Abstract
Interaction between CD40 and the CD40 ligand (CD40L) is critical for the survival and proliferation of B cells during immunopoiesis. However, the role of CD40L in the pathogenesis of malignant lymphomas is ambiguous. Primary mantle cell lymphoma (MCL) cells were cultured in the presence of recombinant human CD40L trimer (huCD40LT), and a significant time- and dose-dependent induction of DNA synthesis was observed in thymidine incorporation assays (n = 7,P < .04). The maximal rate of DNA synthesis was reached at huCD40LT doses of 100 ng/mL and above after 4 days of culture, but a significant increase of DNA synthesis was detected already at doses of 1 ng/mL (P = .03). HuCD40LT never inhibited the basal level of DNA synthesis. These findings established 400 ng/mL of huCD40LT for 4 days as standard conditions in the system. Under these conditions, huCD40LT significantly increased the proportion of cells in the S/G2/M phases of the cell cycle in 4 of 7 studied cases, while the fraction of apoptotic cells remained unchanged (n = 7). HuCD40LT also induced expression of CD80/B7-1, CD86/B7-2, and CD95/Fas and up-regulated the expression of HLA-DR (n = 6). With the use of bromodeoxyuridine incorporation in triple-color flow cytometric analysis, it was found that huCD40LT induced cell-cycle progression in light chain–restricted cells only, of which a median of 14% (range, 0.5% to 29%; n = 4) returned to G0/1 phase DNA content after bromodeoxyuridine incorporation, demonstrating completion of at least one cell cycle in the presence of huCD40LT. Thus, primary clonal MCL cells are activated and can proliferate in the presence of huCD40LT as a single agent.
Introduction
Mantle cell lymphoma (MCL) is a distinct CD5+ B-cell non-Hodgkin lymphoma (NHL) with low chemosensitivity and a very poor prognosis, even when treated with high-dose therapy and autologous bone marrow transplantation.1-4 MCL is characterized by the translocation t(11;14)(q13;q32), leading to overexpression of cyclin D1 and subsequent deregulation of the critical cyclin D1/Retinoblastoma protein pathway of the cell cycle.5-7 Cyclin D1 overexpression, however, is neither tumorigenic per se in mouse models nor related to the proliferative activity in MCL, suggesting that the malignant behavior of the disease depends on further growth deregulation of MCL cells.8,9 CD40/CD40 ligand (CD40L) interaction is critical for the survival and proliferation of B cells engaged in secondary immune responses.10-13 In lymphoma cell lines, activation of CD40 can induce phosphorylation of Retinoblastoma protein, suggesting a role for CD40L in G1/S phase transition and in lymphomagenesis.14 The role of CD40 activation, however, and in particular the data on the effects on growth and apoptosis in normal and malignant B cells are conflicting.15 For example, CD40 ligation results in cell-cycle progression in resting B cells and rescue from apoptosis in normal germinal center B cells.16,17 By contrast, a direct growth-inhibitory effect was observed in diffuse large cell NHL, Burkitt lymphoma (BL), and Epstein-Barr virus–transformed cell lines after stimulation using the soluble CD40L.18-21 However, soluble CD40L induced DNA synthesis in primary follicular lymphoma cells.21 At least 2 factors are important for the response to CD40 ligation. First, different CD40 ligands, agonistic antibodies, or recombinant proteins, as well as different CD40 systems, using transfected and irradiated cell lines for presenting the CD40 ligand, have been shown to influence the effect of CD40 stimulation differently.22-24 The transfected cell lines influence the effect of CD40 ligation by expression or secretion of other B-cell stimulatory molecules. Second, the functional consequences of CD40 signaling appear to be highly dependent on the B-cell type being triggered.15,25 In 2 previous studies of CD40 ligation in MCL, agonistic anti-CD40 antibody and CD40L-presenting cell lines both failed to induce proliferation, as estimated by thymidine incorporation and relative cell numbers, respectively.26,27 Besides growth regulation, CD40L plays a critical role in the activation of antigen-presenting cells, including B cells.28 On normal and malignant B cells, CD40 ligation induces expression of co-stimulatory molecules, such as CD80/B7-1 and CD86/B7-2, the expression of which is essential for appropriate and efficient antigen presentation during cognate B-cell/T-cell interactions, and the generation of secondary immune responses.29-31 Therefore, the possible benefits of ex vivo and in vivo CD40 activation and the subsequent induction of an antigen-presenting phenotype in malignant B cells are under investigation in phase 1 trials in low-grade NHL and in B-cell chronic lymphocytic leukemia (CLL).32-34 Here, we have used a soluble recombinant human CD40L trimer (huCD40LT) to study the functional consequences of CD40 ligation in terms of activation, proliferation, and apoptosis in primary MCL cells in vitro. We demonstrate that huCD40LT as a single agent induces significant proliferation of lymphoma cells in primary MCL cell cultures.
Patients, materials, and methods
Patients
Eight patients diagnosed with MCL in the leukemic phase according to the Revised European-American Lymphoma classification were studied (Table1).1 All patients had been off treatment for more than 30 days at the time of sample procurement. The study was performed in accordance with protocols approved by the regional ethics committee, and all patients gave their informed consent to participate.
Patient characteristics
| UPN# . | Sex/Age/Stage* . | Cytology . | Immunophenotype . | Cyclin D1 . |
|---|---|---|---|---|
| 1 | M/57/IV | Typical | CD5+CD23−, Ig Bright | Not found |
| 2 | F/85/IV | Typical | CD5+CD23−, Ig Bright | Not found |
| 3 | F/88/IV | Typical | CD5+CD23−, Ig Bright | Cyclin D1 RNA↑ |
| 4 | M/74/IV | Typical | CD5+CD23−, Ig Bright | Cyclin D1 RNA↑ |
| 5 | M/59/IV | Typical | CD5+CD23−, Ig Bright | Cyclin D1 RNA↑ |
| 6 | F/61/IV | Typical | CD5+CD23−, Ig Bright | t(11;14) MTC |
| 7 | M/66/IV | Typical | CD5+CD23−, Ig Bright | Cyclin D1 RNA↑ |
| 8 | M/53/IV | Blastic | CD5+CD23−, Ig Bright | t(11;14) MTC |
| UPN# . | Sex/Age/Stage* . | Cytology . | Immunophenotype . | Cyclin D1 . |
|---|---|---|---|---|
| 1 | M/57/IV | Typical | CD5+CD23−, Ig Bright | Not found |
| 2 | F/85/IV | Typical | CD5+CD23−, Ig Bright | Not found |
| 3 | F/88/IV | Typical | CD5+CD23−, Ig Bright | Cyclin D1 RNA↑ |
| 4 | M/74/IV | Typical | CD5+CD23−, Ig Bright | Cyclin D1 RNA↑ |
| 5 | M/59/IV | Typical | CD5+CD23−, Ig Bright | Cyclin D1 RNA↑ |
| 6 | F/61/IV | Typical | CD5+CD23−, Ig Bright | t(11;14) MTC |
| 7 | M/66/IV | Typical | CD5+CD23−, Ig Bright | Cyclin D1 RNA↑ |
| 8 | M/53/IV | Blastic | CD5+CD23−, Ig Bright | t(11;14) MTC |
UPN# indicates unique patient number; M, male; F, female; Ig Bright, moderate to high expression of surface membrane-bound immunoglobulin; cyclin D1 RNA↑, cyclin D1 RNA overexpression; and t(11;14) MTC, translocation (11;14) with breakpoints in the major translocation cluster detected by PCR analysis.
Stage at procurement of peripheral blood samples.
Sample preparation
Mononuclear cells were isolated from peripheral blood samples by density gradient centrifugation, washed, resuspended, and frozen in RPMI 1640 medium (Gibco, Grand Island, NY) containing 45% fetal calf serum and 15% dimethylsulfoxide supplemented with antibiotics. Cells were frozen to −80°C using an automated cell freezer (FTS-systems, Stone Ridge, NY) and stored in liquid nitrogen. Before use, the cells were thawed, washed, and resuspended in RPMI 1640 medium containing 10% fetal calf serum and antibiotics. The percentage of CD5+/CD19+ MCL cells in the mononuclear fraction was always higher than 90%, and no additional non–B-cell depletion was performed.
Polymerase chain reaction (PCR) to detect t(11;14) and cyclin D1 RNA overexpression
Semi-nested PCR analysis of MCL patients carrying the t(11;14) with breakpoints in the major translocation cluster was performed as described previously.3 Cyclin D1 RNA overexpression was detected using a semiquantitative reverse transcription (RT)-PCR, which competitively amplified cyclin D1, D2, and D3, as described.35 Non-MCL cases of NHL, CLL, and normal donor samples were used as negative controls. Six of 8 patients had the t(11;14) translocation or cyclin D1 RNA overexpression by PCR analysis (Table 1). In the remaining 2 patients (nos. 1 and 2), the diagnosis of MCL was based on pathology and the CD5+/CD19+, CD23−, surface immunoglobulin bright immunophenotype, which was detected in all 8 patients (Table 1).
Determination of expression of surface membrane molecules
Phycoerythrin (PE)- or fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies against cell surface markers (kappa and lambda light chains, CD19, CD5, CD23, CD40, CD80, CD86, CD95/Fas, CD3, and CD154/CD40L) (Becton Dickinson, Mountain View, CA; Dako, Glostrup, Denmark) and the relevant isotype-matched controls were used in standard flow cytometry analysis, with inclusion of all events larger than 52 on forward scatter and no additional gating (FACSort; Becton Dickinson). A total of 5000 cells were acquired and analyzed using CellQuest software (Becton Dickinson).
Cell culture assays
Isolated MCL cells were plated at a concentration of 2 × 106 cells per milliliter medium in the presence or absence of various concentrations of purified huCD40LT (Immunex Corporation, Seattle, WA) and anti-CD40L antibodies (Becton Dickinson, Belgium) and incubated at 37°C in a humidified atmosphere containing 5% CO2. For thymidine incorporation, stimulated and unstimulated control cultures were plated in triplicate in 96-well, 200-μL U-bottom plates and pulsed with 1 μCi [methyl-3H] thymidine (3HTdR) (TRK 637; Amersham, Cardiff, UK) 16 hours before harvest, and thymidine incorporation was measured on a direct beta-counter (Matrix 9600; Packard, Meriden, CT). For quantification of cells undergoing programmed cell death (apoptosis) and cells in the S/G2/M phase of the cell cycle, isolated nuclei from cells from single cultures were stained with propidium iodide (PI) and analyzed by flow cytometry, as described.36
Viable cell recovery
PI exclusion assays of whole-cell preparations from single MCL cell cultures were used to define which cells in the scatter profiles were viable with complete exclusion of PI. Two distinct populations were defined in the scatter profiles based on cells with and without PI exclusion. Viable cells were significantly larger and excluded PI completely. In our recovery assay, isolated cells were plated in triplicate in 50-mL culture flasks at a concentration of 2 × 106 cells/mL with and without huCD40LT in a final volume of 5 mL for up to 2 weeks. Cells sized between 8 and 20 μmol/L were counted 3 times using a Coulter Z1 particle counter (Coulter Electronics Limited, Luton, UK). The averages of these 9 counts were used to calculate the viable cell recovery in the cultures at different time points.
Flow cytometric analysis of monoclonality, DNA synthesis, and cell-cycle progression
Multiparameter flow cytometry analysis with combined measurement of clonality, DNA synthesis, and DNA content was carried out essentially as described.37 38 In brief, primary MCL cells were cultured for 4 days in single cultures in the presence or absence of 400 ng/mL of huCD40LT and supplemented with 50 μmol/L bromodeoxyuridine (BrdUrd) (Sigma, St Louis, MO) for an additional 24 hours of culture. After harvest, the cells were first stained with PE-conjugated anti-kappa or anti-lambda antibodies. The cells were then permeabilized and fixed in a mixture of 1% paraformaldehyde and 0.05% Nonidet P40. Incorporation of BrdUrd, as evidence of DNA synthesis, was then detected using anti-BrdUrd FITC-labeled antibodies (Becton Dickinson) after 1 mg/mL DNase I (DN-25; Sigma) digestion for 30 minutes. DNA content was measured using the DNA-specific dye 7-amino-actinomycin-D (7-AAD) (Sigma). For each sample, 20 000 events were acquired and analyzed (FACS Vantage; Becton Dickinson). To exclude nuclei without proliferative capacity, we defined an acquisition gate such that only events from the G1 peak and brighter were acquired. This gate contained approximately 70% of the total events. After gating on lymphoma cells expressing the clonally restricted light chain of the particular MCL case, a combined analysis of DNA synthesis and DNA content was performed.
Statistical analysis
The nonparametric Wilcoxon signed rank sum test was used to compare assay results from samples cultured under different conditions.
Results
HuCD40LT specifically induces DNA synthesis in primary MCL cell cultures
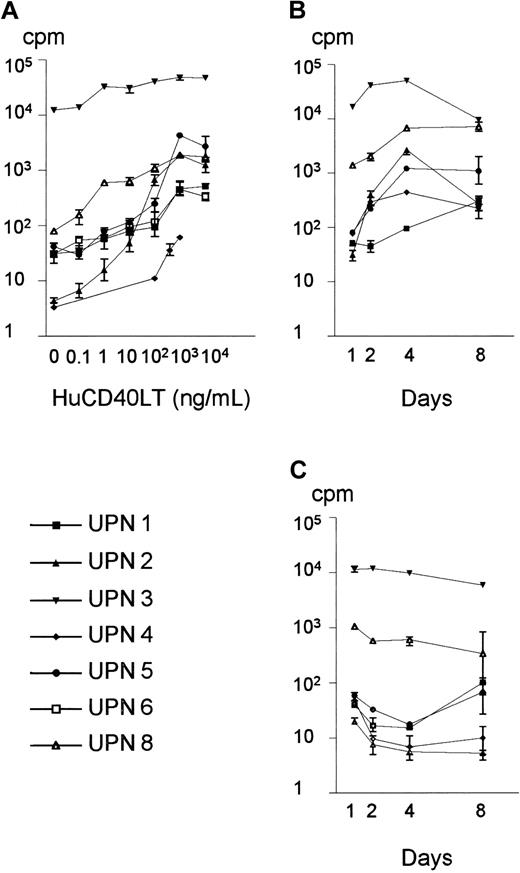
We first applied a thymidine incorporation assay to assess whether huCD40LT could induce DNA synthesis in primary MCL cultures. The quality of the response was homogeneous, and huCD40LT induced dose-dependent thymidine incorporation in all of the 7 MCL samples studied (Figure 1A). The quantity of the response, however, was heterogeneous among the individual cases. The maximal rate of DNA synthesis (mean, 8162 cpm; range, 61-48 000 cpm) was reached at huCD40LT doses between 100 ng/mL and 1000 ng/mL and was significantly higher than the DNA synthesis in the presence of medium alone (mean, 1813 cpm; range, 4-12 500 cpm; P = .03, Wilcoxon) (Figure 1A). We never observed any direct inhibitory effects of huCD40LT on the basal DNA synthesis in primary MCL cell cultures, not even at doses of huCD40LT up to 10 000 ng/mL (Figure 1A). The ability of huCD40LT to induce DNA synthesis in purified leukemic cells was significantly higher in MCL cultures than in cultures from patients with CLL (data not shown). Kinetic studies, using a fixed dose of 400 ng/mL of huCD40LT, showed that the induction of DNA synthesis was also dependent on time and reached a maximal response at day 4 (P < .04, Wilcoxon) (Figure 1B). Significant spontaneous synthesis of DNA could not be demonstrated within the first week of culture (Figure 1C). The specificity of the response was demonstrated in co-cultures, in which the combined presence of 100 μg/mL of anti-CD40L antibody and 400 ng/mL of huCD40LT reduced the huCD40LT-induced DNA synthesis to the level of the unstimulated control (data not shown). On the basis of these results, we chose 400 ng/mL of huCD40LT for 4 days as the standard conditions for further experimentation.
Dose-response assay and kinetics of huCD40LT stimulation of primary MCL cell cultures.
(A) Dose-response assay: Isolated mononuclear cells from 7 MCL patients were plated at 2 × 106 cells/mL and cultured in the absence or presence of increasing doses of huCD40LT for 4 days. Cultured cells were pulsed with 3HTdR 16 hours before harvest and measurement of thymidine incorporation. The maximal response in the presence of huCD40LT (mean, 8162 cpm; range, 61-48 000 cpm) was obtained at doses between 100 and 1000 ng/mL, and huCD40LT-induced 3HTdR incorporation was significantly higher than in the controls (mean, 1813 cpm; range, 4-12 500 cpm) from doses of 1 ng/mL and above (P = .03, Wilcoxon). Data are shown as mean and range of the triplicate cultures. (B) Kinetics: Mononuclear cells from 6 MCL patients were plated at 2 × 106 cells/mL and cultured in the presence of 400 ng/mL of huCD40LT, and 3HTdR incorporation was measured after 1, 2, 4, or 8 days of culture. The difference between days 1 and day 4 was significant (P < .04, Wilcoxon). Data are shown as mean and range of the triplicate cultures. (C) Spontaneous DNA synthesis: Control cultures were plated at 2 × 106cells/mL in medium alone, and 3HTdR incorporation was measured after 1, 2, 4, or 8 days of culture. No significant spontaneous 3HTdR incorporation could be demonstrated as compared with the stimulated cultures. Data are shown as mean and range of triplicate cultures. UPN indicates unique patient number.
Dose-response assay and kinetics of huCD40LT stimulation of primary MCL cell cultures.
(A) Dose-response assay: Isolated mononuclear cells from 7 MCL patients were plated at 2 × 106 cells/mL and cultured in the absence or presence of increasing doses of huCD40LT for 4 days. Cultured cells were pulsed with 3HTdR 16 hours before harvest and measurement of thymidine incorporation. The maximal response in the presence of huCD40LT (mean, 8162 cpm; range, 61-48 000 cpm) was obtained at doses between 100 and 1000 ng/mL, and huCD40LT-induced 3HTdR incorporation was significantly higher than in the controls (mean, 1813 cpm; range, 4-12 500 cpm) from doses of 1 ng/mL and above (P = .03, Wilcoxon). Data are shown as mean and range of the triplicate cultures. (B) Kinetics: Mononuclear cells from 6 MCL patients were plated at 2 × 106 cells/mL and cultured in the presence of 400 ng/mL of huCD40LT, and 3HTdR incorporation was measured after 1, 2, 4, or 8 days of culture. The difference between days 1 and day 4 was significant (P < .04, Wilcoxon). Data are shown as mean and range of the triplicate cultures. (C) Spontaneous DNA synthesis: Control cultures were plated at 2 × 106cells/mL in medium alone, and 3HTdR incorporation was measured after 1, 2, 4, or 8 days of culture. No significant spontaneous 3HTdR incorporation could be demonstrated as compared with the stimulated cultures. Data are shown as mean and range of triplicate cultures. UPN indicates unique patient number.
HuCD40LT increases the number of viable and cycling cells, but does not rescue cells from spontaneous apoptosis, in primary MCL cell cultures
The ability of huCD40LT to induce DNA synthesis in the system is not necessarily equivalent to proliferation of a majority of the cultured cells. In fact, as little as 0.1% residual cells have been noted to contribute significantly to thymidine incorporation in mixed culture systems like the one applied here.39 PI staining and flow cytometric analysis of DNA content were therefore undertaken to assess the effects of huCD40LT on spontaneous apoptosis, cell-cycle progression, and viable cell recovery. No significant changes in the percentage of cells within the sub-G1 peak of the PI histogram, representing cells undergoing apoptosis, were detected in cells cultured under standard conditions and compared with medium alone (Table 2). The percentage of cells in the S/G2/M region of the PI histogram, representing cells in the S, G2, or M phases of the cell cycle, was increased from a median of 6.4% (range, 1.9% to 11.9%) in medium alone to a median of 10.8% (range, 2.1% to 22.5%) in the presence of huCD40LT (Table 2). Although this difference did not reach statistical significance, patients 2, 3, 4, and 7 showed clear increases in the percentages of cells in S/G2/M phases, suggesting that at least some of the proliferative response occurs within the clonal population of MCL cells. The viability of cells increased significantly from a median of 12.2% (range, 3.8% to 39.9%) in the presence of medium alone to a median of 26.5% (range, 8% to 53%) in the presence of huCD40LT, as assessed by flow cytometry–based PI exclusion (P = .03, n = 6, Wilcoxon) (Table 2). Thus, although the majority of cells were not viable at day 4, huCD40LT induced thymidine incorporation and increased the fraction of cycling and viable cells.
Effect of huCD40LT on apoptosis, proliferation, and viability of cells in primary MCL cultures after 4 days of culture
| UPN# . | Apoptosis*(%) . | Proliferation† (%) . | Viability‡(%) . | |||
|---|---|---|---|---|---|---|
| Medium . | huCD40LT . | Medium . | huCD40LT . | Medium . | huCD40LT . | |
| 1 | 4.2 | 3.5 | 3.5 | 2.7 | 5.2 | 8.0 |
| 2 | 14.7 | 20.4 | 6.6 | 10.7 | 10.0 | 53.0 |
| 3 | 42.4 | 26.6 | 11.9 | 22.5 | 3.8 | 17.5 |
| 4 | 34.7 | 37.2 | 10.6 | 17.5 | 6.6 | 17 |
| 5 | 17.9 | 25.5 | 5.2 | 5.6 | 39.9 | 44.1 |
| 6 | 17.5 | 18.7 | 1.9 | 2.1 | 7.8 | 19.2 |
| 7 | 7.9 | 6.2 | 4.9 | 14.5 | ND | ND |
| Median | 19.9 | 19.7 | 6.4 | 10.8 | 12.2 | 26.5 |
| P value | .80 | .08 | .03 | |||
| UPN# . | Apoptosis*(%) . | Proliferation† (%) . | Viability‡(%) . | |||
|---|---|---|---|---|---|---|
| Medium . | huCD40LT . | Medium . | huCD40LT . | Medium . | huCD40LT . | |
| 1 | 4.2 | 3.5 | 3.5 | 2.7 | 5.2 | 8.0 |
| 2 | 14.7 | 20.4 | 6.6 | 10.7 | 10.0 | 53.0 |
| 3 | 42.4 | 26.6 | 11.9 | 22.5 | 3.8 | 17.5 |
| 4 | 34.7 | 37.2 | 10.6 | 17.5 | 6.6 | 17 |
| 5 | 17.9 | 25.5 | 5.2 | 5.6 | 39.9 | 44.1 |
| 6 | 17.5 | 18.7 | 1.9 | 2.1 | 7.8 | 19.2 |
| 7 | 7.9 | 6.2 | 4.9 | 14.5 | ND | ND |
| Median | 19.9 | 19.7 | 6.4 | 10.8 | 12.2 | 26.5 |
| P value | .80 | .08 | .03 | |||
UPN# indicates unique patient number; huCD40LT, 400 ng/mL of soluble recombinant human CD40 ligand trimer; and ND, not done.
Fraction of apoptotic cells as estimated by propidium iodide staining of isolated nuclei and flow cytometry.
Fraction of cells in the S/G2/M phases of the cell cycle as estimated by propidium iodide staining of isolated nuclei and flow cytometry.
Percent viability as determined by propidium iodide exclusion and scatter profiles using flow cytometry.
Therefore, we next assessed whether huCD40LT could also increase the absolute number of cells in primary MCL cultures. Compared with day 0, the absolute number of viably recovered cells from patients 3 and 4 doubled within 1 week of culture in the presence of huCD40LT (data not shown). We were unable to extend the cell growth in these cultures beyond day 8, and washing, replating, and restimulation with huCD40LT did not induce any further increase in viable cell recovery. This failure to induce responses to huCD40LT after 1 week of culture may be a result of MCL lymphoma cell differentiation and CD40 rewiring.13
HuCD40LT induces expression of CD80/B7-1, CD86/B7-2, and CD95/Fas in primary MCL cell cultures
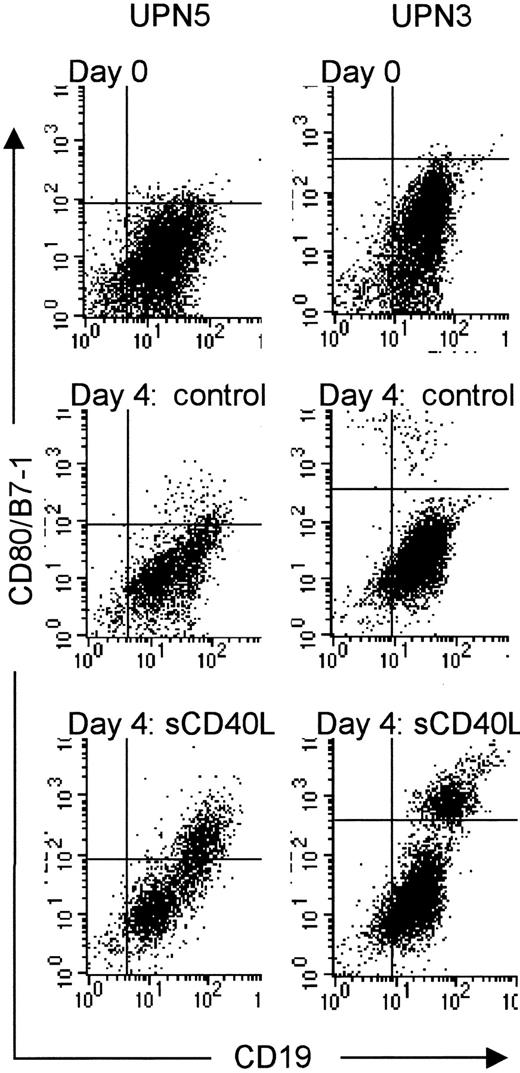
To explore whether the failure to induce extended growth could be caused by loss of CD40 expression, we analyzed the expression of a number of surface membrane markers (see Materials and methods) in primary MCL cell cultures. The vast majority of CD19+ cells expressed readily detectable CD40 throughout 4 days of culture in the presence of huCD40LT in all of the 6 MCL cases studied (data not shown). We never observed coexpression of CD154/CD40L on CD19+ cells regardless of the culture conditions. Consistent with previous reports,29 30 huCD40LT induced significant expression of the costimulatory molecule CD80/B7-1 on CD19+ cells regardless of the proliferative response of the particular MCL case (Figure 2). CD86/B7-2 was similarly induced on CD19+ cells. Along with the up-regulation of the activation markers, we also noted an induced expression of the death receptor CD95/Fas and increased expression of the major histocompatibility complex molecule HLA-DR. All samples remained CD23− regardless of the culture conditions (data not shown).
Induced expression of CD80/B7-1 after huCD40LT stimulation of primary MCL cell cultures.
Isolated mononuclear cells from 6 MCL patients were thawed at day 0 and analyzed using flow cytometry, or seeded at 2 × 106cells/mL and cultured in the presence or absence of 400 ng/mL for 4 days. Before and after culture, the harvested cells were incubated with anti-CD19 (FITC) and anti-CD80/B7-1 (PE) antibodies. A total of 5000 cells were counted using standard flow cytometry analysis. The 2 MCL patients shown represent one case with low (no. 5) and one with high (no. 3) proliferative response to huCD40LT stimulation, respectively. Dot-plot analyses are shown from day 0 and after 4 days of culture, in the absence (day 4: control) or presence of huCD40LT (day 4: huCD40LT) from the 2 MCL patients. sCD40L indicates huCD40LT stimulation.
Induced expression of CD80/B7-1 after huCD40LT stimulation of primary MCL cell cultures.
Isolated mononuclear cells from 6 MCL patients were thawed at day 0 and analyzed using flow cytometry, or seeded at 2 × 106cells/mL and cultured in the presence or absence of 400 ng/mL for 4 days. Before and after culture, the harvested cells were incubated with anti-CD19 (FITC) and anti-CD80/B7-1 (PE) antibodies. A total of 5000 cells were counted using standard flow cytometry analysis. The 2 MCL patients shown represent one case with low (no. 5) and one with high (no. 3) proliferative response to huCD40LT stimulation, respectively. Dot-plot analyses are shown from day 0 and after 4 days of culture, in the absence (day 4: control) or presence of huCD40LT (day 4: huCD40LT) from the 2 MCL patients. sCD40L indicates huCD40LT stimulation.
HuCD40LT induces selective cell-cycle progression in clonal MCL cells in primary MCL cell cultures
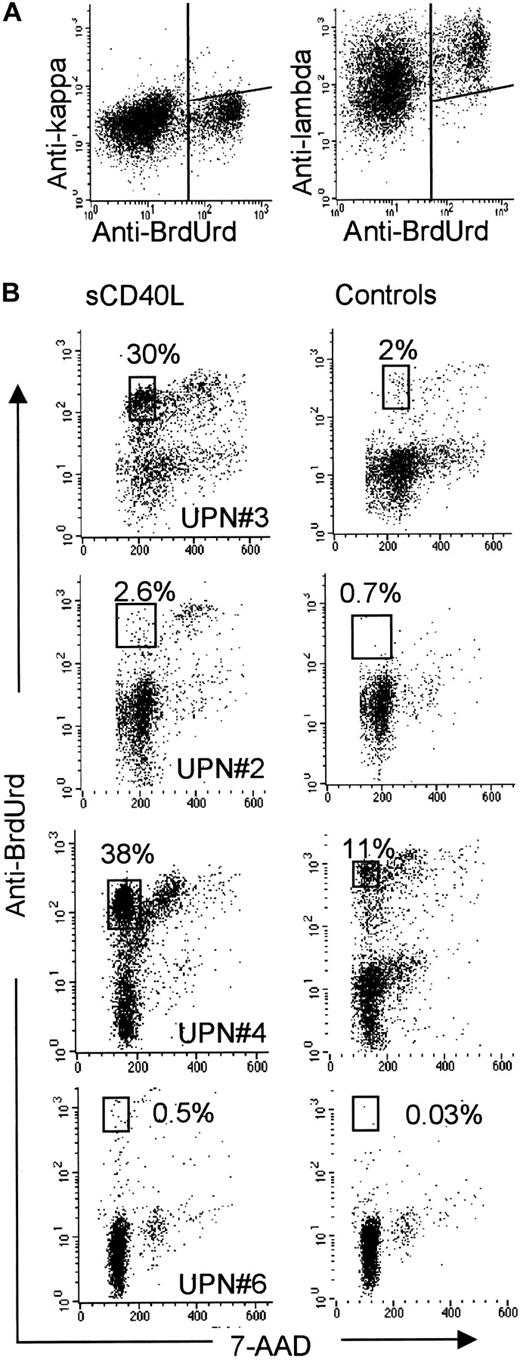
To verify that huCD40LT-induced cell-cycle progression took place in the clonal cells of the primary MCL cultures and not in any residual nonleukemic cells, in particular residual T cells receiving costimulatory signals from MCL cells activated by huCD40LT, we applied a multiparameter flow cytometric assay. In this assay, simultaneous measurement of clonality (light-chain restriction, FL2), DNA synthesis (BrdUrd incorporation, FL1), and DNA content (7-AAD, FL3) was carried out. The kappa and lambda light-chain markers were first used to identify cells in which BrdUrd incorporation occurred in the presence of huCD40LT. As shown in Figure3A, the majority of cells incorporating BrdUrd after 4 days of culture stained positive for a restricted light chain. These results indicate that the cells belonging to the MCL population, and not residual nonclonal cells in the culture system, are the major contributors to the DNA synthesis observed in the other proliferation assays. Simultaneous analysis of BrdUrd incorporation and DNA content (7-AAD) in light chain–restricted cells from the primary MCL cell cultures yielded 2 populations of BrdUrd-positive cells, based on 7-AAD fluorescence intensity: one population of cells in S/G2/M phase and another population in post-mitotic G0/1 phase. As seen in Figure 3B, huCD40LT induced at least a 3-fold increase of clonal BrdUrd-positive G1 cells, which have progressed through and completed the cell cycle within the last 24 hours of the BrdUrd incubation period of the 5-day culture. HuCD40LT induced a median of 14% (range, 0.5% to 29%; n = 4) of G1/BrdUrd-positive cells as compared with the unstimulated control cultures. Because the cells in this analysis were gated for light-chain restriction, this observation can be explained only by huCD40LT-induced proliferation of primary MCL cells.
Induction of cell-cycle progression and proliferation of clonal cells after huCD40LT stimulation of primary MCL cell cultures.
Isolated mononuclear cells from 4 MCL patients were seeded at 2 × 106 cells/mL and cultured in the presence or absence of 400 ng/mL for 4 days. On day 4, the cultures were incubated with bromodeoxyuridine (BrdUrd) for 24 hours. After harvest, the cells were analyzed using multiparameter flow cytometry. (A) Dot-plot analyses of kappa and lambda light-chain expression, respectively, combined with detection of BrdUrd incorporation in one MCL cell culture after stimulation with huCD40LT. (B) Dot-plot analyses of simultaneous detection of BrdUrd incorporation (DNA synthesis) and 7-amino-actinomycin-D (7-AAD) (DNA content) after exclusion of small DNA fragments and of cells that did not clearly express the light chain of the malignant clone of the particular MCL case. Dot-plot analyses of 4 huCD40LT-stimulated and -unstimulated MCL cell cultures are shown (displayed dots represent 25% of total counts). The percentages of cells in the framed regions indicate the fraction of cells that traversed through S-phase with BrdUrd incorporation, completed cell division, and returned to G1-phase. sCD40L indicates huCD40LT stimulation.
Induction of cell-cycle progression and proliferation of clonal cells after huCD40LT stimulation of primary MCL cell cultures.
Isolated mononuclear cells from 4 MCL patients were seeded at 2 × 106 cells/mL and cultured in the presence or absence of 400 ng/mL for 4 days. On day 4, the cultures were incubated with bromodeoxyuridine (BrdUrd) for 24 hours. After harvest, the cells were analyzed using multiparameter flow cytometry. (A) Dot-plot analyses of kappa and lambda light-chain expression, respectively, combined with detection of BrdUrd incorporation in one MCL cell culture after stimulation with huCD40LT. (B) Dot-plot analyses of simultaneous detection of BrdUrd incorporation (DNA synthesis) and 7-amino-actinomycin-D (7-AAD) (DNA content) after exclusion of small DNA fragments and of cells that did not clearly express the light chain of the malignant clone of the particular MCL case. Dot-plot analyses of 4 huCD40LT-stimulated and -unstimulated MCL cell cultures are shown (displayed dots represent 25% of total counts). The percentages of cells in the framed regions indicate the fraction of cells that traversed through S-phase with BrdUrd incorporation, completed cell division, and returned to G1-phase. sCD40L indicates huCD40LT stimulation.
Discussion
In this study, we demonstrate that activation of CD40 by a purified recombinant human CD40 ligand trimer as a single agent induces proliferation of lymphoma cells in primary MCL cell cultures. This conclusion is based on several lines of evidence from 4 different proliferation assays: (1) huCD40LT induced dose- and time-dependent thymidine incorporation; (2) huCD40LT increased the percentage of cells in S, G2, or M phases of the cell cycle; (3) huCD40LT increased the viable cell recovery; (4) essentially all huCD40LT-induced BrdUrd incorporation occurred in cells expressing a restricted light chain; (5) a significant proportion of these BrdUrd-positive, light chain–restricted clonal MCL cells completed the cell cycle within 24 hours, between day 4 and day 5 of the culture period; and (6) we never observed any growth inhibition, not even at very high doses of huCD40LT. The huCD40LT-induced proliferation occurred without any concomitant effects on the degree of spontaneous apoptosis.
The implications of these results are important for 3 aspects of MCL biology: the interpretation of data from different CD40 systems, the response elicited by CD40 in heterogeneous primary cultures, and the possible role of CD40 in the pathobiology and treatment of MCL.
Our results are contradictory to previous studies of the role of CD40 activation in MCL and other NHL lymphomas.18-21,26,27 In NHL cell lines, CD40 ligation induced a direct growth inhibition,18-21 and in 2 MCL studies, the proliferative response of CD40 activation was very weak or absent.26,27These contradictions stress that the interpretation of studies of CD40 function must reflect the applied CD40 activation system as well as the developmental stage of the triggered B cell.15 For example, the agonistic antibody used in one of the MCL studies may significantly underestimate the full effect of CD40 ligation when compared with the huCD40LT.22 The use of irradiated stimulatory cell lines presenting either CD40 ligand or agonistic CD40 antibodies introduces the risk that other presently identified or unidentified biologic substances may influence the observed responses.23 24 Our results clearly demonstrate that a purified recombinant CD40L trimer induces proliferation in primary MCL clonal cells.
Although the MCL cells did respond to additional activation of the interleukin-4 receptor (data not shown), activation through CD40 alone was sufficient to trigger the proliferative response without any requirement for supplementary stimulation through cytokine receptors. This is important because proliferation assays may measure the results of activation of residual T cells in the mixed system.39In particular, the present study confirms that huCD40LT induced phenotypic changes of MCL cells associated with acquisition of an effective antigen-presenting phenotype. Thus, DNA synthesis in mixed primary cell cultures like the one used in this study could be the result of direct huCD40LT-induced growth of MCL cells or, alternatively, could be the result of CD40-induced phenotypic changes and subsequent activation and proliferation of residual T cells, responding to the antigen presented by CD40-activated MCL cells with coexpression of CD80/B7-1 and CD86/B7-2. The simultaneous analysis of phenotype, DNA synthesis, and DNA content did not support the latter possibility. Essentially all BrdUrd incorporation occurred in cells with restricted light-chain expression, which strongly suggests that these cells belong to the malignant clone. Thus, the results of this study are in keeping with a recent study in which primary follicular lymphoma cells were found to undergo proliferation in the presence of huCD40LT.21 This conclusion raises concern for the application of CD40L therapy in vivo in MCL. On the other hand, ex vivo expansion of primary MCL cells with an effective antigen-presenting phenotype may be a useful tool for the application of adaptive immune therapy in MCL.
One unexplained finding in this study was our failure to extend absolute expansion of primary MCL cells beyond 1 week of culture. Despite restimulation, washing, dilution, and replating, the cells from the 2 patients studied with the recovery assay (patients 3 and 4) ceased proliferation within 1 week of culture. This suggests that stimulation through CD40 somehow alters subsequent restimulation through CD40. Whether this phenomenon is caused by MCL lymphoma cell differentiation after huCD40LT stimulation and altered signaling by the receptor or altered gene-expression profiles following the primary CD40-derived signal is not known. Although the quality of the response was comparable among the 8 patients studied, the magnitude of the response was highly heterogeneous (Figures 1 and 3, Table 2). This difference could also be the result of either differential signal transduction through CD40 or differential gene-expression profiles between low- and high-responder cases. To address these issues, we are currently investigating the kinetics and array of gene expression, as well as CD40-mediated signal transduction after huCD40LT stimulation, in low- and high-responder cases of MCL.
Finally, our results demonstrate that activation through CD40 is sufficient to commit a fraction of primary MCL lymphoma cells to cell-cycle progression in vitro, although the size of this fraction is highly variable. In vivo CD40L is induced on activated T cells at different stages of immunopoiesis in secondary lymphoid tissues. The abundance of follicular dendritic cells in MCL-infiltrated lymph nodes may lead to higher numbers of activated T cells and increased CD40L expression, as have been observed in BL.40,41 In line with this, we have previously observed somatic mutations in the heavy chain of the immunoglobulin gene in clonal MCL cells, indicating that antigen selection can take place in at least some cases of MCL.3To do so, the B cell, in which the final transformation took place, must have had the capacity to interact productively with a T cell. Together with the results of the present study, these data suggest that cooperation between inherent cell-cycle deregulatory genetic lesions in MCL and a CD40L-rich microenvironment may be sufficient to induce cell-cycle progression of importance in the pathogenesis of MCL.
Acknowledgments
We thank the Nordic Lymphoma Group and Dr Erik Segel for supplying patient samples. Human recombinant CD40 ligand trimer was kindly provided by Immunex Corporation, Seattle, WA.
Supported by grants from the Danish Medical Research Council (96-018-28), the Danish Cancer Society (94-100-62 and 96-100-09), the John and Birthe Meyer Foundation, Katrine and Vigo Skovgaards Foundation, and A. L. Vengs Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Niels S. Andersen, Department of Hematology L-4041, Rigshospitalet, Blegdamsvej 9, DK-2100 Copenhagen, Denmark; e-mail: nanders@rh.dk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal