppress the angiogenic capacity of the DG75 vector control line. Thus, equal numbers (2.5 × 105) of vector control cells and hIL-10 or vIL-10 lines were mixed and implanted. Coinjection of IL-10 transfectants abolished (0 of 4 for hIL-10 and 0 of 7 for vIL-10 implants) the angiogenic capacity of the vector control line in all cases (Figure 4C).
IL-10 inhibits the angiogenic activity of DG75 sublines.
Five microliters of cell suspensions were implanted into surgically produced corneal micropockets. DG75 vector cell line. ■, vector 4 × 105; ▪, vector 2.5 × 105. (A); DG75 hIL-10 cells and DG75 vIL-10 cells. ⋄, h/L-10 2.5 × 105; ○, IL-10 2.5 × 105. (B); 2.5 × 105 DG75 vector cells admixed with 2.5 × 105 DG75 hIL-10 cells or with 2.5 × 105 DG75 vIL-10 cells. ♦, vector plus hIL-10; ●, vector plus vIL-10. (C). Each data point is the mean of at least 4 implants.
IL-10 inhibits the angiogenic activity of DG75 sublines.
Five microliters of cell suspensions were implanted into surgically produced corneal micropockets. DG75 vector cell line. ■, vector 4 × 105; ▪, vector 2.5 × 105. (A); DG75 hIL-10 cells and DG75 vIL-10 cells. ⋄, h/L-10 2.5 × 105; ○, IL-10 2.5 × 105. (B); 2.5 × 105 DG75 vector cells admixed with 2.5 × 105 DG75 hIL-10 cells or with 2.5 × 105 DG75 vIL-10 cells. ♦, vector plus hIL-10; ●, vector plus vIL-10. (C). Each data point is the mean of at least 4 implants.
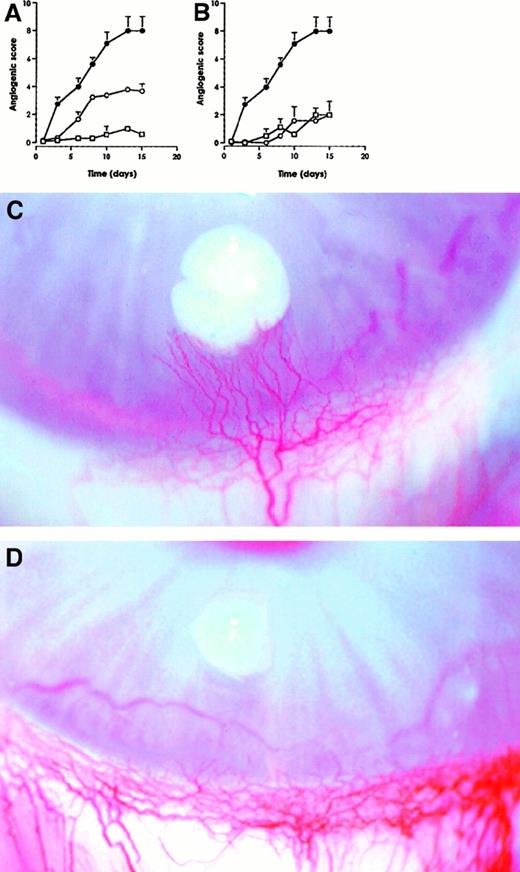
To further investigate the inhibitory effect of IL-10 on angiogenesis, recombinant hIL-10 and vIL-10 proteins were tested for their capacity to modify the angiogenesis induced by VEGF-165. Slow release pellets bearing IL-10 (1000 units per pellet) with or without VEGF-165 (200 ng per pellet) were prepared. Although the 2 forms of IL-10 were devoid of any angiogenic activity per se, angiogenesis induced by VEGF-165 was completely blocked by hIL-10 and inhibited by 50% by vIL-10 (Figure5).
Recombinant hIL-10 and vIL-10 inhibit VEGF-induced angiogenesis.
The 1000 units vIL-10 (A) and hIL-10 (B) were incorporated in slow release pellets in the absence (■) and in the presence (○) of recombinant VEGF-165 (200 ng per pellet), VEGF-165 alone (●). The results represent mean ± SEM of at least 4 independent implants. Representative pictures of VEGF-165–induced neovascularization in the absence (C), and in the presence of hIL-10 (D). Photographs were taken at day 15.
Recombinant hIL-10 and vIL-10 inhibit VEGF-induced angiogenesis.
The 1000 units vIL-10 (A) and hIL-10 (B) were incorporated in slow release pellets in the absence (■) and in the presence (○) of recombinant VEGF-165 (200 ng per pellet), VEGF-165 alone (●). The results represent mean ± SEM of at least 4 independent implants. Representative pictures of VEGF-165–induced neovascularization in the absence (C), and in the presence of hIL-10 (D). Photographs were taken at day 15.
Recombinant interleukin-10 inhibits endothelial cell proliferation
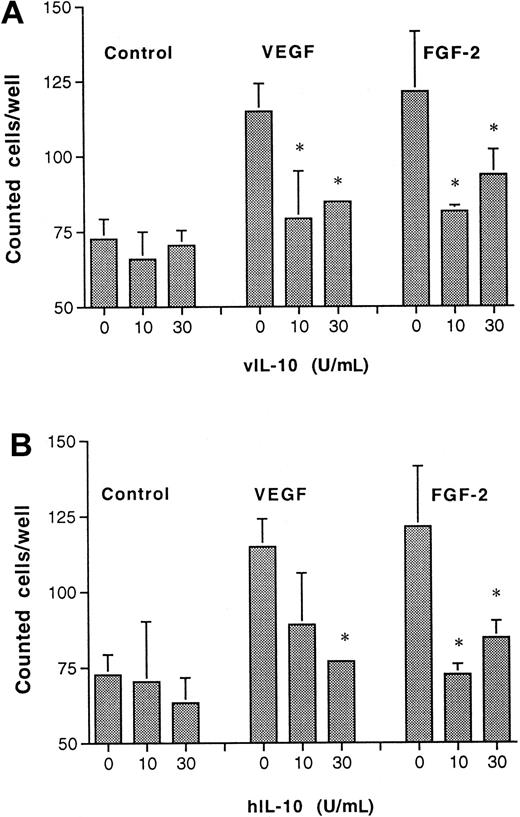
To assess whether the antiangiogenic activity observed in vivo was linked to a direct effect on vascular endothelium, the proliferation of postcapillary endothelial cells (CVECs) in response to IL-10 was evaluated 48 hours after stimulation. Although hIL-10 and vIL-10 (10-30 U/mL) had no inhibitory effect on basal proliferation, both forms were able to inhibit FGF-2– and VEGF-induced growth (Figure6). At higher doses of IL-10 (greater than 30 U/mL), a slight growth-promoting effect was observed (data not shown). Of note, an anti–IL-10 monoclonal antibody (9D7) was able to revert the inhibitory effect of both IL-10 forms on angiogenic factor-induced proliferation (data not shown).
Recombinant vIL-10 and hIL-10 inhibit postcapillary venular endothelial cell proliferation.
Synchronized and subconfluent endothelial cells were cultured with test substances (VEGF or FGF-2) in the absence or in the presence of vIL-10 (A) or hIL-10 (B) for 48 hours. Thereafter, the cells were counted. Data are means ± SEM of 4 experiments. *P < .05 versus proliferation in the absence of IL-10 (Studentt test).
Recombinant vIL-10 and hIL-10 inhibit postcapillary venular endothelial cell proliferation.
Synchronized and subconfluent endothelial cells were cultured with test substances (VEGF or FGF-2) in the absence or in the presence of vIL-10 (A) or hIL-10 (B) for 48 hours. Thereafter, the cells were counted. Data are means ± SEM of 4 experiments. *P < .05 versus proliferation in the absence of IL-10 (Studentt test).
Discussion
In this study, we show that constitutive expression of IL-10 in BL cells significantly suppresses their tumorigenic potential in SCID mice, and relate these observations to a direct antiangiogenic effect of IL-10.
Several studies in murine models have shown that the introduction of the IL-10 gene into tumor cells results in decreased tumorigenicity. The effects of IL-10 are not limited to one tumor cell type, or to syngeneic, allogeneic, or xenogeneic tumor models.9,12,17Here we demonstrate that experimental angiogenesis is inhibited in vitro and in vivo by IL-10–transfected lines, and by recombinant hIL-10 and vIL-10 proteins. Angiogenesis, the physiologic process whereby endothelial cells divide and migrate to form new vessels, is both critical to and significantly parallels tumor growth and metastasis.33-35
By using the rabbit corneal assay for angiogenesis, we demonstrate that the ability of the DG75 line to induce angiogenesis is lost after transfection with either hIL-10 or vIL-10, and that the IL-10 transfectants inhibit the angiogenic capacity of the vector control line when coinjected. We also show that recombinant IL-10 inhibits the angiogenesis induced in vitro and in vivo by purified angiogenic factors. Both IL-10 forms were able to reduce/block the neovascular response induced by VEGF-165. In vitro studies on microvascular endothelial cells demonstrated the direct antiangiogenic activity elicited by IL-10 on proliferation.
In vivo, the effects of IL-10 may be multifold. They can be related to direct inhibition of IL-10 on the angiogenic process per se, or indirectly, by affecting the angiogenic capacity or signals from tumor and/or tumor-infiltrating cells. Compelling evidence indicates that the antiangiogenic effect of IL-10 results from the inhibition of angiogenic factor release/production by the tumor and/or stromal cells. IL-10 induces production of TIMP-1 (an inhibitor of angiogenesis), and inhibits MMP-2 and MMP-9 secretion by prostate cancer cell lines, blocking the induction of microvessel formation in vitro.18,19 Moreover, in murine mammary tumors, the antimetastasic and antitumor activity resulting from IL-10 gene transfer is related to enhanced production of nitric oxide (NO).17 A key determinant of the role of IL-10 in regulation of immune responses lies in its deactivating effect on macrophages. From the many cells and cell products within a tumor that serve as inducers or modulators of angiogenesis, macrophages have emerged as a major component.36 IL-10 prevents macrophage infiltration9 and inhibits the expression of angiogenic factors (VEGF, IL-1β, TNF-α, IL-6, and MMP-9) in tumor-associated macrophages.12 16 These changes correlate with decreased neovascularization of the tumors.
In this study, we demonstrate a direct and indirect contribution of IL-10 in its antiangiogenic activity. Coinjection of IL-10 transfectants inhibited the angiogenicity and tumorigenicity of the mock-transfected line. The local effects of IL-10 were sufficient to inhibit tumor development, whereas the systemic effects only delayed it. Noteworthy, we show that IL-10 can directly inhibit endothelial cell response to angiogenic factors. The molecular mechanisms by which IL-10 inhibits endothelial cells are yet to be defined, we speculate that modification in the expression of receptors for angiogenic factors and/or in activating factor(s) production are potential candidates. In fact, Di Carlo et al16 has shown that VEGF expression is decreased in IL-10–transduced mammary adenocarcinoma tumors. Additionally, IL-10 may affect intracellular signaling pathways. This assumption is supported by the observation that IL-10 inhibits TNF-α–, IL-1β–, and LPS-induced expression of IL-6 and IL-8 in human endothelial cells.37
In this report, there is a partial contribution of NK cells to the antiangiogenic/antitumor effects of IL-10. The tumor incidence was higher in antiasialoGM1-treated mice, though the differences in tumorigenicity between the vector control and IL-10 sublines were still present. NK cells were recently shown to contribute to the antiangiogenic effects of IL-12 through the killing of endothelial cells.38 Because IL-10 did not affect the NK sensitivity of our DG75 transfectants, we propose that a main target for NK cells in vivo may be the endothelial cells involved in neovascularization.
The antitumor effects of IL-10 contrast to the immunomodulatory effects that this cytokine displays in vitro. IL-10 can act as an autocrine factor for human B cell and melanoma cell lines;27-29however, IL-10 had no effect on the in vitro growth of the DG75 B cell line. The tumorigenicity and angiogenicity of the parental cell line were inhibited only when mixed with IL-10–producing cells suggesting that the IL-10 effects relate mostly to its effect in the local immediate environment.
In various tumor models vIL-10, but not IL-10, has been shown to have local immunosuppressive effects.14 39 In this study, hIL-10 and vIL-10 displayed concordant effects both as antitumor and as antiangiogenic agents, suggesting that the differences between vIL-10 and IL-10 may be related to inhibition of immune reactivity by vIL-10.
In conclusion, these results indicate that the potent inhibition of tumor development by IL-10 is a consequence of its direct antiangiogenic activity. These results emphasize the potential benefits of IL-10 in the treatment of BL and other lymphoid malignancies.
Acknowledgments
We thank Drs G. Klein, L. Holmgren, and members of the H.-G. Ljunggren group for helpful discussions. We are thankful for the skillful technical assistance of M. Hagelin and M.-L. Solberg.
Supported by funds from The Swedish Cancer Society, The Lars Hiertas Foundation, The Swedish Medical Research Foundation, The Italian Association for Cancer Research (AIRC), Italian Ministry for University and for Scientific and Technological Research (prot. 9706217225-006), National Research Council (Progetto Finalizzato Biotecnologie), and Azienda Ospedaliera Careggi, Quality Development Project n. 94a. L.C. was supported by The Swedish Institute. J.L.W. was supported by a long-term EMBO fellowship and by the EU.
L.C. and L.M. contributed equally to this paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Maria Teresa Bejarano, Karolinska Institutet, Box 280, S-171 77 Stockholm, Sweden; e-mail: marbej@ki.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal