Abstract
Hirudin, a potent and specific thrombin inhibitor, is a protein of nonhuman origin and therefore potentially immunogenic. The primary objectives of this investigation were to determine the incidence of antihirudin antibodies (ahir-ab) in patients with heparin-induced thrombocytopenia (HIT) who received lepirudin as parenteral anticoagulation and to determine the incidence of death, limb amputation, new thromboembolic complications (TECs), and major hemorrhage in patients who had ahir-ab, compared with patients who were ahir-ab negative. The investigation used data from 2 prospective multicenter studies with the same study protocol, in which HIT patients received 1 of 4 intravenous lepirudin dosage regimens. The treatment duration was 2 to 10 days. Ahir-ab were determined by a newly developed enzyme-linked immunosorbent assay (ELISA). Eighty-seven of 196 evaluable patients (44.4%) had ahir-ab of the IgG class. Development of ahir-ab was dependent on the duration of treatment (ahir-ab–positive patients 18.6 days vs ahir-ab–negative patients 11.8 days; P = .0001). Fewer ahir-ab–positive than ahir-ab–negative patients died (P = .001). Ahir-ab did not cause an increase in limb amputation (P = .765), new TECs (P > .99), or major bleedings (P = .549). In 23 of 51 (45.1%) evaluable patients in whom ahir-ab developed during treatment with lepirudin ( = 12% of all lepirudin treated patients), the ahir-ab enhanced the anticoagulatory effect of lepirudin. Ahir-ab are frequent in patients treated with lepirudin for more than 5 days. Ahir-ab are the first example for a drug-induced immune response causing enhanced activity of a drug. Therefore, during prolonged treatment with lepirudin, anticoagulatory activity should be monitored daily to avoid bleeding complications.
Introduction
Hirudin, an antithrombotic substance produced by the salivary glands of the medicinal leech (Hirudo medicinalis), is the most potent and specific thrombin inhibitor currently known.1
Two r-hirudins have been approved for clinical use recently: desirudin (Revasc, CGP 39393, Ciba Geigy, Basel, Switzerland) for thrombosis prophylaxis of patients after orthopaedic hip surgery2 in the European Union, and lepirudin (Refludan, Hoechst Marion Roussel, Frankfurt, Germany) for further parenteral anticoagulation of patients with heparin-induced thrombocytopenia (HIT) and thromboembolic complications (TECs) in the European Union and the United States.
HIT is a potentially life-threatening adverse effect of heparin treatment. It is generally agreed that HIT is caused by immunologic mechanisms. Typically, immune complexes of IgG antibodies,3 sulfated oligosaccharides (eg, heparin)4,5 and platelet factor 4 (PF4)6,7activate platelets via the Fc-receptor (CD 32) causing intravascular platelet activation, reduced platelet survival, thrombocytopenia,8,9 activation of endothelial cells,7 10 and increased thrombin generation. Affected patients are at risk of new TECs developing.
Although r-hirudins are proteins of nonhuman origin, no antihirudin antibodies (ahir-ab) have been reported in various phase I, II, and III studies.2 11-18 However, most of these patients had been treated with r-hirudin for myocardial infarction or unstable angina for a duration of 72 hours only. r-Hirudins might induce a primary immune response if applied for a longer period, especially in a patient group such as HIT patients, preselected by an immune-mediated adverse drug reaction.
The goals of this investigation were to assess the incidence of ahir-ab in HIT patients treated with lepirudin and the clinical effects of these antibodies.
Patients, materials, and methods
Patients
The investigation included patients with a diagnosis of HIT based on clinical criteria (ie, a decrease in platelet count by more than 30% and/or new TECs during heparin administration) and the presence of HIT antibodies, as defined by a positive heparin-induced platelet activation (HIPA test),19 20 and a definite need for parenteral antithrombotic therapy in therapeutic dose (treatment regimen A1, A2, C) or prophylactic dose (treatment regimen B).
Study design and treatment schedules
Data were obtained in 2 prospective studies21 22following the same protocol. Patients with HIT were treated with 1 of 4 lepirudin (Refludan, Hoechst Marion Roussel) regimens depending on clinical status (Table 1).
Treatment regimen, treatment duration, and incidence of anti-r-hirudin antibodies during first course of hirudin (lepirudin) treatment
| Treatment regimen . | Patients (n) . | Treatment duration median (range) (days) . | Antihirudin-antibody– positive patients/total evaluable patients n (%) . |
|---|---|---|---|
| A1—HIT patients with thrombosis; 0.4 mg/kg bolus then 0.15 mg/kg × h adjusted by aPTT | 116 | 11 (0-104)* | 55/115 (47.8) |
| A2—HIT patients with thrombosis receiving thrombolysis; 0.2 mg/kg bolus then 0.1 mg/kg × h adjusted by aPTT | 9 | 10 (1-57)* | 3/9 (33.3) |
| B—HIT patients without thrombosis; 0.1 mg/kg × h | 61 | 8 (1-67)* | 23/60 (38.3) |
| C—During surgery using cardiopulmonary bypass; 0.25 mg/kg bolus then 5 mg boluses when r-hirudin concentration was below 2.5 μg/mL, followed by regimen B | 12 | 5 (1-25) | 3/12 (25) |
| Total | 198 | 10 (0-104) | 84†/196 (42.9) |
| Treatment regimen . | Patients (n) . | Treatment duration median (range) (days) . | Antihirudin-antibody– positive patients/total evaluable patients n (%) . |
|---|---|---|---|
| A1—HIT patients with thrombosis; 0.4 mg/kg bolus then 0.15 mg/kg × h adjusted by aPTT | 116 | 11 (0-104)* | 55/115 (47.8) |
| A2—HIT patients with thrombosis receiving thrombolysis; 0.2 mg/kg bolus then 0.1 mg/kg × h adjusted by aPTT | 9 | 10 (1-57)* | 3/9 (33.3) |
| B—HIT patients without thrombosis; 0.1 mg/kg × h | 61 | 8 (1-67)* | 23/60 (38.3) |
| C—During surgery using cardiopulmonary bypass; 0.25 mg/kg bolus then 5 mg boluses when r-hirudin concentration was below 2.5 μg/mL, followed by regimen B | 12 | 5 (1-25) | 3/12 (25) |
| Total | 198 | 10 (0-104) | 84†/196 (42.9) |
Length of treatment did not differ between the groups,P = .1.
During or after first course of treatment with lepirudin.
The scheduled treatment duration was 2 to 10 days, but treatment could be prolonged if clinically indicated. After the end of lepirudin treatment, the patient's clinical course was monitored for an additional 2 weeks ± 2 days and a final blood sample was obtained. Patients were assessed clinically on a daily basis. Symptoms of new TECs had to be confirmed by objective methods, ie, phlebography or positive duplex sonography. TECs were not assessed by an adjudicator.
The studies were conducted in accordance with the good clinical practice guidelines of the European Union and the Declaration of Helsinki. The study protocols were approved by the ethics committees of the Medical Councils of the States of the Federal Republic of Germany and by the university ethics committee of the principal investigator (A.G.).
Objectives
The primary objectives of the investigation were (1) to determine the incidence and day of occurrence of ahir-ab in all lepirudin-treated patients and any potentially predisposing factors (age, gender, body weight [bw], mean lepirudin dosage applied per kg bw and h [mg/kg bw × h], treatment duration) and (2) to determine the relevance of ahir-ab for clinical outcome (death, limb amputation, new TECs, major hemorrhage, and allergic reactions) in patients who had ahir-ab during the observation period, compared with ahir-ab–negative patients.
Secondary objectives were to assess the influence of ahir-ab on lepirudin effect as measured by hirudin plasma concentrations and aPTT prolongation; to compare the courses of platelet counts, the required lepirudin dosage, and lepirudin plasma levels in ahir-ab–positive and ahir-ab–negative patients; and to compare the courses in prothrombin fragments F1 + 2 and thrombin-antithrombin (TAT)-complexes in ahir-ab–positive patients and ahir-ab–negative patients.
Laboratory methods
Blood samples were obtained before start of lepirudin treatment (baseline), daily during lepirudin treatment, and 14 days after the end of lepirudin treatment. Platelet counts, aPTT, prothrombin fragment F1 + 2 (Enzygnost, Dade-Behring, Marburg, Germany), and TAT complexes (Enzygnost, Dade-Behring) were measured daily following standard methods. Lepirudin plasma levels and thrombin-hirudin–complex (THC)-concentrations were measured using enzyme-linked immunosorbent assay (ELISA) methodology.23 24
Development of an enzyme-linked immunosorbent assay for detection of antihirudin IgG antibodies
Microtiter plate (Polysorb plates, Nunc) wells were coated with 125 μL of 10 μg/mL lepirudin in coating buffer (8 mmol/L NaH2PO4 × H2O, 53 mmol/L Na2HPO4, pH 7.5) overnight at 18°C to 24°C. Plates were washed 4 times with washing buffer (Product No. OSEW 96, Dade-Behring) and incubated (60 minutes, 37°C) with citrated plasma (100 μL of 1:20 in sample dilution buffer [Product No. OWBE, Dade-Behring]), washed 4 times, and incubated with 100 μL antihuman IgG-POD conjugate (Product No. OWBC 905, Dade-Behring, 60 minutes, 37°C, 1:51 in sample dilution buffer). After washing 4 more times, microtiter plates were incubated with 100 μL per well substrate solution (3,3′,5,5′-tetramethylbenzidine in substrate buffer, Product No. OUVG, Dade-Behring) for 30 minutes at room temperature in the dark. The reaction was stopped by adding 100 μL 0.5 mol/L H2SO4 and optical densities were measured at 450 nm (650 nm reference). Test conditions were established using a rabbit-antihirudin-IgG-Fab′ human IgG (antibody conjugate from the Fab′ portion of antihirudin-rabbit IgG and human-IgG) as a synthetic standard and a positive control. Arbitrary antibody concentrations were obtained by using a standard curve of diluted rabbit–antihirudin-IgG-Fab′ human IgG.
Cutoff values were defined on the basis of the plasma samples from 200 healthy blood donors. Assay results were deemed negative or positive (99% quantile of blood donor data). Each sample with positive test results was retested in a confirmatory assay by preincubation with lepirudin in increasing concentrations and only samples with a concentration-dependent decrease in test signal were judged as specifically ahir-ab positive. To ensure assay reproducibility, all samples and standards were tested in triplicate and only data with intra-assay optical density coefficient of variation less than or equal to 15% were accepted.
Statistical analysis
The day of occurrence of ahir-ab was determined relative to the first day of lepirudin treatment (day 1). The one-dimensional statistical tests performed to analyze for possible dependence of the occurrence of ahir-ab on various factors were as follows: 2 × 3 field chi-square test for gender; Wilcoxon rank sum test for age; chi-square test for treatment regimens A1, A2, B, and C; Wilcoxon rank sum test for treatment duration and mean lepirudin dosages applied per kg bw × h; and the Wilcoxon signed rank test for mean lepirudin dosages applied per kg bw × h before versus after formation of ahir-ab. The median hirudin plasma levels (arbitrary units) before and after formation of ahir-ab were determined by the area under the curve and compared by the Wilcoxon signed rank test. Incidences of death, limb amputations, and new thromboembolic events in ahir-ab–positive patients and ahir-ab–negative patients were compared using Fisher's exact test (2-tailed).
To further define possible predictive factors for development of ahir-ab, the following parameters were compared in ahir-ab positive and ahir-ab negative patients by discriminant analysis25: age, gender, body weight, treatment duration, mean lepirudin dosages (mg/ kg bw × h), aPTT levels, platelet counts, hirudin plasma levels, and concentrations of TAT complexes, prothrombin fragment F1 + 2, and THC.
The following periods were assessed: (a) total observation period = start of lepirudin treatment until end of observation period, (b) treatment period in patients with ahir-ab, (c) time from start of treatment until detection of ahir-ab, (d) time from detection of ahir-ab until end of lepirudin treatment, and (e) time from detection of ahir-ab until end of observation period. Statistical analysis was performed using the SAS package, Version 6.12 (SAS Institute, Cary, NC).
Results
Evaluation of enzyme-linked immunosorbent assay for detection of antihirudin antibodies
According to the 99% quantile definition, the cutoff for a positive antibody assay was defined as 4.55 (arbitrary units according to standard curve). A new standard curve was obtained with each assay. Intra-assay optical density coefficients of variation were less than 10% in all tests. By preincubation of the standard with lepirudin and of all ahir-ab–positive patient plasma samples in different concentrations, a concentration-dependent decrease in test signals occurred, confirming the specificity of the assay for ahir-ab.
Patients and incidence of antihirudin antibodies
A total of 198 consecutive patients (84 male, 114 female) treated with lepirudin were investigated. 185 patients received one treatment course, 12 patients received 2 treatment courses, and one patient received 4 treatment courses. Data regarding treatment schedules, duration of treatment with lepirudin, and incidence of ahir-ab are given in Table 1. Antibody tests could not be performed in 2 patients (one patient with treatment regimen A1, one patient with treatment regimen B). Eighty-seven of the 196 evaluable patients (44.4%) developed ahir-ab of the IgG class (84 patients during or after the first course of lepirudin treatment, 3 patients during the second course).
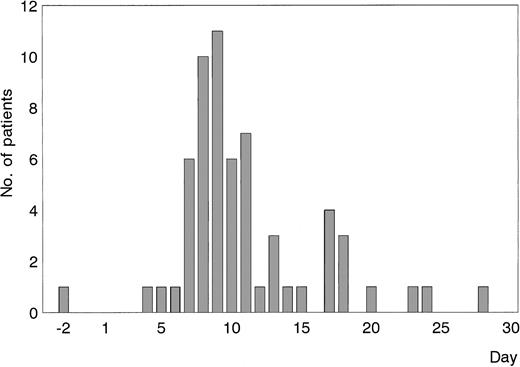
Fifty-four patients became ahir-ab positive during the first course of lepirudin treatment, from day 4 with a maximum at days 8 and 9 (Figure1). Seven patients were first found to be ahir-ab positive on the day of cessation of lepirudin, and 20 patients during the follow-up period. In 2 additional patients, the first day of ahir-ab detection was missing, and in one patient the end of treatment date was missing. One patient was ahir-ab positive before the start of treatment. This patient had not been treated with lepirudin before, but contact with leeches could not be excluded.
First occurrence of ahir-ab of the IgG class measured by an ELISA as described under “Patients, materials, and methods.”
Eighty-four patients had ahir-ab develop during the first course of treatment with r-hirudin. Sixty-one of these were evaluable for determination of ahir-ab on the first day of lepirudin treatment (n = 54) or on the day of cessation of lepirudin (n = 7). The 20 patients who became ahir-ab positive during the follow-up period are not included in this Figure.
First occurrence of ahir-ab of the IgG class measured by an ELISA as described under “Patients, materials, and methods.”
Eighty-four patients had ahir-ab develop during the first course of treatment with r-hirudin. Sixty-one of these were evaluable for determination of ahir-ab on the first day of lepirudin treatment (n = 54) or on the day of cessation of lepirudin (n = 7). The 20 patients who became ahir-ab positive during the follow-up period are not included in this Figure.
Variables influencing occurrence of ahir-ab
Discriminant analysis between ahir-ab–positive and ahir-ab–negative patients showed that ahir-ab development was not influenced by age (P = .265), gender (P = .676), body weight (P = .898), or mean lepirudin dosages per kg bw × h (0.1 mg [range, 0.006-0.324] vs 0.1 mg [range, 0.014-0.227], P > .99). Treatment duration (18.6 days, [range, 2-65] vs 11.8 days [range, 1-68],P = .0001) was, however, associated with the development of ahir-ab.
Clinical associations of ahir-ab
Fewer ahir-ab–positive patients than ahir-ab–negative patients died (1 of 84 [1.2%; 95% CI 0.03%-6%] vs 16 of 112 [14.3%; 95% CI 8%-22%], P = .001). Seven of 112 patients died after day 8, the average time point of development of ahir-abs.
There were no major differences between ahir-ab–positive and ahir-ab–negative patients with regard to limb amputations (6 of 84 [7.1%; 95% CI 3%-15%] vs 6 of 112 [5.4%; 95% CI 2%-11%],P = .765), new TECs (9 of 84 [10.7%; 95% CI 5%-19%] vs 11 of 112 [9.8%; 95% CI 5%-17%], P > .99), or major bleeding complications (11 of 84 [13.1%; 95% CI 7%-22%] vs 19 of 112 [17.0%; 95% CI 10%-25%], P = .549).
Eight patients in the study developed allergic reactions. Three of these patients showed maculopapular exanthema, allergic exanthema on the back and on the leg, or a rash. For the other 5 patients, no data were available. Ahir-ab were not detected in any of these patients.
Ahir-ab and anticoagulation
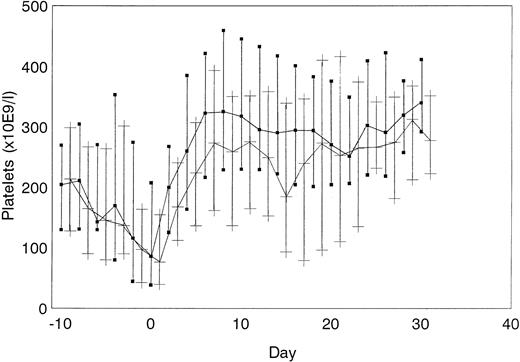
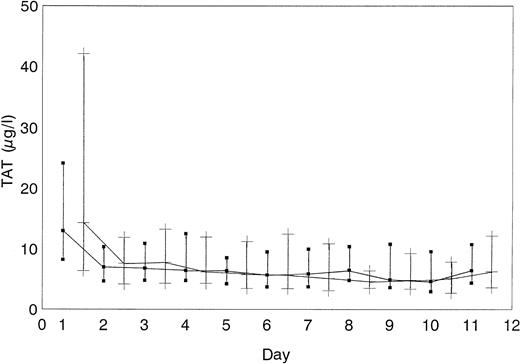
The course of platelet count normalization did not differ between the 2 groups (Figure 2). Concentrations of F1 + 2 fragments (data not shown) and TAT complexes (Figure 3) did not differ between ahir-ab–positive and ahir-ab–negative patients, indicating that there was no increased thrombin generation in ahir-ab positive patients, compared with ahir-ab–negative patients. Patients in whom ahir-ab developed during treatment with lepirudin (n = 54) required a dosage of 0.13 mg/kg bw × h lepirudin (median) before ahir-ab formation and a dosage of 0.09 mg/kg bw × h lepirudin (median) after ahir-ab formation (P = .1). The median lepirudin plasma levels in these patients also did not change after ahir-ab formation (637.7 to 579.7 arbitrary units, P = .1).
Platelet count normalization.
Course of platelet count normalization in ahir-ab–positive (▪) and in ahir-ab–negative patients (+) (median and quartiles are given).
Platelet count normalization.
Course of platelet count normalization in ahir-ab–positive (▪) and in ahir-ab–negative patients (+) (median and quartiles are given).
TAT complex concentrations.
TAT complex concentrations did not differ between ahir-ab–positive (▪) and in ahir-ab–negative patients (+) (median and quartiles are given).
TAT complex concentrations.
TAT complex concentrations did not differ between ahir-ab–positive (▪) and in ahir-ab–negative patients (+) (median and quartiles are given).
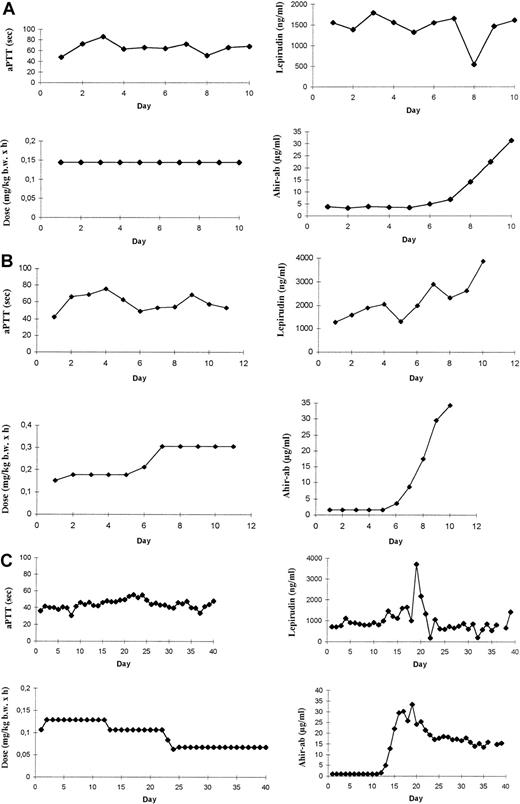
However, the patients could be classified into 3 groups. No relationship between ahir-ab and aPTT levels were observed in 25 of the 51 evaluable patients (49.0%) with ahir-ab during treatment with lepirudin. In 3 of 51 patients (5.9%), concomitant to the increase in ahir-ab, either the aPTT decreased from 80 to 62 seconds, whereas lepirudin dosage was stable or the lepirudin dosage had to be increased by 20% and 22%, respectively, to maintain the aPTT in the therapeutic range. And in 23 of 51 patients (45.1%) associated to the development of ahir-ab, a sharp increase in aPTT (n = 8) was observed when lepirudin dosage was stable, or, if the dosage was adjusted according to aPTT values, a decrease in lepirudin dosage by 45% (mean) (range, 17% to 90%) was necessary to maintain the aPTT within the therapeutic range (n = 15). Data of 3 representative patients are given in Figure 4A,B,C.
APTT, lepirudin dosages, hirudin plasma levels, and ahir-ab concentrations.
Courses of aPTT (sec), lepirudin dosages (mg/kg bw × h), hirudin plasma levels (ng/mL), and ahir-ab concentrations (μg/mL) are given for (A) a patient with ahir-abs but no effect on aPTT levels; (B) a patient with a decrease in aPTT concomitant with the increase in ahir-ab concentration; and (C) a patient with a decrease in lepirudin dosage after formation of ahir-abs to maintain the aPTT within the therapeutic range.
APTT, lepirudin dosages, hirudin plasma levels, and ahir-ab concentrations.
Courses of aPTT (sec), lepirudin dosages (mg/kg bw × h), hirudin plasma levels (ng/mL), and ahir-ab concentrations (μg/mL) are given for (A) a patient with ahir-abs but no effect on aPTT levels; (B) a patient with a decrease in aPTT concomitant with the increase in ahir-ab concentration; and (C) a patient with a decrease in lepirudin dosage after formation of ahir-abs to maintain the aPTT within the therapeutic range.
Re-exposure to lepirudin
Eight patients with persistent ahir-ab required reexposure to lepirudin for parenteral anticoagulation. Two of the patients were ahir-ab negative at the beginning of reexposure to lepirudin, whereas 3 patients remained ahir-ab positive up to reexposure. For 3 additional patients, data with regard to the ahir-ab levels at the beginning of reexposure to lepirudin were not available.
None of these reexposed patients experienced allergic symptoms, death, limb amputation, a new TEC, or major bleeding during reexposure to lepirudin.
Persistence of ahir-ab
Follow-up samples were taken 6 months after the start of lepirudin treatment from 45 patients with ahir-ab. Fifteen patients (33.3%) showed a positive antibody assay in the follow-up sample. In one patient, ahir-ab were still present 415 days after cessation of lepirudin treatment.
Discussion
Prospective data from 198 patients treated with the r-hirudin lepirudin for a median duration of 10 days were assessed with a newly developed ELISA for detection of ahir-ab of the IgG class. The ELISA specificity was demonstrated by inhibition of antibody binding by free lepirudin. The intra-assay variation was less than 10%.
Of the 196 evaluable patients, 44.4% had ahir-ab. These findings are in contrast to data obtained before large clinical trials with recombinant hirudins were performed and indicated low immunogenicity of r-hirudin. No ahir-IgG or -IgM antibodies were observed in baboons after 4 intravenous injections of natural or r-hirudin at day 1, 3, 8, and 42.26 In this study, tests for detection of hirudin-specific antibodies were performed using serum samples obtained before, after each, and 1 week after the last injection. In humans, no r-hirudin–specific antibodies were found after a single parenteral bolus exposure to natural hirudin in 12 healthy volunteers.27 Similarly, in a study with 263 healthy immunocompetent volunteers without a history of allergy to hirudin, no ahir-IgG-ab were detectable after exposure to the r-hirudin desirudin (CGP 39393, Revasc, Ciba Geigy Ltd, Basel, Switzerland) subcutaneously, as an intravenous bolus, or as a continuous infusion for 6 or 72 hours.28 Even after a second exposure to desirudin in 195 volunteers and a third exposure in 5 volunteers, no ahir-ab were measured. In this study, the blood samples for measurement of hirudin-specific antibodies were obtained from all volunteers immediately before and 28-56 days after exposure to CGP 39393. Finally, no ahir-ab were detected in a study of 39 patients with coronary artery disease 2 weeks after a 6-hour infusion of rhirudin (CGP 39393).14
In each of these studies, patients received r-hirudin for fewer than 4 days. Our data indicate that this period is too short to induce an ahir-ab response in nonimmunized patients as ahir-ab were detectable at the earliest after 4 days treatment. Patients in the 2 studies investigated here received lepirudin for an average of 10 days and the treatment time was longer in ahir-ab positive than in ahir-ab negative patients (18.6 days vs 11.8 days; P = .0001), corroborating the hypothesis that the duration of hirudin application is a major factor determining the development of ahir-ab.
As HIT causes a massive increase in generation of thrombin29 as demonstrated by the high TAT levels of our patients (Figure 3), enhanced generation of THCs might be a further reason for the high incidence of ahir-ab in HIT patients, compared with other patient groups treated with r-hirudin.
Although the medical history of the patients did not reveal an increased incidence of autoimmune or allergic diseases, HIT patients may have an increased risk of developing an immune response to drugs.
In the same patient population (ie, HIT patients treated with lepirudin), Huhle et al30 and Song et al31 also found a high incidence of ahir-ab. The higher percentage of ahir-ab–positive patients (20 of 24; 74.1%) found by Huhle et al30 might be caused by the small number of patients investigated.
In addition to treatment duration, production mode and purity of a r-hirudin preparation might also be cofactors influencing immunization. However, no data are available on ahir-ab in patients treated with any other r-hirudins under similar study conditions.
Clinically, ahir-ab–positive patients showed a decreased death rate. This observational finding was not a predefined study endpoint. We speculate that this was either a chance finding or the possible explanation (i) that patients with a poor prognosis already had a suppressed immune system because of the severity of their underlying disease, which prevented formation of ahir-ab or (ii) that the formation of ahir-ab may be beneficial. Early mortality of ahir-ab–negative patients did not account for these differences. Ahir-ab–positive patients did not show an enhanced risk of limb amputations, new TECs, or major bleeding complications, compared with ahir-ab–negative patients. Also, symptoms of allergic reactions were only observed in ahir-ab–negative patients. As this investigation assessed only ahir-abs of the IgG class, this does not exclude ahir-ab of the IgE- or IgA-class.
The most important finding we made was the necessity to reduce the lepirudin dosage (up to 90%) after generation of ahir-ab in 23 of 51 evaluable patients (45%) who had developed ahir-ab during treatment with lepirudin, corresponding to 12% of all lepirudin-treated patients. Because r-hirudin is largely excreted renally,32we speculate that ahir-ab/lepirudin complexes are too large for renal excretion, resulting in prolongation of the half-life of the drug. This has been shown for recombinant hirudin (CGP 39393) in a 3-month intravenous tolerability study in dogs,33 PEG conjugated r-hirudin,34 and for r-hirudin genetically fused to albumin with higher molecular weight.35 It is possible that the reduced renal excretion of r-hirudin after complex formation with ahir-abs is compensated in some patients by a neutralizing effect of the ahir-ab or phagocytosis of lepirudin–ahir-ab complexes.
This prospective study shows that ahir-ab occurred in nearly half of the patients with HIT who received the r-hirudin lepirudin for further parenteral anticoagulation for more than 5 days. This is of clinical importance because lepirudin has recently been approved for HIT patients in the European Union and the United States. Furthermore, r-hirudin might be used in a large number of patients for thrombosis prophylaxis after hip replacement surgery.36 Reexposing patients with preformed antibodies to lepirudin did not cause allergic reactions in this study; however, the number of patients investigated is too small to exclude the possibility of allergic reactions. All patients who receive lepirudin a second time should therefore be monitored initially for anaphylactic reactions.
The investigation also is the first example demonstrating that drug-induced antibodies can enhance the pharmacologic activity of a drug. The current data do not indicate that lepirudin-treated patients need to be monitored for ahir-ab for safety reasons. However, although r-hirudin allows consistent dosage administration in most patients, daily monitoring of aPTT on and after day 5 of treatment is suggested because ahir-ab may enhance the pharmacologic effect of r-hirudin, thus increasing the risk of bleeding complications.
Supported by the Deutsche Forschungsgemeinschaft Gr 1096/2-3, by a research stipendium of the Sandoz foundation, and by Hoechst Marion Roussel.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andreas Greinacher, Institute for Immunology and Transfusion Medicine, Ernst-Moritz-Arndt-University, Sauerbruchstr./Diagnostikzentrum, 17487 Greifswald, Germany; e-mail:greinach@mail.uni-greifswald.de.