Abstract
Immunotherapy trials targeting the induction of tumor-reactive T-cell responses in cancer patients appear to hold significant promise. Because nonmutated lineage-specific antigens and mutated idiotypic antigens may be coexpressed by tumor cells, the use of autologous tumor material to promote the broadest range of antitumor T-cell specificities has significant clinical potential in cancer vaccination trials. As a model for vaccination in the cancer setting, we chose to analyze the promotion of T-cell responses against Epstein-Barr virus (EBV)-transformed B-lymphoblastoid cell line (B-LCL)–derived antigens in vitro. A series of bulk antigenic formats (freeze–thaw lysate, trifluoroacetic acid lysate, extracted membranes, affinity-purified MHC class I– and class II–presented peptides, acid-eluted peptides) prepared from EBV B-LCLs were tested for their ability to stimulate EBV B-LCL–reactive CD4+ and CD8+ T lymphocytes in vitro when pulsed onto autologous dendritic cells (DCs). DC presentation of freeze–thaw lysate material derived from (either autologous or allogeneic) EBV B-LCLs with an Mr of 10 kd or larger stimulated optimal anti-EBV B-LCL responsiveness from freshly isolated CD4+ and CD8+ peripheral blood T cells. These in vivo “memory” T-cell responses were observed only in EBV-seropositive donors. CD4+ T-cell responses to lysate-pulsed DCs were Th1 type (ie, strong interferon-γ and weak interleukin-5 responses). While CD8+ T-cell responses were also observed in interferon-γ Elispot assays and in cytotoxicity assays, these responses were of low frequency unless the DC stimulators were induced to “mature” after being fed with tumor lysates. Optimal-length, naturally processed, and MHC class I– or class II–presented tumor peptides were comparatively poorly immunogenic in this model system.
Introduction
Effective vaccines designed to treat cancer or alternate malignancies should elicit both CD4+ and CD8+ T-cell responses to epitopes derived from tumor- or pathogen-associated antigens.1 This may be most effectively accomplished by accessing or implementing autologous dendritic cells (DCs) in the design of the vaccine. DCs have been shown to efficiently stimulate both primary and secondary CD4+and CD8+ T-cell immune responses and are therefore considered to represent potent biologic adjuvants for application to vaccination trials.2 After so-called immature DCs capture and process antigens in the periphery, they migrate to lymphoid organs. Terminally differentiated, or “mature,” DCs stimulate antigen-specific T cells via the presentation of peptide antigens in association with HLA class I and II molecules, the provision of T-cell costimulation, and the secretion of T-cell growth and differentiation cytokines. DC maturation may be induced by a number of stimuli, including pathogens, cognate T-cell interaction, or proinflammatory cytokines.3
Immature DCs efficiently acquire and process exogenous antigens (such as those extracted from tumor or transformed cells) and can be easily matured into optimal T-cell stimulatory antigen-presenting cells.4 5 Given these characteristics, we have evaluated the ability of this induction system (DCs plus “tumor” extracts) to promote “tumor”-specific CD4+ and CD8+T-cell immune responses in vitro using Epstein-Barr virus (EBV)-transformed B-lymphoblastoid cell line (B-LCL) as a model “tumor.” The results of this EBV B-LCL model system allow for the construction of vaccines for the treatment or prevention of cancer or alternate malignancies, such as the EBV-associated malignancies posttransplantation lymphoproliferative disorder (PTLD), Burkitt lymphoma, Hodgkin lymphoma, and undifferentiated nasopharyngeal carcinoma.
Material and methods
Donors and cell lines
The donors IP1, IP2, and IP3 were healthy individuals without evidence of acute EBV infection. As determined by Western blotting (kindly performed by Dr David Rowe, Department of Infectious Diseases and Microbiology, Graduate School of Public Health, University of Pittsburgh), sera from donors IP1, IP2, and IP3 were positive for immunoglobulin (Ig) G antibodies to the EBV protein EBNA-1 (titer 1:100-1:250) and negative for reactivity against EBV viral capsid antigens. According to standard HLA serotyping procedures, donor IP1 was typed HLA-A2,32; B7,62; Cw3; DR4,15; donor IP2 was typed HLA-A11,68; B35,44; DR1,5; and donor IP3 was typed HLA-A1; B8; Cw7; DR3,13.
LCLs were established by EBV (B95.8 strain) transformation of peripheral blood mononuclear cells (PBMCs) in the presence of 0.1 μg/mL cyclosporine (Sandoz, Basel, Switzerland). Anti-μ B-cell blasts were generated by stimulating PBMCs with 10 μg/mL rabbit antihuman IgM immunobeads (Irvine Scientific, Santa Ana, CA) in the presence of 100 U/mL recombinant human interleukin-4 (rhIL-4; Schering-Plough, Kenilworth, NJ). Phytohemagglutinin (PHA)-activated T-cell blasts were prepared by stimulating PBMCs with 5 μg/mL PHA (Sigma, St. Louis, MO). Cell lines were maintained in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mmol/L L-glutamine, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 1 mmol/L sodium pyruvate. All cell culture reagents were purchased from Life Technologies (Gaithersburg, MD).
Antigen-presenting cells
For the generation of DCs, PBMCs were isolated by density centrifugation on Ficoll-Hypaque gradients (LSM, Organon-Teknika, Durham, NC) for 25 minutes at 880g at room temperature and were washed 4 to 5 times in Hank's balanced salt solution (HBSS; Life Technologies) to remove platelets. CD4+ or CD8+T cells were positively isolated from PBMCs using immunomagnetic CD4/CD8 MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and were directly applied as T-cell responders in Elispot assays or cryopreserved until used. The remaining cells were resuspended at 107/mL in AIM-V medium (Life Technologies) and were incubated for 90 minutes in 75-cm2 tissue culture flasks (37°C, 5% CO2). After removal of nonadherent cells, plastic adherent cells were cultured (37°C, 5% CO2) in 10 mL of DC medium (AIM-V medium supplemented with 1000 U/mL recombinant human granulocyte-macrophage colony-stimulating factor [rhGM-CSF] and 1000 U/mL rhIL-4; both from Schering-Plough). At day 3, cells were fed with 5 mL of fresh DC medium. At day 6, nonadherent cells were rinsed off the flasks and cultured for 48 hours in 6-well plates (Costar, Corning, NY) at a final concentration of 5 × 105 cells per well in 3 mL of DC medium. DCs generated in this way had an “immature” phenotype (no expression of CD83 and low to intermediate expression of CD54, CD80, CD86, HLA class I and II) as assessed by flow cytometry. To obtain “mature” DCs (high expression of CD54, CD80, CD83, CD86, HLA class I and II), we followed a procedure described recently by Jonuleit et al.4 According to this protocol, a cytokine cocktail consisting of 10 ng/mL recombinant human tumor necrosis factor-α (rhTNF-α; Sigma), 10 ng/mL rhIL-1β (Genzyme, Cambridge, MA), 1000 U/mL rhIL-6 (Genzyme), and 1 μg/mL prostaglandin E2(PGE2; Sigma) was added to the culture medium on day 6, and DCs were harvested on day 8. To pulse DCs with antigens, bulk antigenic formats were irradiated (15 000 rad) on ice and were added at a ratio of 100 LCL/B- or T-blast cell equivalents to 1 DC directly after DCs were seeded in fresh culture medium on day 6. In general, immature and mature DCs were harvested on day 8, washed twice in AIM-V, and added to responder T lymphocytes in Elispot assays or long-term T-cell cultures. Monocytes were used as comparative antigen-presenting cells (APCs). These cells were positively isolated from fresh PBMCs by immunomagnetic CD14 MicroBeads according to the manufacturer's instructions and were cultured for 48 hours in 6-well plates at a final concentration of 1 × 106 cells per well in 3 mL AIM-V supplemented with 1000 U/mL rhGM-CSF.
Bulk antigenic formats
Autologous LCL cells, B-, or T-cell blasts were expanded in RPMI/10% FCS, washed, and subsequently recultured for an additional 3 days in AIM-V to remove calf serum proteins and to reduce the number of FCS-derived HLA-presented epitopes on the cell surface. At the time of cell harvest, cells (109 each) were washed twice with HBSS prior to extraction of cell-associated antigens using the procedures indicated below.
Freeze–thaw lysate
Cells were resuspended in 2 mL of HBSS and lysed by 5 freeze (on methanol and dry ice)–thaw (room temperature) cycles. Total cell disruption was microscopically validated using trypan blue staining. After sonication for 10 minutes, lysate was centrifuged at 15 000g (30 minutes, 4°C). Supernatant (SN; without top lipid layer) was recovered and fractionated on Centricon-10 ultrafiltration devices (Amicon, Cambridge, MA) by centrifugation at 3000 rpm for 2 to 3 hours at 4°C. Upper (10-kd proteins or larger) and lower (smaller than 10-kd proteins/peptides) fractions were individually harvested and stored at −70°C until use.
Trifluoroacetic acid lysates
Cells were resuspended in trifluoroacetic acid (TFA) 0.1% or 1% in distilled, deionized water (ddH2O) and dounce homogenized until qualitative cell disruption had occurred (typically 150-200 cycles). The resulting lysate was sonicated for 10 minutes, followed by centrifugation at 15 000g over 30 minutes at 4°C. SN (without top lipid layer) was removed and placed on Centricon-10 ultrafiltration devices as outlined above. After centrifugation at 3000 rpm for 2 to 3 hours at 4°C, top and bottom fractions were recovered, lyophilized in a Labconco Speed-Vac until near dryness, and resuspended in 1 mL phosphate-buffered saline (PBS)/10% dimethyl sulfoxide (DMSO). Lysate was stored at −70°C until use.
Extraction of cell membranes
Pelleted membranes resulting from centrifugation of TFA 1% lysates were extracted using 1% TFA in 90% acetonitrile (ACN)/9% ddH2O overnight at 4°C following an additional centrifugation at 15 000g at 4°C over 30 minutes. The SN was recovered, lyophilized to remove organic solvent, and resuspended in 1 mL PBS/10% DMSO. Extract was stored at −70°C until use.
Extraction of naturally processed peptides from viable cells
Cells were incubated with 50 mL citrate-phosphate buffer, pH 3.0,6 for 1 minute following centrifugation over 3 minutes at 2000 rpm. To remove remaining cell fragments, the SN was spun down at 4000 rpm over 10 minutes (both at 4°C). Cell-free SN containing eluted peptides was concentrated on a SepPak C18 cartridge (Millipore, Bedford, MA) according to the manufacturer's instructions. Bound peptides were eluted by 60% (vol/vol) followed by 100% (vol/vol) acetonitrile (in ddH2O) and concentrated in a Speed-Vac. They were resuspended in 1 mL PBS/10% DMSO and stored at −70°C until use.
Extraction of naturally processed peptides from affinity-purified HLA-A2.1 and HLA-DR molecules
Pellets from 1.5 × 109 LCL cells were lysed in 20 mL Chaps detergent (Sigma, 5% in ddH2O) containing protease inhibitors (Boehringer Mannheim, Mannheim, Germany) for 45 minutes on ice. After centrifugation at 2000 rpm for 10 minutes, followed by 15 000g for 30 minutes (both at 4°C), SN was passed through chromatography columns filled with either Sepharose beads coupled with monoclonal antibodies (mAbs) BB7.2 (anti-HLA-A2.1) or L243 (anti-HLA-DR monomorphic). Antibodies were coupled to Sepharose-4B matrix (Sigma) per the manufacturer's instructions. Matrices were then treated with 0.1% TFA (in ddH2O) for 15 minutes at room temperature to denature the major histocompatibility complex (MHC) peptide complexes, allowing for the harvest of soluble peptides. After initial centrifugation to pellet the Sepharose beads (3000 rpm, 10 minutes), SN was recovered and fractionated on Centricon-3 ultrafiltration devices over 2 to 3 hours at 4°C. Top (3 kd or larger) and bottom (smaller than 3 kd) fractions were lyophilized and resuspended in 1 mL PBS/10% DMSO and then stored at −70°C until use.
Flow cytometry
For immunophenotyping, DC or T-cell responders were washed in HBSS supplemented with 1% bovine serum albumin and 0.1% NaN3 and incubated (30 minutes at 4°C) with one of the following antibodies: fluorescein isothiocyanate (FITC)-conjugated anti-HLA class I (Serotec, Oxford, England), phycoerythrin (PE)-conjugated anti-HLA-DR (Becton Dickinson, Mountain View, CA), FITC-conjugated anti-CD8 (Becton Dickinson), PE-conjugated anti-CD54 (Becton Dickinson), FITC-conjugated anti-CD80 (Ancell, Bayport, MN), PE-conjugated anti-CD83 (Coulter-Immunotech, Miami, FL), and FITC-conjugated anti-CD86 (PharMingen, San Diego, CA). Unconjugated anti-CD45RO and anti-CD45RA mAbs were obtained from the Sixth International Leukocyte Typing Workshop and were used in indirect immunofluorescence assays. Cells were also stained with corresponding isotype-matched control mAb (PharMingen). For indirect staining, FITC-conjugated goat antimouse IgG F(ab)2antibody (Becton Dickinson) was used (30 minutes, 4°C). Surface expression was analyzed using a FACScan flow cytometer (Becton Dickinson) and Lysis II software. Data were collected on 10 000 viable cells.
T-cell cultures
CD4+ and CD8+ T lymphocytes were positively isolated from PBMCs by immunomagnetic CD4/CD8 MicroBeads and were seeded at 3 × 106 cells per well in 24-well plates (Costar). Autologous irradiated DCs (105 per well) prepulsed with a freeze–thaw lysate (10 kd or larger) of LCLs, B- or T-cell blasts (for loading, see above), or intact autologous irradiated LCL cells (7.5 × 104 per well) were then added. Radiation dose was 2500 rad for DCs and 4000 rad for LCLs. Culture medium was AIM-V supplemented with 5% human AB serum (Sigma) at a final volume of 2 mL/well. For cultures containing CD8+ T cells, 1000 U/mL rhIL-6 (Sandoz) and 1 ng/mL rhIL-12 (Genetics Institute, Bedford, MA) were added on day 0.7 Cultures containing CD4+ T cells were supplemented on day 3 with 10 IU/mL rhIL-2 (Chiron, Emeryville, CA). Responding T cells were restimulated on day 7 and day 14 using irradiated, antigen-pulsed DCs or irradiated LCL cells at a responder-to-stimulator ratio of 30:1 (DC) or 40:1 (LCL) in AIM-V medium containing 10 IU/mL IL-2 and 5 ng/mL rhIL-7 (Genzyme).
Elispot assays for interferon-γ and IL-5
Elispot assays were performed as previously described8 using capture mAbs antihuman interferon (IFN)-γ (1-D1K; Mabtech, Stockholm, Sweden) or antihuman IL-5 (18051D; PharMingen) and detection biotinylated mAbs antihuman IFN-γ (7-B6-1; Mabtech) or antihuman IL-5 (18522D; PharMingen). Nonirradiated autologous monocytes (4 × 104 per well), immature or mature DCs (2 × 104 per well) prepulsed with bulk antigenic formats (for loading, see above), or autologous LCL cells (5 × 104 per well, not irradiated) were used as stimulator cells. CD4+ and CD8+ T-cell responders were positively isolated from PBMCs by immunomagnetic CD4/CD8 MicroBeads and were more than 95% pure. Control wells contained unstimulated T cells, T cells in the presence of unloaded APC, and LCL cells alone. Spot numbers were automatically determined with the use of a computer-assisted video image analyzer (Zeiss-Kontron, Jena, Germany).9 To calculate the number of T cells responding to a particular antigen, the mean numbers of spots induced by DCs alone were subtracted from mean spot numbers induced by antigen-loaded DCs. For statistical evaluation, at test for unpaired samples was used. Values of P< .05 were considered significant.
Cytotoxicity assays
CD8+ responder populations were tested for their cytolytic activity after 2 weekly (days 7, 14) restimulations on days 23 to 25 against LCLs, PHA blasts, and the natural killer target K562 in a standard 6-hour 51Cr release assay.6 In some assays, natural killer activity was blocked by the addition of 40 000 nonlabeled K562 per well. Blocking antibodies W6/32 (anti-HLA class I) and L243 (anti-HLA-DR, class II) were added at 20 μg/well.
Results
Subcellular fractions of EBV B-LCLs contain immunogenic antigens
In IFN-γ Elispot analyses, we generally observed that autologous EBV B-LCLs induced strong spot production when admixed with purified blood-derived T cells obtained from EBV-seropositive, healthy individuals. In donor IP1, for example, the frequencies of LCL-reactive T lymphocytes were 203 per 105 for CD4+ T cells and 845 per 105 for CD8+ T cells (data not shown), suggesting that the autologous EBV B-LCLs express immunogenic HLA class I and class II complexes presenting viral epitopes recognized by donor T cells.
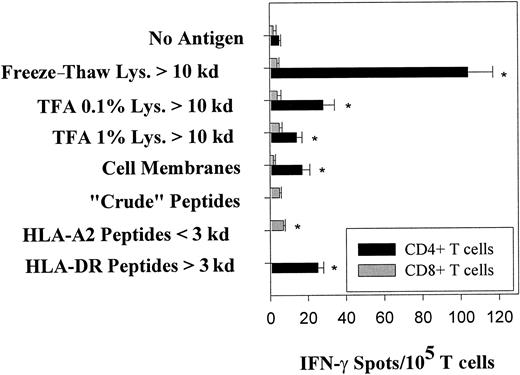
For the purposes of DC-based vaccine construction, we sought to determine those extracts that might be obtained from a given target cell (ie, tumor, EBV B-LCL) to effectively promote CD4+ and CD8+ T-cell reactivity against target antigens. In our model system, we prepared lysates from 109 EBV B-LCL cells of donor IP1 (IP1-LCL) by freeze–thaw cycles or mechanical disruption in 0.1% or 1% TFA, with a subsequent fractionation of extracted proteins/peptides into material with Mr smaller than 10 kd or Mr 10 kd or larger performed using ultrafiltration devices. In addition to lysates, naturally processed peptides were isolated from viable IP1-LCL cells or from HLA-A2.1 and HLA-DR complexes affinity-purified from IP1-LCL by acid-denaturation. Eluted peptides were divided into small and large peptides (ie, those with Mr smaller than 3 kd or Mr 3 kd or larger) using 3-kd ultrafiltration devices. These EBV B-LCL–derived bulk antigenic proteins/peptides were then loaded on autologous immature, endocytic DCs and were screened for recognition by purified CD4+ and CD8+ “memory” T cells freshly isolated from the blood of donor IP1 using IFN-γ Elispot assays. For CD4+ lymphocytes, the strongest reactivity was directed against the freeze–thaw lysate fraction containing molecules larger than 10 kd (98 spot-forming lymphocytes per 105CD4+ T cells; Figure 1). As shown in Figure 2, CD4+ T cells of donor IP1 exclusively recognized the freeze–thaw lysate prepared from autologous EBV B-LCL cells and not freeze–thaw lysates derived from comparable numbers of autologous B-cell or T-cell blasts, suggesting an EBV-associated reactivity for these effector cells. To trigger significant IFN-γ spot formation in CD4+ T cells, autologous immature DCs were prepulsed with an LCL freeze–thaw lysate at a ratio of 1 or more EBV B-LCL cell equivalents per DC. Compared with freeze–thaw lysates, all LCL-derived TFA lysate fractions 10 kd or larger induced lower but still significant IFN-γ spot production among the CD4+ T cells of donor IP1 (Figure 1). Of major interest, significant T-cell responsiveness was also observed against peptides removed from HLA-DR molecules (3 kd or larger) by acid dissociation (22 per 105 CD4+ T cells). In sharp contrast, when LCL-derived bulk antigenic formats were loaded on immature DCs and screened with the CD8+ T cells of donor IP1, only low, if any, IFN-γ spot production was detected (less than 10 per 105 CD8+ T cells).
Freshly isolated T cells from EBV-seropositive healthy donor IP1 react against bulk antigenic formats prepared from autologous EBV B-LCL cells and presented by autologous DCs.
EBV B-LCL–derived bulk antigens (for preparation, see “Materials and methods”) were pulsed onto autologous immature DCs at a ratio of 100 tumor cell equivalents per DC and were screened for reactivity using CD4+ and CD8+ T-cell responders purified from the blood of donor IP1 (HLA-A2,32; B7,62; Cw3; DR4,15) in IFN-γ Elispot assays. Protein/peptide yields from 109 EBV B-LCL cells were in the range of the following: freeze–thaw lysates, 30 to 50 mg; TFA lysates, 10 to 30 mg; and eluted naturally presented peptides, 0.5 to 1 mg. Control wells contained T cells with untreated DCs. After a culture period of 20 hours, IFN-γ spots were developed and counted by computer-assisted video image analysis. Each bar represents the mean spot number of triplicates ± SD with 105 CD4+ T lymphocytes or CD8+ T lymphocytes initially seeded per well. The numbers of antigen-reactive T cells per 105 T lymphocytes are calculated by subtraction of mean spot numbers induced by untreated DCs from mean spot numbers induced by antigen-loaded DCs (asterisks indicate significant results, ie, P < .05). No T-cell responses were observed for freeze–thaw and TFA lysate fractions smaller than 10 kd, acid-eluted HLA-A2 peptide fraction 3 kd or larger, and acid-eluted HLA-DR peptide fraction smaller than 3 kd. Spot production was not detected when T cells were incubated with EBV B-LCL–derived bulk antigens in the absence of DCs. Results were confirmed in 4 independent experiments.
Freshly isolated T cells from EBV-seropositive healthy donor IP1 react against bulk antigenic formats prepared from autologous EBV B-LCL cells and presented by autologous DCs.
EBV B-LCL–derived bulk antigens (for preparation, see “Materials and methods”) were pulsed onto autologous immature DCs at a ratio of 100 tumor cell equivalents per DC and were screened for reactivity using CD4+ and CD8+ T-cell responders purified from the blood of donor IP1 (HLA-A2,32; B7,62; Cw3; DR4,15) in IFN-γ Elispot assays. Protein/peptide yields from 109 EBV B-LCL cells were in the range of the following: freeze–thaw lysates, 30 to 50 mg; TFA lysates, 10 to 30 mg; and eluted naturally presented peptides, 0.5 to 1 mg. Control wells contained T cells with untreated DCs. After a culture period of 20 hours, IFN-γ spots were developed and counted by computer-assisted video image analysis. Each bar represents the mean spot number of triplicates ± SD with 105 CD4+ T lymphocytes or CD8+ T lymphocytes initially seeded per well. The numbers of antigen-reactive T cells per 105 T lymphocytes are calculated by subtraction of mean spot numbers induced by untreated DCs from mean spot numbers induced by antigen-loaded DCs (asterisks indicate significant results, ie, P < .05). No T-cell responses were observed for freeze–thaw and TFA lysate fractions smaller than 10 kd, acid-eluted HLA-A2 peptide fraction 3 kd or larger, and acid-eluted HLA-DR peptide fraction smaller than 3 kd. Spot production was not detected when T cells were incubated with EBV B-LCL–derived bulk antigens in the absence of DCs. Results were confirmed in 4 independent experiments.
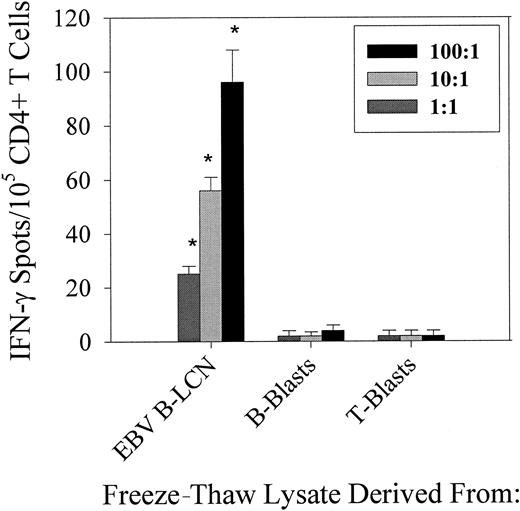
Autologous DCs pulsed with EBV B-LCL–derived freeze–thaw lysate induces EBV-specific CD4+T-cell responses in an antigen dose-dependent manner.
CD4+ T cells directly isolated from blood lymphocytes of healthy anti-EBV–positive donor IP1 were seeded at 105cells per well and were tested for reactivity against freeze–thaw lysate fractions 10 kd or larger prepared from autologous LCL cells, B-, or T-cell blasts in IFN-γ Elispot assays. For antigen processing and presentation, autologous immature DCs were prepulsed with freeze–thaw cell lysates at the ratio of cell equivalents per DCs of 100:1, 10:1, or 1:1. Resulting spots were evaluated and presented as described in Figure 1. Results were confirmed in 3 independent experiments.
Autologous DCs pulsed with EBV B-LCL–derived freeze–thaw lysate induces EBV-specific CD4+T-cell responses in an antigen dose-dependent manner.
CD4+ T cells directly isolated from blood lymphocytes of healthy anti-EBV–positive donor IP1 were seeded at 105cells per well and were tested for reactivity against freeze–thaw lysate fractions 10 kd or larger prepared from autologous LCL cells, B-, or T-cell blasts in IFN-γ Elispot assays. For antigen processing and presentation, autologous immature DCs were prepulsed with freeze–thaw cell lysates at the ratio of cell equivalents per DCs of 100:1, 10:1, or 1:1. Resulting spots were evaluated and presented as described in Figure 1. Results were confirmed in 3 independent experiments.
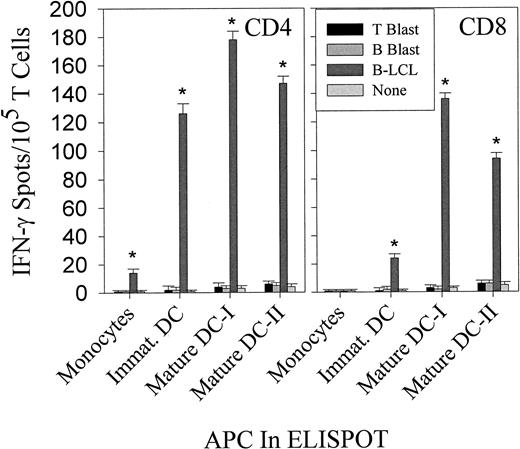
Mature DCs loaded with LCL-derived freeze–thaw lysates stimulate both CD4+ and CD8+ T-cell responses to EBV B-LCL antigens
We compared autologous monocytes with immature and mature DCs for their ability to induce IFN-γ spot production by purified CD4+ and CD8+ T lymphocytes after being pulsed with freeze–thaw lysates (10 kd or larger) from autologous EBV B-LCLs or B-cell or T-cell blasts. As shown in Figure3, mature DCs were the only APC capable of stimulating both CD4+ and CD8+ T cells reactive against epitopes derived from autologous EBV B-LCL freeze–thaw lysates. Of note, mature DCs that were prepulsed with EBV B-LCL lysates at the time when maturation was initiated (ie, mature DC-I) exhibited stronger T-cell stimulatory capacity than DCs that were loaded with EBV B-LCL lysates after DC maturation (ie, mature DC-II) was already achieved. Accordingly, for all subsequent experiments involving mature DCs, day 6 autologous immature DCs were first fed with freeze–thaw lysates (10 kd or larger) and then matured in vitro for 2 days using TNF-α, IL-1β, IL-6, and PGE2. In the control groups evaluated, autologous monocytes pulsed with EBV B-LCL lysates were less efficient in inducing significant IFN-γ spot production in donors' T cells, and T-cell responsiveness to lysates prepared from autologous B- or T-cell blasts was not observed. Interestingly, CD4+ and CD8+ T cells isolated from both donors displayed cross-reactivity against autologous mature DCs pulsed with allogeneic EBV B-LCL lysates, whereas they did not recognize lysates prepared from the corresponding matched allogeneic B-cell or T-cell blasts pulsed onto autologous DCs (Figure4).
Comparison of the ability of autologous monocytes and immature and mature DCs to stimulate CD4+ and CD8+ T-cell responses against EBV B-LCL freeze–thaw lysates in IFN-γ Elispot assays.
CD4+ and CD8+ T cells were directly isolated from the blood of EBV-seropositive healthy donor IP2 and were seeded at 105 cells per well. Autologous monocytes, immature DCs, or mature DCs were not pulsed or were pulsed with lysates derived from autologous EBV B-LCL cells, B-, or T-cell blasts (both 10 kd or larger) as indicated and were added to microwells containing T-cell responders. For maturation, immature DCs were treated on day 6 with TNF-α, IL-1β, IL-6, and PGE2 for 48 hours (see “Materials and methods”). Mature DC-Is were pulsed with lysate during the 48 hours of maturation from immature DC. Mature DC-IIs were first matured for 48 hours and then pulsed with the lysate for an additonal 48 hours prior to addition to Elispot wells. Resulting spots developed after 20-hour incubation were evaluated and presented as described in Figure 1. Each bar represents the mean spot number of triplicates ± SD with 105 CD4+ T lymphocytes or CD8+ T lymphocytes initially seeded per well. The data shown are from 1 representative experiment of 5 performed using donors IP1 and IP2.
Comparison of the ability of autologous monocytes and immature and mature DCs to stimulate CD4+ and CD8+ T-cell responses against EBV B-LCL freeze–thaw lysates in IFN-γ Elispot assays.
CD4+ and CD8+ T cells were directly isolated from the blood of EBV-seropositive healthy donor IP2 and were seeded at 105 cells per well. Autologous monocytes, immature DCs, or mature DCs were not pulsed or were pulsed with lysates derived from autologous EBV B-LCL cells, B-, or T-cell blasts (both 10 kd or larger) as indicated and were added to microwells containing T-cell responders. For maturation, immature DCs were treated on day 6 with TNF-α, IL-1β, IL-6, and PGE2 for 48 hours (see “Materials and methods”). Mature DC-Is were pulsed with lysate during the 48 hours of maturation from immature DC. Mature DC-IIs were first matured for 48 hours and then pulsed with the lysate for an additonal 48 hours prior to addition to Elispot wells. Resulting spots developed after 20-hour incubation were evaluated and presented as described in Figure 1. Each bar represents the mean spot number of triplicates ± SD with 105 CD4+ T lymphocytes or CD8+ T lymphocytes initially seeded per well. The data shown are from 1 representative experiment of 5 performed using donors IP1 and IP2.
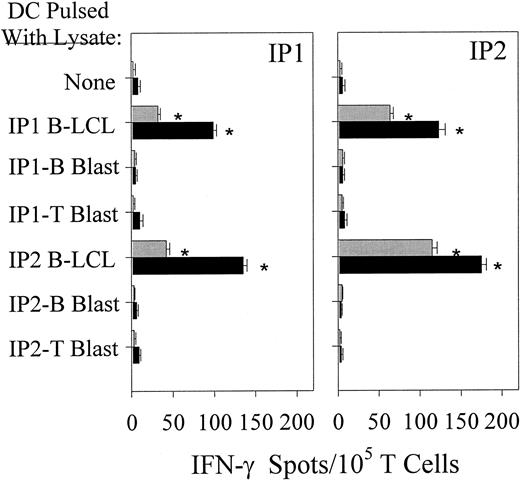
Mature DCs cross-present LCL-derived epitopes derived from freeze–thaw lysates to freshly isolated CD4+and CD8+ T cells.
Immature DCs generated from donors IP1 and IP2 were loaded with lysate fractions 10 kd or larger prepared from either donors' EBV B-LCL or B-cell/T-cell blasts. After maturation was induced (see “Materials and methods”), DCs were added to freshly isolated autologous CD4+ and CD8+ T cells in 20-hour IFN-γ Elispot assays. Resulting spots were developed and counted as described in Figure 1. Each bar represents the mean spot number of triplicates ± SD per 105 CD4+ T lymphocytes (▪) or CD8+ T lymphocytes (░) initially seeded per well. Calculation of lysate-responsive T-cell frequencies were performed as outlined in Figure 1. Results were confirmed in 4 independent experiments.
Mature DCs cross-present LCL-derived epitopes derived from freeze–thaw lysates to freshly isolated CD4+and CD8+ T cells.
Immature DCs generated from donors IP1 and IP2 were loaded with lysate fractions 10 kd or larger prepared from either donors' EBV B-LCL or B-cell/T-cell blasts. After maturation was induced (see “Materials and methods”), DCs were added to freshly isolated autologous CD4+ and CD8+ T cells in 20-hour IFN-γ Elispot assays. Resulting spots were developed and counted as described in Figure 1. Each bar represents the mean spot number of triplicates ± SD per 105 CD4+ T lymphocytes (▪) or CD8+ T lymphocytes (░) initially seeded per well. Calculation of lysate-responsive T-cell frequencies were performed as outlined in Figure 1. Results were confirmed in 4 independent experiments.
To further confirm the specificity of the T-cell response to allogeneic EBV B-LCL lysates obtained in donors IP1 and IP2 (Figure 4) and in several other healthy EBV carriers evaluated (results not shown), we performed IFN-γ Elispot analyses on CD4+ and CD8+ T cells freshly isolated from the blood of 3 EBV-seronegative donors. Reactivity against allogeneic EBV B-LCL lysates was not observed in any of these individuals, irrespective of whether immature or mature DCs were used as APC (data not shown), suggesting the absence of anti-EBV “memory” T cells.
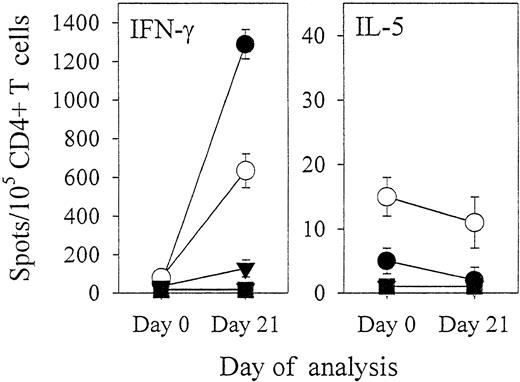
We next investigated how effectively repeated in vitro stimulations of CD4+ and CD8+ T cells with EBV B-LCL lysate-pulsed mature DCs were able to generate effector T lymphocytes exhibiting reactivity against EBV B-LCL target cells. CD4+T cells purified from donor IP1 were stimulated weekly with autologous mature DCs preloaded with freeze–thaw lysates (10 kd or larger) derived from autologous EBV B-LCLs or T-cell blasts. In parallel, T cells were also stimulated on a weekly basis with the autologous EBV B-LCL. Compared with freshly isolated CD4+ T cells of donor IP1, day 21–cultured CD4+ responder lymphocytes induced with autologous DCs and lysate prepared from the autologous EBV B-LCL showed a 6- to 13-fold increase in the frequency of T cells recognizing autologous EBV B-LCL cells or EBV B-LCL lysate-pulsed DCs as determined by IFN-γ Elispot assays (Figure 5). In contrast, this responder lymphocyte population did not recognize DCs pulsed with freeze–thaw lysates (10 kd or larger) prepared from autologous B- or T-cell blasts. To provide further evidence that these CD4+ T-cell responders induced by EBV B-LCL lysate-pulsed DCs were directed against EBV B-LCL antigens, we analyzed their reactivity against naturally processed peptides acid-eluted from affinity-purified HLA-DR complexes of the autologous EBV B-LCL. Compared with freshly isolated CD4+ T cells of donor IP1, day 21 EBV B-LCL lysate-induced CD4+ T lymphocytes showed an 8-fold increase in the frequency of T cells recognizing naturally processed HLA-DR–associated peptides derived from the autologous EBV B-LCL (Figure 5). T-cell reactivity against EBV B-LCL lysates was Th1 type because most CD4+ T cells secreted IFN-γ, with only a few secreting IL-5 (Figure 5). In the control groups evaluated, CD4+ T-cell responders stimulated on a weekly basis with autologous DCs preloaded with the autologous T-cell blast lysate (10 kd or larger) did not respond to autologous EBV B-LCL cells, EBV B-LCL lysate-pulsed DCs, or HLA-DR–associated natural peptides derived from the autologous EBV B-LCL (data not shown). In comparison with freshly isolated CD4+ T cells of donor IP1, a 15-fold increase in the frequency of T cells reactive against autologous EBV B-LCL cells was observed in day 21–cultured CD4+ responder lymphocytes that had been stimulated on a weekly basis with intact autologous EBV B-LCL cells (data not shown).
CD4+ T cells reactive against autologous EBV B-LCL cells may be efficiently induced by repeated stimulations with LCL lysate-pulsed mature autologous DCs.
CD4+ T cells freshly isolated from EBV-seropositive donor IP1 were stimulated on a weekly regimen (days 0, 7, and 14) with autologous mature DCs prepulsed with freeze–thaw lysate (10 kd or larger) prepared from autologous EBV B-LCL. Freshly isolated (day 0) CD4+ T cells and T lymphocyte responders harvested on day 21 of culture (both seeded in triplicates at 105 and 104 cells per well) were analyzed in IFN-γ and IL-5 Elispot assays. T-cell reactivity was screened against intact autologous EBV B-LCL (●); against autologous mature DCs preloaded with freeze–thaw lysates (10 kd or larger) isolated from autologous EBV B-LCL (○), B-cell blasts (▾), or T-cell blasts (▪); and against autologous mature DCs pulsed with naturally processed peptides acid-eluted from affinity-purified HLA-DR complexes of the autologous EBV B-LCL (■). Resulting spots were developed and evaluated as described in Figure 1. Spot production observed in microwells where CD4+ (responder) lymphocytes were seeded with the autologous EBV B-LCL or with mature DCs loaded with the EBV B-LCL lysate was completely blocked by the addition of the anti-HLA-DR (class II) antibody L243 (100 μg/mL) but not by the anti-HLA class I antibody W6/32 (100 μg/mL). Results were confirmed in 2 independent experiments.
CD4+ T cells reactive against autologous EBV B-LCL cells may be efficiently induced by repeated stimulations with LCL lysate-pulsed mature autologous DCs.
CD4+ T cells freshly isolated from EBV-seropositive donor IP1 were stimulated on a weekly regimen (days 0, 7, and 14) with autologous mature DCs prepulsed with freeze–thaw lysate (10 kd or larger) prepared from autologous EBV B-LCL. Freshly isolated (day 0) CD4+ T cells and T lymphocyte responders harvested on day 21 of culture (both seeded in triplicates at 105 and 104 cells per well) were analyzed in IFN-γ and IL-5 Elispot assays. T-cell reactivity was screened against intact autologous EBV B-LCL (●); against autologous mature DCs preloaded with freeze–thaw lysates (10 kd or larger) isolated from autologous EBV B-LCL (○), B-cell blasts (▾), or T-cell blasts (▪); and against autologous mature DCs pulsed with naturally processed peptides acid-eluted from affinity-purified HLA-DR complexes of the autologous EBV B-LCL (■). Resulting spots were developed and evaluated as described in Figure 1. Spot production observed in microwells where CD4+ (responder) lymphocytes were seeded with the autologous EBV B-LCL or with mature DCs loaded with the EBV B-LCL lysate was completely blocked by the addition of the anti-HLA-DR (class II) antibody L243 (100 μg/mL) but not by the anti-HLA class I antibody W6/32 (100 μg/mL). Results were confirmed in 2 independent experiments.
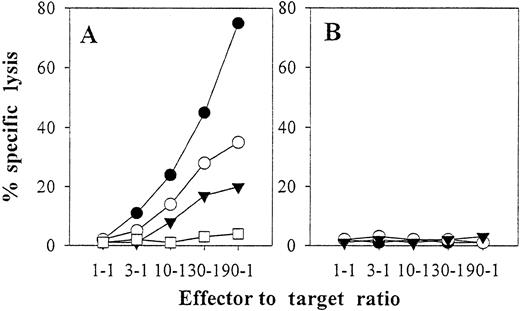
CD8+ T cells were stimulated weekly with autologous intact EBV B-LCL cells or with autologous mature DCs preloaded with freeze–thaw lysates (10 kd or larger) prepared from autologous EBV B-LCL, autologous T-cell blasts, or allogenic EBV-B LCL. Responder lymphocytes generated in this way were predominantly CD45RO+ as assessed by flow cytometry, indicating the expansion of “memory” CD8+ T lymphocytes. When we tested the cytolytic activity of day 23–cultured T-cell responders, maximum reactivity was observed against the autologous EBV B-LCL if T-cell cultures were stimulated with intact EBV B-LCL cells (Figure6). Lower, but still significant, levels of lysis against autologous EBV B-LCL cells were obtained if responder lymphocytes were instead induced with autologous mature DCs preloaded with freeze–thaw lysates prepared from either autologous or allogenic EBV B-LCLs (Figures 6 and 7). In contrast, responder T cells stimulated with autologous mature DCs prepulsed with the freeze–thaw lysate derived from autologous T-cell blasts did not recognize autologous EBV B-LCL cells (Figure 6). Further, cytolytic activity against autologous T-cell blasts was not observed in any of the responder lymphocyte populations tested.
Autologous mature DCs pulsed with EBV B-LCL cells versus freeze–thaw lysates stimulate anti-EBV CD8+ CTL in vitro.
CD8+ T cells were purified from the blood of EBV-seropositive donor IP2 and were then repetitively stimulated on a weekly basis (days 0, 7, and 14) with autologous intact EBV B-LCL cells (●) or with autologous mature DCs preloaded with freeze–thaw lysates (10 kd or larger) prepared from autologous EBV B-LCL (○), autologous T-cell blasts (■), or allogeneic EBV-B LCL of donor IP1 (▾). On day 23 of culture, responder lymphocytes were harvested and were tested in a 6-hour 51Cr release assay at the indicated effector-to-target ratios for cytolytic activity against autologous EBV B-LCL (A) or autologous T-cell blasts (B) in the presence of a 20-fold excess of nonlabeled K562 competitors. For all responder lymphocyte cultures, lysis of labeled K562 in the presence of a 20-fold excess of nonlabeled K562 was below 5% at all effector-to-target ratios evaluated (not shown). The data depicted are from 1 representative experiment of 3 performed.
Autologous mature DCs pulsed with EBV B-LCL cells versus freeze–thaw lysates stimulate anti-EBV CD8+ CTL in vitro.
CD8+ T cells were purified from the blood of EBV-seropositive donor IP2 and were then repetitively stimulated on a weekly basis (days 0, 7, and 14) with autologous intact EBV B-LCL cells (●) or with autologous mature DCs preloaded with freeze–thaw lysates (10 kd or larger) prepared from autologous EBV B-LCL (○), autologous T-cell blasts (■), or allogeneic EBV-B LCL of donor IP1 (▾). On day 23 of culture, responder lymphocytes were harvested and were tested in a 6-hour 51Cr release assay at the indicated effector-to-target ratios for cytolytic activity against autologous EBV B-LCL (A) or autologous T-cell blasts (B) in the presence of a 20-fold excess of nonlabeled K562 competitors. For all responder lymphocyte cultures, lysis of labeled K562 in the presence of a 20-fold excess of nonlabeled K562 was below 5% at all effector-to-target ratios evaluated (not shown). The data depicted are from 1 representative experiment of 3 performed.
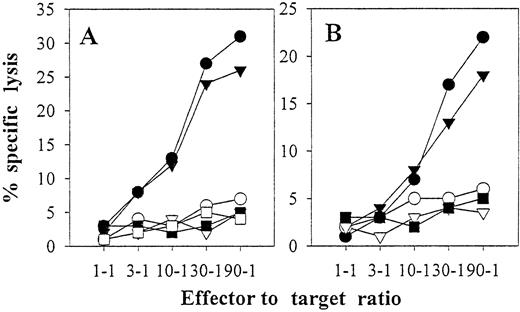
Autologous mature DCs pulsed with autologous or allogeneic EBV B-LCL freeze–thaw lysates stimulate anti-EBV CD8+ CTL in vitro.
Purified CD8+ lymphocytes from EBV-seropositive donor IP3 were stimulated with autologous DCs loaded with freeze–thaw lysates prepared from autologous IP3 EBV B-LCL (A) or allogeneic IP2 EBV B-LCL (B) as described in “Materials and methods” and Figure 6. Six-hour51Cr-release assays were performed on day 24 (10 days after restimulation on day 14). K562 cells were not added as cold-target inhibitors because T-cell specific lysis of K562 was less than 5% at all effector-to-target ratios (data not shown). The percent specific lysis is reported against IP1 EBV B-LCL (▪), IP2 EBV B-LCL (▿), IP3 EBV B-LCL (●), IP3 EBV B-LCL in the presence of blocking mAb directed against MHC class I (W6/32, ○) or MHC class II (L243, ▾) molecules, or IP3 T blasts (■). Donors IP1, IP2, and IP3 are completely mismatched for HLA class I.
Autologous mature DCs pulsed with autologous or allogeneic EBV B-LCL freeze–thaw lysates stimulate anti-EBV CD8+ CTL in vitro.
Purified CD8+ lymphocytes from EBV-seropositive donor IP3 were stimulated with autologous DCs loaded with freeze–thaw lysates prepared from autologous IP3 EBV B-LCL (A) or allogeneic IP2 EBV B-LCL (B) as described in “Materials and methods” and Figure 6. Six-hour51Cr-release assays were performed on day 24 (10 days after restimulation on day 14). K562 cells were not added as cold-target inhibitors because T-cell specific lysis of K562 was less than 5% at all effector-to-target ratios (data not shown). The percent specific lysis is reported against IP1 EBV B-LCL (▪), IP2 EBV B-LCL (▿), IP3 EBV B-LCL (●), IP3 EBV B-LCL in the presence of blocking mAb directed against MHC class I (W6/32, ○) or MHC class II (L243, ▾) molecules, or IP3 T blasts (■). Donors IP1, IP2, and IP3 are completely mismatched for HLA class I.
CD8+ T cells stimulated by autologous DCs pulsed with either auto- or allo-EBV B-LCL freeze–thaw lysates killed in a class I–restricted manner (Figure 7) and recognized autologous EBV B-LCL targets but not completely HLA-mismatched allogeneic EBV B-LCL (Figure 7). The inability of cytotoxic T lymphocytes (CTL) induced by autologous DCs plus allogeneic EBV B-LCL lysates to recognize the EBV B-LCL from which the lysate was derived (Figure 7B) argues strongly against the induction of allospecific CTL using this in vitro induction protocol.
Discussion
Among bulk antigenic formats prepared from autologous EBV B-LCLs, freeze–thaw lysates were clearly the most efficient in stimulating CD4+ T lymphocytes when processed and presented by autologous DCs (Figure 1). The superior immunogenicity of freeze–thaw lysates is not intuitively obvious but may reflect differential antigen extraction efficiency, differential retention of immunogenic proteins in freeze–thaw lysates, differential uptake of freeze–thaw antigens by DCs, or the differential presence of DC activators in freeze–thaw lysates5 10 among other reasons.
The fact that lysates obtained from identically grown B- or T-cell blasts were not recognized by CD4+ T cells argues against reactivity directed towards epitopes derived from autoantigens or FCS proteins (Figures 2-5). Furthermore, freshly isolated T-cell responses directed against EBV B-LCL–derived material was only observed in EBV-seropositive donors, supporting the anti-EBV “specificity” of these “memory” T-cell reactivities. Immune reactivity against EBV B-LCL lysates was primarily Th1 type because most responder T cells secreted IFN-γ and only a few secreted IL-5 (Figure 5). Although the frequency of T cells recognizing intact autologous EBV B-LCL cells was clearly higher among CD8+ T cells than among CD4+ T cells, EBV B-LCL–derived antigens loaded on immature DCs induced only low, if any, IFN-γ spot production by CD8+ T cells (Figure 1). In contrast, strong anti-EBV reactivity was observed for CD4+ T cells (Figure 1). In donors IP1 and IP2, we compared autologous monocytes, immature, and mature DCs for their ability to stimulate T-cell reactivity against freeze–thaw lysate-derived epitopes prepared from autologous EBV B-LCLs. Maximum IFN-γ spot production by both CD4+ and CD8+ T cells was observed when DCs were matured after they were fed with EBV B-LCL lysates (Figure 3). CD4+ and CD8+ responder T cells stimulated on a weekly regimen with mature DCs prepulsed with EBV B-LCL lysates specifically recognized EBV B-LCL cells (IFN-γ production [Figure 5] and cytolytic activity [Figure 6]). With the use of computer-assisted video image analysis,9 we also measured the size of IFN-γ spots (reflective of the magnitude of cytokine secreted at the single-cell level) produced by CD4+ and CD8+ T cells after the addition of EBV B-LCL lysate-loaded immature or mature DCs. Spots that appeared in the presence of lysate-pulsed mature DCs had the largest areas, whereas spots occurring with lysate-pulsed immature DCs were comparatively smaller (data not shown). Intuitively, mature DCs that express significantly higher levels of HLA and costimulatory molecules have a superior ability to induce IFN-γ production in EBV-specific “memory” T cells compared with immature DCs. Because reactivation of “memory” antitumor T cells may represent a primary goal of tumor vaccines, DC-based approaches should arguably implement mature DCs. In approaches using tumor lysates, DC maturation should be induced prior to or concurrent with lysate delivery to DC. Based on data provided in the current study, freeze–thaw lysates are vastly superior to TFA lysates when provided to DCs to elicit specific T-cell immune responses.
Interestingly, T cells isolated from EBV-seropositive donors responded to autologous DCs pulsed with freeze–thaw lysates prepared from either autologous or allogeneic EBV B-LCLs but not DCs loaded with lysates derived from the corresponding B- or T-cell blasts (Figure 4). We confirmed this finding in several other healthy individuals previously infected with EBV (data not shown). The fact that “memory” T-cell reactivity against allogeneic EBV B-LCL lysates was only observed in anti-EBV–positive individuals (and not in donors seronegative for EBV; data not shown) provides further evidence that T cells responding to EBV B-LCL lysate-loaded DCs recognize EBV-related antigens. In addition, these results suggest that EBV B-LCL lysates, irrespective of the donor from which they were derived, contain “shared” antigens that can yield epitopes that are “cross-presented” by DCs and recognized by CD4+ and CD8+ T cells. This phenomenon might be explained by the general observation that reactivity against lysates was exclusively found in lysate fractions containing molecules larger than 10 kd (Figure 1). Naturally processed and presented HLA-binding oligopeptides are expected to be smaller than 10 kd, whereas the EBV B-LCL lysate fractions 10 kd and larger obviously contain large “shared” EBV proteins that after appropriate processing and presentation by autologous, mature DCs are recognized by the individual T-cell systems. The finding that EBV B-LCL lysates can stimulate LCL-specific CD8+ T-cell responses is in agreement with earlier studies by others demonstrating that MHC class I presentation of exogenous, soluble antigens can be achieved by professional APC both in vitro and in vivo but requires high concentrations of antigens.11-15This has also recently been documented for alternate “antigens” such as nonreplicating microbes16 and apoptotic or infected cells,17 in which DCs were observed to process and present epitopes in MHC class I complexes that are derived from these endocytosed organisms that conceptually have limited access to the DC cytosol. Of note, by processing dying cells, DCs were even able to “cross-prime” T cells exhibiting specificity for “shared” viral antigens.17
In general, the use of tumor freeze–thaw lysates as a source of antigen for pulsing autologous DCs appears to represent an attractive approach to optimally activate a broad repertoire of antigen-specific CD4+ and CD8+ T cells. This is particularly compelling for prospective clinical vaccines designed to treat cancer histologies for which well-characterized tumor antigens are limited in number or are yet to be defined. Further, this approach incorporates any idiotypic epitopes or antigens that may derive from mutational events associated with the tumorigenic process of a given individual. It has recently been reported that certain human melanoma vaccines generated from mechanical or freeze–thaw lysates can stimulate melanoma-specific T cells.18-20 There is also evidence from murine studies that DCs pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo.21 22 Indeed, our own preliminary data support the ability of this procedure to promote the expansion of CD4+and CD8+ T cells specific for melanoma, renal cell carcinoma, or squamous cell carcinoma of the head and neck from patient peripheral blood lymphocyte responders (W.J.S., unpublished data).
The use of tumor (autologous or allogeneic) lysate as an antigen source for vaccine construction circumvents the need for viable fresh tumor cells and the need to establish tumor cell lines in vitro, which may prove logistically difficult to acquire or time-consuming to produce. Because human cancers have been shown to elicit multi-epitope–specific immune responses in vivo, the approach of using tumor lysates pulsed onto DCs would offer the potential advantage of inducing a broader T-cell response to tumor-associated antigens than could be achieved by pulsing DCs with a single or with several defined synthetic tumor peptides. This strategy potentially lessens the possibility of immune escape by evolving tumors in the face of a broader, polyspecific antitumor T-cell immune response. In addition, greater potential exists for the simultaneous presentation of CTL-defined and T-helper–defined epitopes by lysate-pulsed DCs for adoptive application in clinical vaccines. This may be particularly true for mature DCs. After maturation, DCs express enhanced levels of HLA and costimulatory molecules and heightened cytokine production that may optimally activate and maintain both CD4+ and CD8+antigen-specific T cells in vivo.3 In this regard, although several HLA class I–presented tumor-associated epitopes have been defined by human CTL,23 limited knowledge exists about the identity of CD4+ T-cell–defined tumor-associated epitopes. This represents a glaring deficiency in our knowledge base because there is clear evidence from both in vitro and in vivo studies that the successful induction of durable cellular immunity in chronic diseases (ie, viral infections or cancer) requires the activation of both antigen-specific CD4+ and antigen-specific CD8+ T cells.24 25
The use of tumor lysates as a vaccine component, however, has the potential disadvantage that this approach might induce pathologic autoimmune reactivity to normal tissue antigens as a consequence of the processing and presentation of “housekeeping” or “lineage-associated” epitopes by autologous DCs. However, our studies evaluating T-cell responsiveness to DCs loaded with EBV B-LCL lysates were unable to demonstrate responder T-cell cross-reactivity against B- or T-cell blasts. Furthermore, DCs pulsed with lysates derived from T-cell blasts were unable to promote the expansion of reactivity to “self” T-cell–associated antigens. This may reflect the comparative threshold density of a given epitope presented by MHC molecules on the surface of a tumor cell (ie, overexpressed antigens) versus normal cells.26 27
The use of this approach, applied in clinical vaccine trials, may be of significant value in the treatment of cancer or transformed cells such as EBV-associated lymphomas observed in PTLD,28,29which is a frequent tumor in allograft recipients that develops mostly after prolonged immunosuppression. There is also evidence that EBV plays a major role in the etiology of Burkitt's lymphoma, Hodgkin's lymphoma, and undifferentiated nasopharyngeal carcinoma.30The rationale for using EBV B-LCL lysates as a vaccine in patients suffering from EBV-associated tumors derives from the observation that at least some of the latent EBV proteins expressed in EBV B-LCL represent potential targets for viral-specific T-cell responses in EBV-positive malignancies.31 32 We observed that CD4+ and CD8+ T cells reactive against autologous EBV B-LCL cells could be coordinately generated by in vitro stimulation with mature DCs preloaded with lysates from allogenic (HLA completely mismatched) EBV B-LCL. Importantly, this anti-EBV B-LCL reactivity occurred in the absence of detectable allospecific T-cell reactivity (cytokine secretion or cytotoxicity). This encourages the potential use of a single “off-the-shelf” standard EBV B-LCL lysate preparation to be applied to DCs in generating a general vaccine for these tumor-bearing patients irrespective of their HLA type. This may prove logistically attractive in the clinical setting, where the generation of autologous EBV B-LCL for clinical application is not always attained and requires extended culture periods of 4 to 5 weeks. Overall, these observations may be extrapolated to alternative tumor histologies using either freshly resected tumor material or a reference lineage-matched tumor cell line from which to generate the lysate for clinical application.
Acknowledgments
The authors thank Drs Lisa Salvucci Kierstead, Russell Salter, and Jan Mueller-Berghaus for careful review and helpful discussion in the generation of this manuscript.
Supported by National Institutes of Health grant CA 57840 (W.J.S.), a clinical investigator award from the Cancer Research Institute (W.J.S.), CNR-NATO grant 216.1919 (L.G.), NATO collaborative research grant CRG.CRG 973153 (L.G., W.J.S.), and a fellowship from the Deutsche Forschungsgemeinschaft (He 2896/1-1; W.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Walter J. Storkus, W1555 Biomedical Sciences Tower, University of Pittsburgh School of Medicine, 200 Lothrop St, Pittsburgh, PA 15261; e-mail: storkuswj@msx.upmc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal