Abstract
Infection of peripheral blood mononuclear cells (PBMNCs) has been demonstrated to be a crucial event in the vertical transmission of viruses, and it is known that hepatitis C virus (HCV) can infect PBMNCs. The relationship between vertical transmission of HCV and the presence of positive and negative strands of HCV-RNA in the PBMNCs of HCV-carrier mothers was investigated using reverse transcriptase–polymerase chain reaction (RT-PCR). During the study, 13 consecutive mothers who transmitted infection to their offspring and 53 consecutive mothers who did not were examined. The positive strand of HCV-RNA was identified in the PBMNCs of all mothers who transmitted the infection and in 13 of 53 mothers who did not (P < 10−6). The HCV-RNA−strand was found in 5 of 13 mothers who transmitted the infection, and the strand was not found in the mothers who did not transmit the infection (P = .0001). Neither maternal PBMNC infection nor HCV transmission to the offspring was significantly related to the viral genotype or to the maternal viral load. These data show that maternal PBMNC infection by HCV and viral replicative activity in PBMNCs are important factors in the transmission of HCV from mother to child. The mechanism through which HCV infection of PBMNC favors vertical transmission of the virus is still incompletely understood.
Introduction
Since the introduction of hepatitis C virus (HCV) screening of donated blood units, vertical transmission seems to be the most common route of HCV infection in the pediatric age group.1 The importance of certain risk factors in enhancing vertical HCV transmission has been previously discussed. The rate of newborn infection clearly seems to increase with the rising prevalence of human immunodeficiency virus (HIV) infection in mothers.2-4 On the other hand, a history of maternal intravenous drug use itself, independent of HIV-1 infection, may be an important predisposing factor for perinatal transmission of HCV.5 Similarly, a previous history of maternal posttransfusion hepatitis is highly related to vertical transmission.5 In contrast, other factors, such as breast feeding or vaginal delivery, do not seem to influence the rate of vertical transmission.2,5 Anti-HCV+ mothers may transmit the virus to their offspring during gestation or delivery. Infection may occur through maternal blood, which contains free HCV particles and occasionally HCV-infected cells as well.6
Several studies have provided convincing evidence for the occurrence of active HCV infection in peripheral blood mononuclear cells (PBMNCs), hematopoietic progenitor cells, and bone marrow.7-9Recently it has been demonstrated that lymphocytes and hepatocytes have a specific receptor for HCV envelope protein E210; however, the mechanisms that induce HCV entry into lymphocytes have yet to be clearly elucidated. It has been shown that cellular infection is crucial in mother-to-infant HIV transmission, and it has been suggested that maternal cells may play an important role in other vertical viral infections.11 At present, the contribution of free virions or infected cells to HCV transmission from mother to child is still unknown. In this study we looked for evidence of HCV genomic sequences (both the positive- and negative-strand RNA) in PBMNCs of HCV-infected mothers and the relationship of these findings to vertical transmission of HCV.
Patients and methods
Patients
We studied 13 HIV-1− HCV-RNA+ mothers who transmitted HCV infection to their offspring. The control group comprised 53 consecutive HIV-1− HCV-RNA+mothers whose babies did not become infected. All the mothers were positive for HCV antibodies, as detected by a third-generation enzyme-linked immunosorbent assay (ELISA) (Ortho Diagnostic System Inc., Raritan, NJ) and Western blot analysis (Innogenetics, Zwijndrecht, Belgium). Viral infection was confirmed in all mothers by polymerase chain reaction (PCR) detection of HCV-RNA. No evidence of other active viral infection was documented in these mothers. None of the children had received blood product transfusion. In children, detection of HCV-RNA at 2 different times or the persistence of anti-HCV beyond 18 months of age was assumed to be evidence of vertical transmission. In all children clinical evaluation and biochemical, hematologic, and virologic testing were performed at 3- to 6-month intervals during a follow-up of 2 years or longer.
Separation of PBMNCs and RNA extraction
PBMNCs were obtained from all of the mothers at the time of delivery. PBMNCs were isolated from 10 mL of heparinized whole blood by centrifugation over density gradient (Lymphoprep; Nycomed Pharma AS, Oslo, Norway) and were washed 3 times after isolation in 10 mL phosphate-buffered saline (PBS). Aliquots of paired plasma and 1 × 106 PBMNCs were stored at −70°C until use. Total RNA was extracted from 100 μL plasma, 500 μL final PBMNC washing, and 1 × 106 unthawed PBMNCs by RNA fast II (Molecular Systems, San Diego, CA).
Determination of HCV genotypes, viral load, and specific amplification of positive- and negative-strand HCV-RNA
Viral genotypes were determined in serum samples and PBMNCs from all the mothers and from HCV-infected children with a line probe assay (Innogenetics). Quantitative analysis of RNA was performed by a Amplicor HCV Monitor (Roche Diagnostic Systems, Branchburg, NJ).
The presence of positive- and negative-strand HCV-RNA in plasma and PBMNC samples was detected by strand-specific reverse transcriptase (RT)-PCR. The antisense 1A (5′-GATGCACGGTCTACGAGACCTG-3′) and sense 1B (5′-AACTACTGTCTTCACGCAGAA -3′) outer primers were used to reverse transcribe the positive and negative strands of HCV-RNA, respectively. The cDNA was amplified subsequently by nested PCR as previously described.12 To avoid the detection of a “false” negative strand, the following methods were used after cDNA synthesis: (1) heat treatment for 1 hour at 95°C to inactivate RT activity and (2) 1 unit ribonuclease (RNase) A (Promega, Madison, WI) at 37°C for 30 minutes to digest RNA and to prevent possible RT and amplification of positive strands by Thermus aquaticus(Taq) polymerase. To avoid false-positive results by contamination, the preventative measures of Kwok and Higuchi13 were followed strictly, and negative controls were included in all experiments.
Statistical analysis
The data were processed with the SPSSX statistical package (SPSS Inc, Chicago, IL). RNA copy numbers were reported as the median and range. The differences were evaluated by the nonparametric Mann-Whitney U test, and the differences in frequencies were evaluated by the χ2 test or Fisher exact test.
Results
Detection of positive and negative strands of HCV-RNA in mothers and the relationship with vertical transmission
All mothers included in the study had HCV-RNA+ sera. The HCV-RNA+ strand was demonstrated in the PBMNCs from all 13 mothers whose babies became infected and in 13 of 53 mothers whose babies did not (P < 10−6). The HCV-RNA− strand was demonstrated in PBMNCs of 5 of 13 mothers who transmitted HCV to their babies and in none of the mothers who did not (P = .0001) (Table1). The HCV-RNA− strand was never found in sera of any of the mothers studied. Neither positive nor negative HCV-RNA strands were ever found in the final PBMNC washing.
Relationship between the presence of positive- or negative-strand HCV in maternal PBMNCs and vertical HCV transmission
| HCV strand . | Infants infected (n = 13), no. . | Infants not infected (n = 53), no. . | P . |
|---|---|---|---|
| Positive | 13 | 13 | < .000001 |
| Negative | 5 | 0 | .0001 |
| HCV strand . | Infants infected (n = 13), no. . | Infants not infected (n = 53), no. . | P . |
|---|---|---|---|
| Positive | 13 | 13 | < .000001 |
| Negative | 5 | 0 | .0001 |
Analysis of the genotype distribution in PBMNCs and sera of mothers and infected infants
HCV genotypes were analyzed in the PBMNCs and sera of all mothers. The results are shown in Table 2. When infection of a child occurred, the same HCV genotype was found in the mother-child pair. None of the patients were infected by 2 or more different HCV genotypes. The same viral genotype was always found in PBMNCs and sera. Even though genotype 1 was more frequently identified in mothers whose babies became infected, the difference was not statistically significant (P = .09). Similarly, there was no difference in genotype found between mothers whose lymphocytes were HCV-RNA+ and mothers whose lymphocytes were not, even though genotype 1b was more frequently associated with PBMNC infection (Table 3).
Correlation between mother-to-child infection and viral genotype
| Genotype . | Mothers who infected their offspring (n = 13), no. (%) . | Mothers who did not infect their offspring (n = 53), no. (%) . | P . |
|---|---|---|---|
| 1a | 4 (30.8) | 11 (20.7) | .33 |
| 1b | 6 (46.1) | 17 (32.2) | .26 |
| 2a/2c | 1 (7.7) | 4 (7.5) | .68 |
| 3a | 1 (7.7) | 13 (24.5) | .17 |
| 4c/4d | 1 (7.7) | 8 (15.1) | .43 |
| Genotype . | Mothers who infected their offspring (n = 13), no. (%) . | Mothers who did not infect their offspring (n = 53), no. (%) . | P . |
|---|---|---|---|
| 1a | 4 (30.8) | 11 (20.7) | .33 |
| 1b | 6 (46.1) | 17 (32.2) | .26 |
| 2a/2c | 1 (7.7) | 4 (7.5) | .68 |
| 3a | 1 (7.7) | 13 (24.5) | .17 |
| 4c/4d | 1 (7.7) | 8 (15.1) | .43 |
Relation between maternal PBMNC infection and viral genotype
| Genotype . | Mothers with PBMNC HCV infection (n = 26), no. (%) . | Mothers without PBMNC HCV infection (n = 40), no. (%) . | P . |
|---|---|---|---|
| 1a | 4 (15.4) | 11 (27.5) | .19 |
| 1b | 13 (50.0) | 10 (25.0) | .07 |
| 2a/2c | 1 (3.9) | 4 (10.0) | .34 |
| 3a | 3 (11.5) | 11 (27.5) | .10 |
| 4c/4d | 5 (19.2) | 4 (10.0) | .23 |
| Genotype . | Mothers with PBMNC HCV infection (n = 26), no. (%) . | Mothers without PBMNC HCV infection (n = 40), no. (%) . | P . |
|---|---|---|---|
| 1a | 4 (15.4) | 11 (27.5) | .19 |
| 1b | 13 (50.0) | 10 (25.0) | .07 |
| 2a/2c | 1 (3.9) | 4 (10.0) | .34 |
| 3a | 3 (11.5) | 11 (27.5) | .10 |
| 4c/4d | 5 (19.2) | 4 (10.0) | .23 |
Correlation between PBMNC infection and viral load in mothers
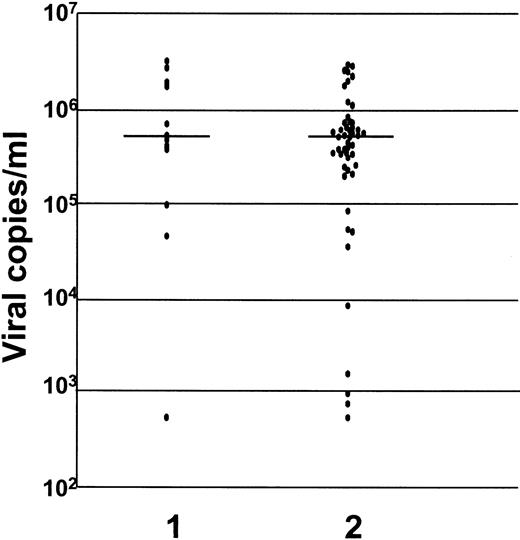
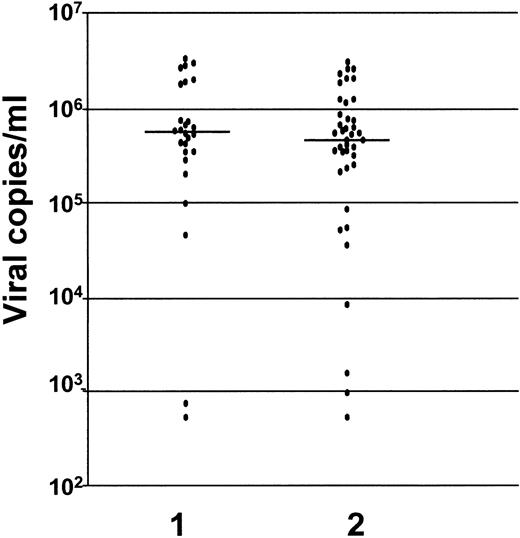
There was no difference in the serum viral load between the mothers who infected their babies (5.4 × 105 RNA copies per mL; range, 0.005-34 × 105 RNA copies per mL) and mothers who did not (5.2 × 105 RNA copies per mL; range, 0.005-30 × 105 RNA copies per mL) (P = .23) (Figure 1). Similarly there was no difference in serum viral load between mothers whose lymphocytes harbored the virus (5.6 × 105 RNA copies per mL; range, 0.005-34 × 105 RNA copies per mL) and mothers whose lymphocytes did not (4.6 × 105 RNA copies per mL; range, 0.005-31 × 105 RNA copies per mL) (P = .4) (Figure 2).
Levels of HCV-RNA in sera of mothers who transmitted HCV infection to their offspring and in mothers who did not.
Transmitted HCV infection is indicated by 1; nontransmission of HCV is indicated by 2 (P = .23). The levels are measured as viral copies per mL.
Levels of HCV-RNA in sera of mothers who transmitted HCV infection to their offspring and in mothers who did not.
Transmitted HCV infection is indicated by 1; nontransmission of HCV is indicated by 2 (P = .23). The levels are measured as viral copies per mL.
Levels of HCV-RNA in sera of mothers whose lymphocytes harbored the virus and mothers whose lymphocytes did not.
Virus harbored in lymphocytes is indicated by 1; nonharbored virus is indicated by 2 (P = .4). The levels are measured as viral copies per mL.
Levels of HCV-RNA in sera of mothers whose lymphocytes harbored the virus and mothers whose lymphocytes did not.
Virus harbored in lymphocytes is indicated by 1; nonharbored virus is indicated by 2 (P = .4). The levels are measured as viral copies per mL.
Discussion
The data presented show that the presence of HCV-RNA in maternal PBMNCs is highly associated with transmission of HCV to the newborn, and the presence of negative-strand HCV-RNA, a marker for HCV replication in maternal PBMNCs, is associated with vertical transmission. These data also confirm the previous observation that PBMNC infection by HCV does not correlate with viral load or HCV genotype.14,15 PBMNC infection by HCV has been reported by several groups,6,7 and viral replication in infected PBMNCs has been shown as well.14,15 The role of PBMNC infection by HCV is controversial, but it has been demonstrated that lymphocytes may act as an HCV reservoir and play a key role in the relapse of HCV disease after liver transplantation16 or after the discontinuation of interferon therapy.17
Recent studies11 examining the vertical transmission of HIV-1 demonstrated that the exposure of offspring to HIV-1 in utero and during birth may involve free cell virus or, perhaps more likely, cell-associated virus. There is good evidence that the fetus is exposed to maternal blood, and maternal cells may be found in cord blood samples at a frequency of 1 of 104-105nucleated cells.18 It has been shown that maternal infection is crucial in mother-to-infant HIV-1 transmission.11 Perinatal transmission of HIV-1 infection by direct passage of maternal-infected T cells has been demonstrated in experimental models,19,20 and this is considered to be the major source of infection when the virus is transmitted vertically.21
The persistence of maternal-infected cells in fetal blood may therefore facilitate the infection of newborn target cells. This situation may be favored by particular conditions. In fact, it has been demonstrated that HLA concordance between mother and infant may favor HIV-1 infection of offspring through an extended survival of maternal cells in the newborn.11,22 The same authors suggest that a similar mechanism may be effective in other viral infections that are transmitted vertically.11
Mother-to-child HIV-1 transmission is influenced by viral phenotype.23 In most cases a single or a few closely related maternal HIV-1 variants are transmitted to the fetus.24 A similar behavior has been demonstrated for HCV transmission.25-27 It may be suggested that in both infections, certain viral phenotypes have some selective advantages in determining both the infection of particular maternal target cells and the viral transmission to the offspring.
Furthermore our experiments showed that viral replicative activity in maternal PBMNCs is related to mother-to-infant transmission of HCV. In fact, the presence of a negative strand in PBMNCs of mothers is highly associated with HCV transmission to newborns. We consider detection of the HCV-RNA− strand in infected cells to be a marker of active viral replication.15 The absence of a negative strand in serum samples from all mothers included in this study actually suggests that its presence in PBMNCs is a marker of active replication and not uptake of cellular debris.
Although the presence of positive- and negative-strand HCV-RNA has been investigated by other authors, at present the role played by PBMNC infection in chronicity of HCV liver disease and in liver damage is still unclear.14 Similarly, the clinical or virologic aspects that favor virus entry into PBMNCs and the mechanisms through which PBMNC infection may favor vertical transmission need to be clarified.
Acknowledgments
We thank Sergio Nanni and Luigi Sgarra for expert technical assistance.
Partially supported by a grant from the Italian Scientific and Technological Research Department, University of Florence, Florence, Italy (04.01.00309).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Chiara Azzari, Department of Pediatrics, Via Luca Giordano 13, I-50132 Florence, Italy; e-mail:m.resti@ao-meyer.toscana.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal