Abstract
The von Willebrand factor propeptide, vW AgII, has been shown to be required for the formation of vWF multimers and sorting of vWF to storage granules; whether these 2 processes are independent events has been unclear. Chimeric constructs of human and canine vWF were developed to further define these processes and to determine whether they are independent intracellular events. Cells expressing only mature vWF (Δpro) produced vWF dimers that were not stored in AtT-20 cells; whereas the expression of vW AgII alone resulted in vW AgII granular storage. Expression of vW AgII in trans with Δpro resulted in the multimerization of vWF and colocalized storage of vW AgII and vWF. Expression of canine vW AgII in trans orcis with human Δpro resulted in the multimerization of human vWF, with no storage of human vWF but with normal storage of canine vW AgII. This dissociation of functions indicates that the signals for multimerization of vWF are different from the signals for trafficking of vWF to storage and demonstrates that vWF storage and multimerization are 2 independent intracellular processes. vW AgII contains the signal(s) required for trafficking to storage, and only through interaction with vW AgII is vWF chaperoned into granules.
Introduction
Von Willebrand factor (vWF) is a large, adhesive glycoprotein that performs 2 essential roles in hemostasis. It mediates the attachment of platelets, through their glycoprotein Ib receptor, to subendothelial tissue at the site of vascular injury, and it serves as the carrier protein for coagulation factor VIII, protecting it from proteolytic degradation by plasma enzymes.1,2 Decreased levels or defects in vWF are identified in patients with von Willebrand disease, a common hereditary bleeding disorder.3
vWF is synthesized exclusively in megakaryocytes and endothelial cells.4,5 The pre-pro-vWF molecule is synthesized as a 22-amino acid signal peptide, 741-amino acid propeptide (also known as von Willebrand antigen II, vW AgII), and the 2050-amino acid mature vWF protein. Pro-vWF exhibits considerable internal homology and is composed of 4 types of domains (A-D) linked as follows: NH2-D1-D2-D′-D3-A1-A2-A3-D4-B1-B2-B3-C1-C2-COOH. The propeptide consists of D1 and D2 domains, and the mature vWF subunit begins with the D′ domain.1,2 The precursor pre-pro-vWF protein undergoes an extensive series of intracellular modifications. In the endoplasmic reticulum, the signal peptide is cleaved and pro-vWF forms C-terminal dimers. On migration through the Golgi and post-Golgi compartments, vWF is subjected to further modifications, including carbohydrate processing, sulfation, and amino-terminal multimerization of C-terminal dimers.1,6,7 Proteolytic processing yields the propeptide known as vW AgII and mature vWF multimers. Both amino-terminal multimerization and propeptide cleavage are thought to occur in the trans-Golgi network (TGN).8 The paired dibasic amino acid-cleaving enzyme (PACE or furin) is localized to the TGN and has been identified as the vWF proteolytic processing enzyme.9-11 vWF is stored in α-granules in megakaryocytes or Weibel-Palade bodies in endothelial cells.12-15
The large propeptide is required for the formation of vWF multimers and the sorting of vWF to storage granules.16,17 Both propeptide domains, D1 and D2, have been shown to be necessary for multimerization.18 Deletion of either D1 or D2 or removing the entire propeptide results in the expression of only C-terminal dimers that are not sorted to storage granules.16-18 The homologous D-domains are rich in cysteine residues, with alignment of 23 cysteines between the 4 D-domains.19 The D1 and D2 domains contain vicinal cysteines similar to those found at the active site of disulfide isomerases. The propeptide may engage in intrinsic disulfide isomerase activity that is involved in catalyzing the disulfide bond-forming events of vWF and multimerization.20 Propeptides of several enzymes and hormones have been shown to mediate the folding of their mature protein molecules.21-24
The propeptide is required for targeting vWF to storage granules.18,25 The propeptide remains noncovalently associated with vWF and is found in a 1:1 stoichiometric ratio in Weibel-Palade bodies.26,27 This continued association suggests that the 2 proteins traffic together and that the propeptide may play a role in trafficking vWF to secretory granules. Such a function has been demonstrated for the propeptide of prosomatostatin that has been shown to be necessary for the storage of mature somatostatin protein.28,29 The mechanism by which proteins are sorted to storage granules is not well defined. Secretory proteins appear to condense in the TGN, and this aggregation may be a key event for the formation of protein storage granules.30-33Another hypothesis proposes that there exists targeting sequence(s) on the stored protein that interacts with specific receptor(s) to initiate storage.30-33 The propeptide vW AgII may serve a role in promoting the aggregation of vWF, facilitating granular storage, it may contain the targeting signal(s) necessary for sorting into the storage pathway, or it may do both.25
In the current study, chimeric constructs of human and canine vWF cDNA were constructed and transfected into AtT-20 cells to further assess the vWF multimerization and storage processes and to determine whether they are independent events. Our results establish that vW AgII not only independently mediates multimerization, it also functions as an intracellular chaperone, directing vWF to regulated storage. The portion of the sequence within vW AgII that mediates multimerization is different from the signal(s) responsible for the association with, and the subsequent sorting of, vWF to storage granules in AtT-20 cells. Furthermore, our results substantiate that the proteolytic cleavage of vW AgII from vWF occurs before the sorting of vWF to storage granules.
Materials and methods
Construction of expression plasmids
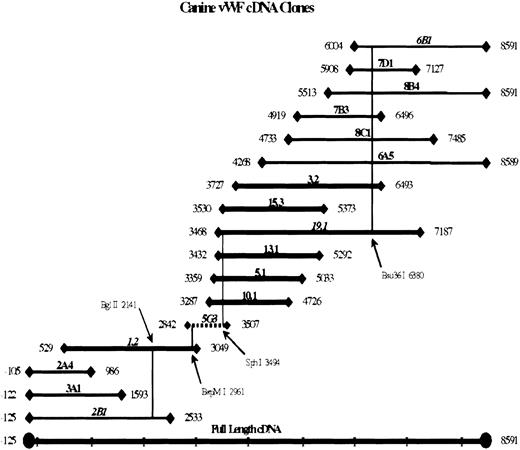
A λgt11 canine heart cDNA library (a kind gift of David Mancusso, Washington University, St. Louis, MO) was screened using a mixture of human and canine vWF cDNA random-primed32P-labeled probes. The 3 probes used were the 1614-bpBsu36I/PvuI fragment, the 1012-bpPvuI/Bsu36I fragment of human vWF cDNA, and the 1127-bp Bsu36I/Asp718I fragment of a chimeric human–canine vWF cDNA construct. Bases 3690 to 4481 of this plasmid are derived from a polymerase chain reaction (PCR) amplification of vWF exon 28 from canine genomic gDNA. Inserts were released from hybridization-positive λ clones by digestion with EcoRI and subcloned into the EcoRI site of pGEM-7Zf(+) (Promega, Madison, WI). End-sequencing of the inserts revealed that 7 clones (shown as bold lines in Figure 1) contained portions of the canine vWF cDNA sequence. Internal sequences of the inserts were obtained by sequence walking, synthesizing additional primers as new sequences became available. A second round of library screening was performed using probes prepared from the most distal 5′ and 3′ sequences obtained in the initial screening. Nine additional vWF-containing clones were obtained and sequenced (shown as thin lines in Figure 1), extending coverage into both 5′- and 3′-untranslated sequences.
Canine vWF cDNA clones.
Clones obtained by screening a λgt11 canine heart cDNA library are shown schematically with reference to the full-length cDNA. The 5′ and 3′ ends of the inserts are indicated, with base 1 the adenine of the initiator methionine. Bold lines represent clones obtained in a first round of screening using mixed human and canine vWF probes. A second round of screening was performed using 2 new canine probes consisting of the 5′ end of clone 1.2 and the 3′ end of clone 19.1. Thin lines represent clones from the rescreening. Clone 5G3 was obtained by PCR amplification across the only remaining gap, between bases 3049 and 3287, and is shown as a dashed line. Clones 2B1, 1.2, 5G3, 19.1, and 6B1 were linked as a full-length cDNA in a series of ligations to produce the construct pKVneo. Restriction site junctions are depicted as vertical lines.
Canine vWF cDNA clones.
Clones obtained by screening a λgt11 canine heart cDNA library are shown schematically with reference to the full-length cDNA. The 5′ and 3′ ends of the inserts are indicated, with base 1 the adenine of the initiator methionine. Bold lines represent clones obtained in a first round of screening using mixed human and canine vWF probes. A second round of screening was performed using 2 new canine probes consisting of the 5′ end of clone 1.2 and the 3′ end of clone 19.1. Thin lines represent clones from the rescreening. Clone 5G3 was obtained by PCR amplification across the only remaining gap, between bases 3049 and 3287, and is shown as a dashed line. Clones 2B1, 1.2, 5G3, 19.1, and 6B1 were linked as a full-length cDNA in a series of ligations to produce the construct pKVneo. Restriction site junctions are depicted as vertical lines.
The 16 clones obtained span the entire canine vWF cDNA sequence, with the exception of a 238-bp gap between bases 3049 and 3287. The canine heart cDNA library was PCR amplified to obtain the sequence across this gap. The PCR product was cloned into pCR 2.1 vector using the TA-cloning kit (Invitrogen, Carlsbad, CA), resulting in clone 5G3 containing bases 2842 to 3507 of canine vWF cDNA (shown as a dashed line in Figure 1). The sequence in common with other clones matches exactly.
Overlapping clones were assembled into a full-length canine vWF cDNA using standard cloning methods. The vertical lines in Figure 1 indicate the restriction enzyme junctions used for reconstruction of the full-length cDNA from clones 2B1, 1.2, 5G3, 19.1, and 6B1 as indicated. The complete cDNA sequence was submitted to GenBank (accession #U66246). The expression plasmid pKVneo was created by inserting the 8.7-kb AvrII/NotI fragment of full-length canine vWF cDNA (bases –78 to 8591) intoXbaI/NotI-digested pCIneo (Promega).
The human vWF expression plasmid pHVneo consists of the full-length cDNA insert from pVW198 in pCIneo.34 pVW198 was digested with AvrII (base −83) and DraI (base 8640) and ligated to the compatible XbaI and EcoRV sites of pCIneo.
The vWF signal sequence (ending at base 66) was joined directly to the mature vWF sequence (starting at base 2290), deleting the entire propeptide sequence (Δpro) using a strategy based on the type IIS restriction enzyme BsmBI.35 36 Human Δpro vWF was assembled by joining a pair of PCR products containing customized cohesive ends at the desired splice site using pVW198 as the amplification template. The 280-bp “left-half” PCR extended 3′ from base 66 using an antisense primer containing a BsmBI site (in bold) 5′-agt cgtctc tCTAC A AAG GGT CCC TGG CAA AAT GAG-3′ and sense primer Hu-s-pVW198(−214)–(−187) in the flanking plasmid sequence. The 886-bp “right-half” PCR extended 5′ from base 2290 using a sense primer containing aBsmBI site 5′-cgc cgtctc tGTAG C CTA TCC TGT CGG CCC CCC ATG-3′ and antisense vWF primer a3176-3144:KpnI 5′-GA GGg taC CAC CAT CGT CTG CTT CAT GAT GTT G-3′. The fidelity of all PCR-derived segments incorporated into final constructs was confirmed by DNA sequencing. After joining the 2 PCR products at the compatible cohesive overhangs created byBsmBI digestion (underlined in the primer sequences above), the AvrII/BamH vWF cassette (bases −83 to 66/2290-2717) was substituted for the fragment (bases -83 to 2717) of pHVneo to assemble the final Hu-Δpro expression plasmid. The K9-Δpro plasmid was created in a similar manner.
Chimeras were created consisting of human propeptide in cis(ie, on the same molecule) with canine mature vWF (Hu/K9-vWF) and the converse (K9/Hu-vWF). The conserved NcoI site at base 2309 was used to join the propeptide sequence of each species to the mature vWF sequence of the other. Because the amino acid sequence between the propeptide cleavage site (base 2290) and the NcoI site is completely conserved between dogs and humans, the protein produced consists of the human propeptide contiguous with mature canine vWF, and the converse.
Human and canine constructs expressing propeptide-only (vW-AgII) were produced by PCR amplifications from cDNA templates using a mutagenic primer to place a stop codon at the normal propeptide cleavage site. Insertion of a T after base 2289 creates the TAG stop codon. This insertion, together with 2 silent mutations in the C-terminal arginine codon, created a unique NheI site. The human AgII/Stop PCR product was ligated to the remainder of the human propeptide cDNA at the unique HindIII site (base 2235). The Hu-vW-AgII expression construct was completed by inserting the fragment produced by digestion with AvrII (base −83) and NheI (at the newly created Stop) into the compatible XbaI site of pCIneo. The canine AgII/Stop PCR product was joined to the 5′-end of the canine propeptide sequence at the unique BssHII site (base 1751), then digested with AvrII and NheI and ligated into the XbaI site of pCIneo.
Cell culture
Two cell lines were used in this study: human embryonic kidney cells (HEK293T), which were kindly provided by David Ginsburg (University of Michigan, Ann Arbor, MI), and mouse pituitary tumor cells (AtT-20/D16v-F2, CRL 1795; American Type Culture Collection). Both cell lines were cultured at 37°C in an atmosphere of 5% CO2. AtT-20 cells were grown in Dulbecco modified Eagle's medium with high glucose supplemented with 10% fetal bovine serum and 2 mmol/L l-glutamine. HEK293T cells were grown in minimal essential medium Eagle (Life Technologies, Grand Island, NY) with Earle's salts and L-glutamine supplemented with 10% fetal bovine serum.
Mammalian cell transfections
AtT-20 and HEK293T cells were transiently transfected with expression plasmid DNA using LipofectAMINE with the LipofectAMINE PLUS reagent (Life Technologies). Typically, 24 hours before transfection, 3 × 105 cells were plated in a 35-mm dish. Cells were incubated with 1 μg DNA, 3 μg PLUS reagent, and 16 μg LipofectAMINE according to the manufacturer's instructions and were diluted in a final volume of 2.2 mL in OptiMEM serum-free medium (both Life Technologies) for 3 to 5 hours at 37°C. The medium containing the DNA–lipid complexes was then replaced with complete medium, and the cells were incubated for an additional 72 hours. Negative controls were either nontransfected or mock transfected with expression vector lacking the insert. Conditioned media were harvested from the cells, centrifuged to remove debris, and frozen at −80°C for further analysis. Transfected AtT-20 cells, which have been shown to store heterologously expressed proteins, were fixed for immunofluorescent staining.25
Antibodies
Monoclonal antibodies AvW-5, AvW-17, 105.4, and the polyclonal anti-vWF antibodies all were produced by our laboratory. The monoclonal AvW-5 and 105.4 and the polyclonal anti-vWF antibodies recognize both human and canine vWF. Antipropeptide (vW AgII) monoclonal antibodies 239.1 to 239.11 were also produced in our laboratory; 239.1, 239.7, 239.8, and 239.11 recognize canine and human propeptide, whereas the others recognize only human propeptide.
Immunofluorescence staining
Transfected AtT-20 cells were analyzed for the intracellular location of vWF and its propeptide, vW AgII, with immunofluorescent antibody staining and confocal laser scanning microscopy in the Imaging Core of the Medical College of Wisconsin. Cells were grown in 35-mm dishes, fixed using 3.7% (vol/vol) buffered formalin, permeabilized in 1% Triton X-100 (in 20 mmol/L HEPES, 300 mmol/L sucrose, 50 mmol/L NaCl, and 3 mmol/L MgCl2 · 6H2O, pH 7.0), and blocked in 2% normal donkey serum in HBSS. Cells were incubated at room temperature for 90 minutes in primary antibodies and then for 1 hour in secondary antibodies. Purified polyclonal anti-vWF antibody, diluted to 5 μg/mL, and a mix of 3 to 7 anti-vW AgII monoclonal antibodies, diluted to 2 [gm]g/mL each in HBSS/1% BSA, were used as primary antibodies. Secondary antibodies used were donkey antirabbit and antimouse IgG (H+L) [F(Ab')2] fragments (Jackson Immunoresearch) conjugated with Texas Red and fluorescein isothiocyanate (FITC), respectively, and diluted to 1:1000 and 1:200 in HBSS/1% BSA. Cells were mounted under glass coverslips with Vecta-shield (Vector Labs, Burlingame, CA). Immunofluorescence detection was performed by confocal microscopy as previously described using an MRC 600 confocal laser imaging system equipped with a krypton–argon laser (Bio-Rad, Hercules, CA) or an epifluorescence microscope (Nikon, Melville, NY).37
Multimer analysis
The conditioned medium of transfected HEK293T or AtT-20 cells was analyzed for vWF by electrophoresis through a 0.8% (w/v) HGT(P) agarose (DMC Bioproducts, Rockland, ME) stacking gel and 2% (wt/vol) HGT(P) agarose running gel containing 1% sodium dodecyl sulfate (SDS) for 16 hours at 40 V using the Laemmli buffer system.38Proteins were then transblotted to Immobilon-P (Millipore, Medford, MA) at 30 V for 30 minutes followed by 60 V for 2.5 hours in 25 mmol/L Tris, 200 mmol/L glycine, 20% methanol, and 4% (wt/vol) SDS. After transfer, membranes were blocked with 5% nonfat dry milk and incubated overnight with anti-vWF monoclonal antibodies AvW-5, AvW-17, and 105.4 at a concentration of 1 μg/mL each. Membranes were then incubated for 2 hours with horseradish peroxidase-conjugated goat antimouse IgG (Pierce, Rockford, IL), developed with Pierce SuperSignal Chemiluminescent substrate, and bands were visualized by exposure to x-ray film (BioMax film; Eastman Kodak, Rochester, NY).
Results
The sequence of canine vWF was obtained by screening a λgt11 canine heart cDNA library. As illustrated in Figure 1, overlapping clones were obtained and sequenced in both directions. Seven clones (shown as bold lines) were initially obtained from hybridization-positive clones. A second round of screening yielded 9 additional clones (thin lines) that extended into the 5′- and 3′-untranslated sequence. The sequence of a final 238-bp gap was obtained through PCR amplification (dashed line). A full-length cDNA construct was constructed from overlapping clones, with the restriction enzyme junctions indicated by vertical lines.
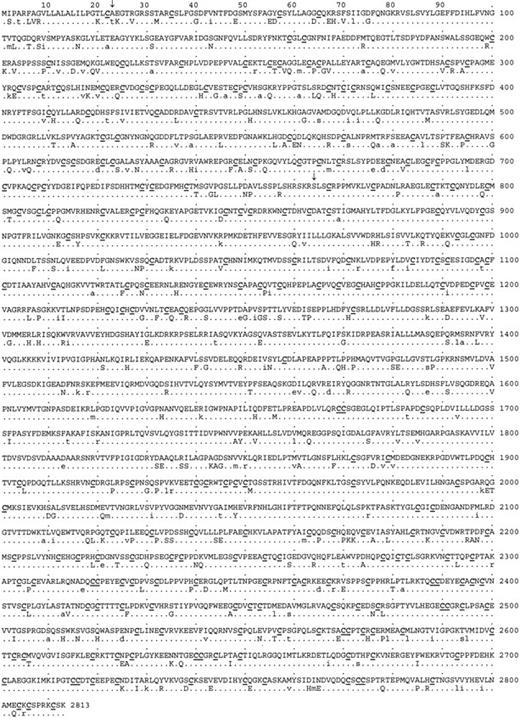
cDNA sequences of human and canine pro-vWF were 87.1% identical. Translation into protein yielded polypeptides that were 86.2% identical, with an additional 4.5% conservative amino acid changes. A comparison of human and canine protein sequences is shown in Figure2. Absolute conservation of the number and position of all 234 cysteine residues implied that the folding and overall shape of the human and canine proteins was probably similar. Mature vWF, exhibiting 87.4% identity and 4.2% conservative substitutions, was conserved to a somewhat greater extent than its propolypeptide (82.8% identity and 5.4% conservative substitutions for the vW AgII sequences). This may reflect that more stringent structural constraints have been imposed on the maintenance of function for vWF than for its propeptide.
Comparison of human and canine vWF amino acid sequence.
The protein sequence of human vWF is similar to canine vWF. The continuous amino acid sequence of human pre-pro-vWF is shown, with the differences in the canine sequence below. Conserved residues are represented by a dot, conservative substitutions are shown as lowercase letters, and nonconservative substitutions are shown as capital letters. The 234 cysteine residues, all of which are conserved, are underlined. Signal peptide and propeptide cleavage sites are represented by ↓ symbols. Canine AgII has an RGD sequence beginning at residue 531, in addition to the residues at amino acids 698 in AgII and 2507 in mature vWF, which exist in both the human and canine proteins.
Comparison of human and canine vWF amino acid sequence.
The protein sequence of human vWF is similar to canine vWF. The continuous amino acid sequence of human pre-pro-vWF is shown, with the differences in the canine sequence below. Conserved residues are represented by a dot, conservative substitutions are shown as lowercase letters, and nonconservative substitutions are shown as capital letters. The 234 cysteine residues, all of which are conserved, are underlined. Signal peptide and propeptide cleavage sites are represented by ↓ symbols. Canine AgII has an RGD sequence beginning at residue 531, in addition to the residues at amino acids 698 in AgII and 2507 in mature vWF, which exist in both the human and canine proteins.
To further investigate the processes of multimerization and storage, human (Hu) and canine (K9) constructs were developed that expressed the full-length pro-vWF (fl-vWF), the propeptide only (vW-AgII), or the mature vWF molecule only (Δpro). Insertion of a stop codon and deletion of the mature portion of the vWF molecule of either human or canine sequence left the 22-amino acid signal peptide followed by the 741-amino acid propeptide in Hu-vW-AgII and K9-vW-AgII constructs. Hu-Δpro plasmids encoded vWF from which the entire propeptide region was deleted, and it consisted of the signal peptide followed directly by the sequence encoding mature vWF. The vW-AgII and Δpro constructs were expressed both individually and intrans with one another (ie, cotransfected). Chimeric constructs composed of human and canine sequence were also developed by exchanging the vW AgII and mature portions of vWF. Hu/K9-vWF encoded the human signal peptide and propeptide in cis (on the same molecule) with mature canine vWF; conversely, K9/Hu-vWF encoded the canine signal peptide and propeptide in cis with mature human vWF. The conditioned medium from HEK293T cells transfected with these constructs was used for multimer analysis because high levels of protein expression were obtained using these cells. The mouse pituitary cell line, AtT-20, correctly processes vWF and has the ability to direct it to regulated storage. Plasmids were transfected into this cell line to examine intracellular localization of vW AgII and vWF.
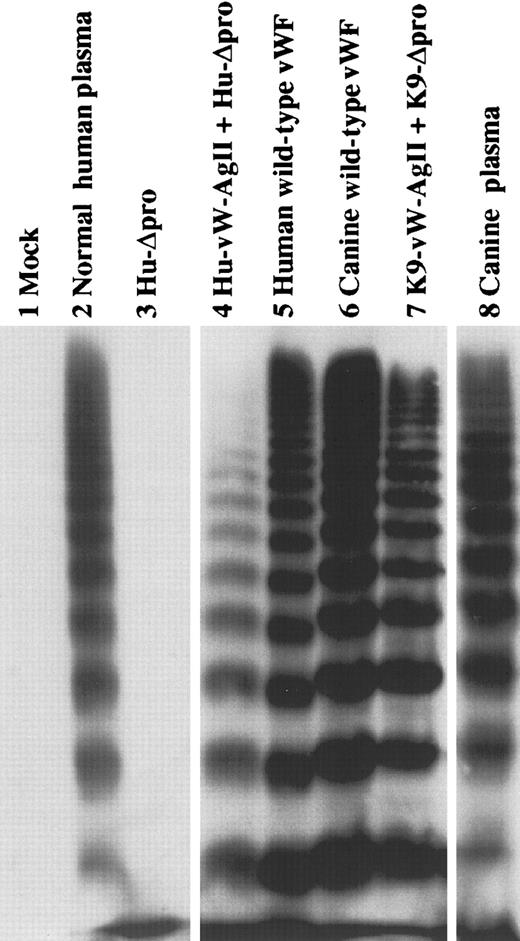
The multimeric structure of vWF in the conditioned medium of cells transfected with the human constructs—fl-Hu-vWF, Hu-Δpro, and Hu-Δpro in trans with Hu-vW-AgII—was determined using a 2% agarose–SDS gel. As seen in Figure3, Hu-Δpro expressed alone did not form multimers.18 Hu-Δpro expressed in trans with Hu-vW-AgII produced a range of multimers similar to the pattern obtained from the expression of fl-Hu-vWF.
Multimeric structure of expressed human and canine vWF.
Canine vWF has a multimer structure similar to that of human vWF. The multimeric structure of expressed vWF constructs was analyzed nonreduced on a 2% agarose–SDS gel. All samples were run on the same gel, and intervening (nonrelevant) lanes have been removed for clarity. The mock-transfected control is shown in lane 1. Normal human plasma vWF (lane 2) shows a full range of multimers, whereas human Δpro expressed alone did not form multimers (lane 3). Expression intrans with human vW AgII resulted in full multimerization (lane 4), similar to that expressed for human wild-type vWF (lane 5). Expressed canine wild-type vWF forms multimers (lane 6) similar to canine Δpro expressed in trans with canine vW AgII (lane 7). Canine plasma vWF is shown in lane 8.
Multimeric structure of expressed human and canine vWF.
Canine vWF has a multimer structure similar to that of human vWF. The multimeric structure of expressed vWF constructs was analyzed nonreduced on a 2% agarose–SDS gel. All samples were run on the same gel, and intervening (nonrelevant) lanes have been removed for clarity. The mock-transfected control is shown in lane 1. Normal human plasma vWF (lane 2) shows a full range of multimers, whereas human Δpro expressed alone did not form multimers (lane 3). Expression intrans with human vW AgII resulted in full multimerization (lane 4), similar to that expressed for human wild-type vWF (lane 5). Expressed canine wild-type vWF forms multimers (lane 6) similar to canine Δpro expressed in trans with canine vW AgII (lane 7). Canine plasma vWF is shown in lane 8.
Because the multimeric structures of canine and human vWF have been found to be similar, we next evaluated the multimeric structure of the vWF produced from our canine constructs.39 Full-length canine vWF (fl-K9-vWF) and K9-Δpro were transfected individually, and K9-Δpro and K9-vW-AgII were cotransfected. Cells transfected with fl-K9-vWF expressed a full range of multimers, similar to fl-Hu-vWF and to normal canine plasma (shown in Figure 3). K9-Δpro, expressed intrans with K9-vW-AgII, was also fully multimerized, similar to the expression of the human constructs in trans.
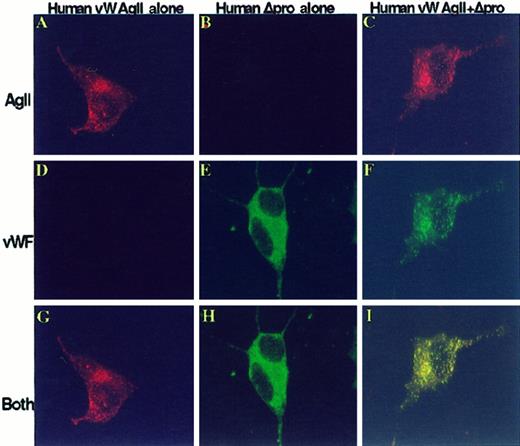
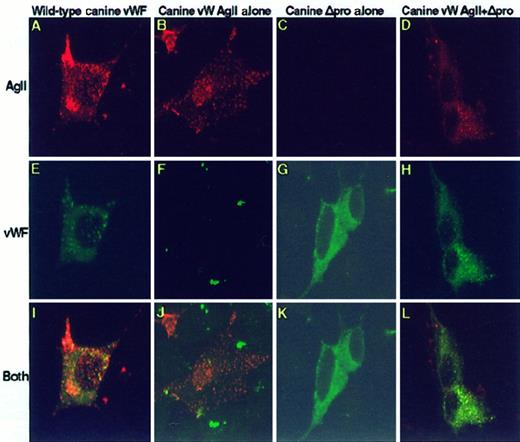
To examine the role of vW AgII in the targeting of vWF to storage, cells were either individually transfected or cotransfected with Hu-vW-AgII, Hu-Δpro, or both. Cells were immunostained with monoclonal antibodies to vW AgII and polyclonal antibody to vWF followed by Texas Red-labeled antimouse and FITC-labeled antirabbit IgG. The stained cells were subsequently examined by confocal microscopy. Figure 4 shows the images obtained by confocal microscopy. Cells expressing only Hu-vW-AgII produced vW AgII-containing storage granules (Figure 4A,D,G), but these cells did not express vWF, and no staining for vWF was detected. Thus, when expressed alone, vW AgII trafficked to storage granules. In cells expressing only the mature vWF without propeptide (Hu-Δpro), only cytoplasmic staining of vWF was seen (Figure 4B,E,H); no vWF-containing granules were produced. Expression of vW AgII in trans with Δpro resulted in granular storage of both human vW AgII and vWF, as seen in Figure 4C,F. Merging the separate images obtained from vWF/FITC and vW AgII/TXR revealed that vW AgII was colocalized with the vWF in granules (shown in yellow in Figure 4I).
The propeptide, vW AgII, trafficks vWF to granular storage in AtT-20 cells.
vW AgII functions as an intracellular chaperone. AtT-20 cells were transiently transfected with human propeptide alone, vW-AgII, human mature vWF alone, or Hu-Δpro or were cotransfected with Hu-vW-AgII + Hu-Δpro. After 72 hours, cells were fixed, permeabilized, dual stained with a mix of monoclonal antibodies to vW AgII and a polyclonal antibody to vWF, and detected with Texas Red-conjugated donkey antimouse IgG and FITC-conjugated donkey antirabbit IgG by confocal microscopy. (A-C) Cells stained for vW AgII. (D-F) vWF staining. The merges of vW AgII stain and vWF stain are shown in G to I. Colocalization of vW AgII and vWF is shown in yellow. Expression of vW AgII alone resulted in granular storage of vW AgII, as shown in A, indicating that vW AgII contains the epitope or conformation necessary for sorting to regulated storage; no vWF was detected (D). Expression of mature vWF alone (B, E, H) showed only a cytoplasmic staining pattern of vWF; no granular storage was observed. Expression of vW AgII in trans with mature vWF resulted in granular storage of both vW AgII and vWF (C, F, I). Furthermore, the 2 proteins were colocalized in storage granules, as shown in I. This suggests that vW AgII contains the necessary signal(s) for sorting to storage, and only through interaction with vW AgII is vWF sorted to storage. Total magnification, 1410×.
The propeptide, vW AgII, trafficks vWF to granular storage in AtT-20 cells.
vW AgII functions as an intracellular chaperone. AtT-20 cells were transiently transfected with human propeptide alone, vW-AgII, human mature vWF alone, or Hu-Δpro or were cotransfected with Hu-vW-AgII + Hu-Δpro. After 72 hours, cells were fixed, permeabilized, dual stained with a mix of monoclonal antibodies to vW AgII and a polyclonal antibody to vWF, and detected with Texas Red-conjugated donkey antimouse IgG and FITC-conjugated donkey antirabbit IgG by confocal microscopy. (A-C) Cells stained for vW AgII. (D-F) vWF staining. The merges of vW AgII stain and vWF stain are shown in G to I. Colocalization of vW AgII and vWF is shown in yellow. Expression of vW AgII alone resulted in granular storage of vW AgII, as shown in A, indicating that vW AgII contains the epitope or conformation necessary for sorting to regulated storage; no vWF was detected (D). Expression of mature vWF alone (B, E, H) showed only a cytoplasmic staining pattern of vWF; no granular storage was observed. Expression of vW AgII in trans with mature vWF resulted in granular storage of both vW AgII and vWF (C, F, I). Furthermore, the 2 proteins were colocalized in storage granules, as shown in I. This suggests that vW AgII contains the necessary signal(s) for sorting to storage, and only through interaction with vW AgII is vWF sorted to storage. Total magnification, 1410×.
To examine the storage of canine vWF, AtT-20 cells were transfected with pKVneo, and K9-vW-AgII and K9-Δpro individually and intrans. Anti-vW AgII monoclonal antibodies that cross-react with canine vW AgII were used for immunostaining. Anti-vWF polyclonal antibodies also cross-react with canine vWF and were used for immunodetection of the canine vWF protein. Cells expressing the fl-K9-vWF exhibited colocalized storage of vW AgII and vWF, as shown in Figure 5, panels A, E, and I. Cells expressing only K9-vW-AgII demonstrated granular storage of vW AgII (Figure 5B,F,J), similar to results obtained with human vW AgII. Expression of K9-Δpro alone did not result in storage of vWF; only cytoplasmic staining was observed (Figure 5C,G,K). Expression of K9-vW-AgII in trans with K9-Δpro resulted in colocalized storage of vWF and vW AgII, as observed with the human constructs (Figure 5D,H,L). The mechanism of storage of vW AgII and vWF appears to be similar in dogs and humans.
Intracellular distribution of canine vW AgII and vWF.
Canine constructs were transiently expressed in AtT-20 cells. Cells were fixed, permeabilized, dual stained with monoclonal antibodies to vW AgII and a polyclonal antibody to vWF that cross-react with canine and are detected with Texas Red-conjugated donkey antimouse IgG and FITC-conjugated donkey antirabbit IgG by confocal microscopy. (A-D) Cells stained for vW AgII. (E-H) vWF staining. Merges of vW AgII stain and vWF stain are shown in I to L; colocalization of vW AgII and vWF is shown in yellow. Cells expressing full-length canine vWF (A, E, I) exhibited granular storage of both vW AgII and vWF, which were colocalized as shown in I. Cells expressing canine vW AgII alone (B, F, J) displayed granular storage of vW AgII, whereas no vWF was detected. Expression of canine mature vWF resulted in a cytoplasmic staining pattern of canine vWF; no granular storage was observed (C, G, K). Co-expression of canine vW AgII and canine mature vWF (D, H, L) resulted in colocalized granular storage of both vW AgII and vWF. These results demonstrate that vW AgII chaperones vWF to regulated storage in dogs as in humans. Total magnification, 1410×.
Intracellular distribution of canine vW AgII and vWF.
Canine constructs were transiently expressed in AtT-20 cells. Cells were fixed, permeabilized, dual stained with monoclonal antibodies to vW AgII and a polyclonal antibody to vWF that cross-react with canine and are detected with Texas Red-conjugated donkey antimouse IgG and FITC-conjugated donkey antirabbit IgG by confocal microscopy. (A-D) Cells stained for vW AgII. (E-H) vWF staining. Merges of vW AgII stain and vWF stain are shown in I to L; colocalization of vW AgII and vWF is shown in yellow. Cells expressing full-length canine vWF (A, E, I) exhibited granular storage of both vW AgII and vWF, which were colocalized as shown in I. Cells expressing canine vW AgII alone (B, F, J) displayed granular storage of vW AgII, whereas no vWF was detected. Expression of canine mature vWF resulted in a cytoplasmic staining pattern of canine vWF; no granular storage was observed (C, G, K). Co-expression of canine vW AgII and canine mature vWF (D, H, L) resulted in colocalized granular storage of both vW AgII and vWF. These results demonstrate that vW AgII chaperones vWF to regulated storage in dogs as in humans. Total magnification, 1410×.
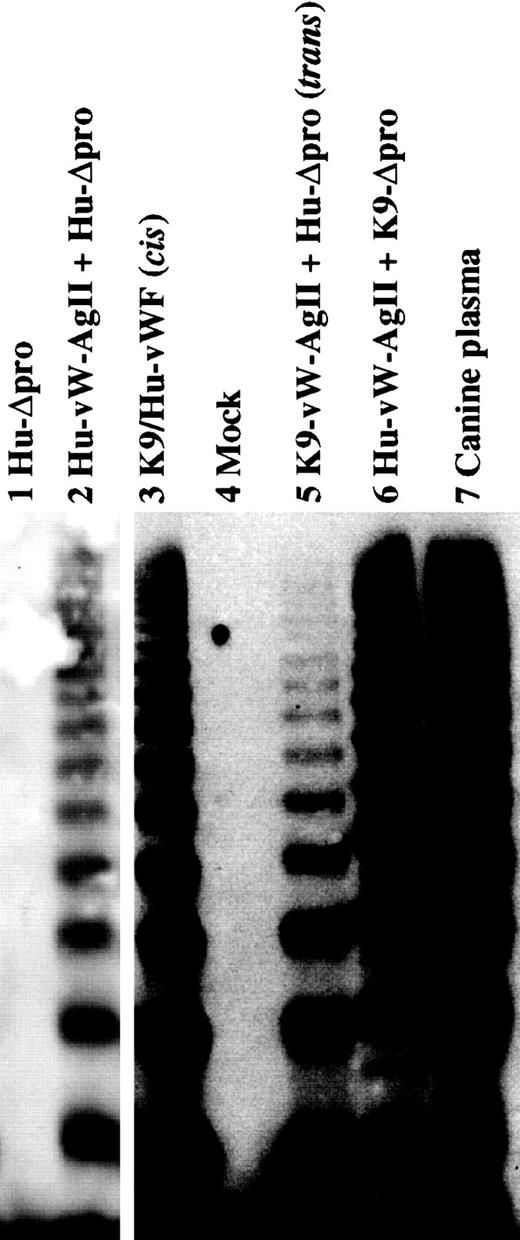
The interaction of human propeptide expressed in cis with mature canine vWF and the converse were investigated by creating the chimeric constructs Hu/K9-vWF and K9/Hu-vWF, respectively. In addition to the cis constructs, Hu-vW-AgII was also expressed intrans with K9-Δpro, and K9-vW-AgII was expressed intrans with Hu-Δpro. Multimerization of vWF was analyzed by SDS–agarose gel electrophoresis (Figure6). Expression of Hu-vW-AgII intrans with K9-Δpro resulted in fully multimerized canine vWF. The cis construct Hu/K9-vWF demonstrated a similar multimer structure to the trans expressed proteins (data not shown). Similarly, K9-vW-AgII expressed in either cisor trans with Hu-Δpro resulted in multimerization of vWF. The expression in trans produced a lower density of high molecular weight multimers, indicating that the multimerization process was less efficient in the trans expression than in the cis expression.
Interspecies multimerization of vWF.
The interspecies multimerization of vWF was examined by expressing canine vW AgII in trans with human mature vWF and the converse and by analyzing the conditioned medium nonreduced on a 2% agarose–SDS gel. All samples were run on the same gel, and intervening (nonrelevant) lanes have been removed for clarity. The mock-transfected control is shown in lane 4. Human mature vWF expressed alone did not form multimers (lane 1), whereas expression in trans with human vW AgII resulted in multimerization (lane 2). The canine propeptide multimerizes human mature vWF when expressed in cis(lane 3) or trans (lane 5). Expression of the human propeptide in trans with canine mature vWF results in multimerized canine vWF (lane 6), similar to that found in canine plasma (lane 7).
Interspecies multimerization of vWF.
The interspecies multimerization of vWF was examined by expressing canine vW AgII in trans with human mature vWF and the converse and by analyzing the conditioned medium nonreduced on a 2% agarose–SDS gel. All samples were run on the same gel, and intervening (nonrelevant) lanes have been removed for clarity. The mock-transfected control is shown in lane 4. Human mature vWF expressed alone did not form multimers (lane 1), whereas expression in trans with human vW AgII resulted in multimerization (lane 2). The canine propeptide multimerizes human mature vWF when expressed in cis(lane 3) or trans (lane 5). Expression of the human propeptide in trans with canine mature vWF results in multimerized canine vWF (lane 6), similar to that found in canine plasma (lane 7).
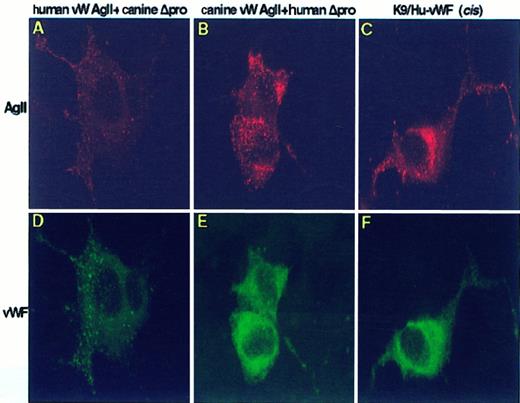
To assess the interspecies storage capability, chimeric constructs incis and constructs of either species in transwere expressed, immunostained, and examined by confocal microscopy (Figure 7). In cells expressing human vW AgII in cis (data not shown) and in trans with canine vWF, human vW AgII and canine vWF were colocalized in storage granules, as shown in Figure 7 (A,D). Thus, the human propeptide functions both to multimerize and sort canine vWF to regulated storage. In contrast, cells expressing canine vW AgII in cis or intrans with human vWF exhibited granular storage of the canine vW AgII, but human vWF was not stored; only cytoplasmic staining of vWF was observed (Figure 7B,C,E,F). Although canine vW AgII does not sort human vWF to storage, it does function in cis and intrans to direct multimerization of human vWF.
Intracellular distribution of chimeric vWF species.
AtT-20 cells were transiently transfected with chimeric vWF constructs, immunostained, and examined by confocal laser scanning microscopy for intracellular distribution of vW AgII and vWF. (A-C) Cells stained for vW AgII. (D-F) vWF staining. The human propeptide expressed intrans with canine mature vWF resulted in granular storage of both human vW AgII and canine vWF (A and D). In contrast, expression of canine propeptide in trans with human mature vWF resulted in granular storage of only canine vW AgII (B). Canine vW AgII did not traffick human mature vWF to granular storage; only a cytoplasmic staining pattern was observed (E). This indicates that canine vW AgII does not have the necessary signal or conformation required for interaction with human mature vWF to sort it to storage. Similar results were obtained when canine vW AgII was expressed incis with human mature vWF, K9/Hu-vWF, as seen in C and F. The canine vW AgII was sorted to granular storage, whereas the human vWF displayed only a cytoplasmic staining pattern. This indicates that propeptide cleavage occurs before the formation of storage granules. Total magnification, 1410×.
Intracellular distribution of chimeric vWF species.
AtT-20 cells were transiently transfected with chimeric vWF constructs, immunostained, and examined by confocal laser scanning microscopy for intracellular distribution of vW AgII and vWF. (A-C) Cells stained for vW AgII. (D-F) vWF staining. The human propeptide expressed intrans with canine mature vWF resulted in granular storage of both human vW AgII and canine vWF (A and D). In contrast, expression of canine propeptide in trans with human mature vWF resulted in granular storage of only canine vW AgII (B). Canine vW AgII did not traffick human mature vWF to granular storage; only a cytoplasmic staining pattern was observed (E). This indicates that canine vW AgII does not have the necessary signal or conformation required for interaction with human mature vWF to sort it to storage. Similar results were obtained when canine vW AgII was expressed incis with human mature vWF, K9/Hu-vWF, as seen in C and F. The canine vW AgII was sorted to granular storage, whereas the human vWF displayed only a cytoplasmic staining pattern. This indicates that propeptide cleavage occurs before the formation of storage granules. Total magnification, 1410×.
All full-length constructs, including chimeric cisconstructs, contain an intact propeptide cleavage site, and propeptide was cleaved as confirmed by reduced SDS-PAGE (data not shown). Intracellular processing of the K9/Hu-vWF molecule is particularly revealing. The cross-species interactions permit C-terminal dimerization, N-terminal multimerization, and propeptide cleavage. However, the confocal images in Figure 7 demonstrate that the canine vW AgII, which is cleaved from human vWF, is trafficked to storage, whereas the cytoplasmic staining pattern of human vWF indicates that vWF is not sorted to storage. This lack of colocalization illustrates that prosequence cleavage occurs before granule formation, most likely in the TGN and that the postcleavage association of vW AgII with vWF is the mechanism by which vWF is sorted to storage granules.
Discussion
The molecular mechanisms for sorting proteins to the regulated secretory pathway have not been well defined. In the TGN, secretory proteins appear to condense, and this aggregation has been proposed as the initiating event in the formation of storage vesicles.31-33,40 Another proposal is that regulated secretory proteins contain targeting signal(s) that interact with cellular receptor(s) to mediate direction to storage.31-33,40 Our results demonstrate that the propeptide vW AgII plays an active role in trafficking vWF to storage vesicles in AtT-20 cells. When expressed alone, vW AgII is sorted to regulated storage. This suggests that vW AgII possesses the signal(s) or conformation that interacts with membrane receptor(s), resulting in its granular storage. Voorberg et al41 previously showed expression of the propeptide in trans with mature vWF results in granular storage of vWF in monkey kidney CV-1 cells. We now demonstrate that expression in trans in AtT-20 cells results in granular storage of vW AgII, colocalized with vWF. vW AgII appears to function as an intracellular chaperone: vW AgII contains the required signal(s) for targeting to storage, and only through association with vW AgII is vWF brought into storage.
Vischer et al8 have proposed that multimerization promotes vWF retention in the TGN, favoring storage by prolonging the availability of vWF aggregates for incorporation into Weibel-Palade bodies. Voorberg et al41 observe a direct correlation between the multimeric structure of vWF and its sorting to storage granules. Our results do not exclude aggregation as a component of granular storage but, rather, eliminate the multimerization of vWF as a prerequisite. Expression of canine vW AgII in trans orcis with human mature vWF produced multimeric vWF that was not sorted to storage in AtT-20 cells, whereas normal storage of canine vW AgII was observed. Multimerization alone is clearly insufficient for the formation of granules. Our laboratory recently characterized a mutation in the propeptide that affected the multimerization of vWF but had no effect on vWF storage or propeptide cleavage.42This mutation consists of a single amino acid substitution of a serine for a tyrosine in vW AgII, Y87S. Expression of the mutated vW AgII in either cis or trans with mature vWF (Δpro) demonstrated a loss of multimerization of vWF; only dimers were produced. However, the mutation had no effect on granular storage. The vWF dimers produced were sorted to granules colocalized with vW AgII. These results demonstrate that multimerization is not a requirement for the storage of vWF. Wagner et al25 also report C-terminal vWF deletion mutants shown to form dimers trafficked to regulated storage. In addition, insertion of a glycine that disrupted vicinal cysteine motifs within vW AgII resulted in the loss of multimerization of vWF, but not the loss of granular storage.20 These mutations all result in unpolymerized dimeric vWF species subsequently sorted to regulated storage. Previous work by Journet et al18 showed lack of granular storage when either or both propeptide D domains are deleted. Our results using Δpro constructs confirm this. In contrast to the dimers formed after deletion of the propeptide, the vWF dimers discussed above were generated from mutant pro-vWF molecules containing an intact propeptide and cleavage site. As a result of the chaperone function of vW AgII, these vWF species were trafficked to storage regardless of their multimeric state.
vWF most likely continues to associate with vW AgII in the TGN, and both proteins are subsequently cotransported to storage by virtue of the sorting signal on vW AgII. Mature vWF and vW AgII are found in a 1:1 stoichiometric ratio in Weibel-Palade bodies.26,27 At pH 6.4, in the presence of calcium, mature vWF and vW AgII are noncovalently associated, whereas at pH 7.4 this interaction is not sustained.8 The conditions promoting association mimic those found in the TGN, which is thought to have a pH between 6.17 and 6.45 and a calcium concentration of approximately 10 mmol/L.32,33 The intragranular pH of other secretory vesicles has been shown to be equally or more acidic than the TGN, which should promote continued association within the storage compartment.43 44 This pH-dependent association is consistent with our results demonstrating the chaperoning capability of vW AgII when coexpressed with mature vWF.
The independent nature of the processes of multimerization and storage is further defined by interspecies interactions in bothcis and trans. Human vW AgII functions to multimerize and traffic canine vWF to storage granules. In contrast, expression of canine vW AgII in either cis ortrans with human mature vWF resulted in the multimerization of vWF and the granular sorting of canine vW AgII, but no corresponding storage of the human vWF (cytoplasmic staining). Although canine vW AgII contains the required signals for sorting itself to storage and to facilitate the multimerization of human vWF, it apparently does not contain the signal or conformation necessary to further associate with human vWF and sort it to storage. This dissociation of functions indicates that the interactions required for the multimerization of vWF are clearly different from those involved in the storage of vWF, and it further demonstrates that multimerization and storage are indeed independent intracellular processes.
Both multimerization and cleavage of vW AgII from vWF are thought to occur in the TGN before the formation of Weibel-Palade bodies.8 Furin/PACE, the likely propeptide-cleaving enzyme, has been localized to the TGN.9 Confocal imaging of AtT-20 cells, expressing the cis construct K9/Hu-vWF, demonstrates unequivocally that propeptide cleavage occurs before granule formation. Cells transfected with this construct exhibited granular storage of canine AgII but no corresponding granular storage of human vWF (cytoplasmic staining). If propeptide cleavage occurred within the storage granule, one would expect to see both canine vW AgII and human vWF, but this was not observed. The propeptide cleavage reaction appears to go to completion before the formation of granules with efficient separation and sorting of canine vW AgII. Further support can be found in studies involving the role of vWF in the binding and stabilization of factor VIII. Kaufman et al45,46 have shown that the cleavage of vWF propeptide is necessary for factor VIII binding and stabilization. Rosenberg et al37 demonstrated that factor VIII transfected in AtT-20 cells trafficks to regulated storage in a vWF-dependent manner. Because propeptide cleavage is required for factor VIII binding, the presence of factor VIII in storage granules suggests that propeptide cleavage occurs before the final formation of storage granules.37vWF processing follows the order of C-terminal dimerization, N-terminal multimerization, propeptide cleavage, factor VIII binding, and, finally, storage granule formation.
The propeptide vW AgII plays a pivotal role in the intracellular processing of vWF, facilitating multimerization of vWF and chaperoning it to granular storage. Experiments combining homologous reaction partners from different species allow the level of functionality to be correlated with the degree of evolutionary sequence conservation or divergence. Maintenance of function indicates that key structural motifs have been preserved and that only nonvital residues have been altered. Conversely, an interspecies loss of function indicates that any conserved sequence is insufficient to maintain essential interactions and that whatever differences have occurred cause some level of structural disruption. In the multiple associations of the AgII molecule, we find examples of both possibilities. The human and canine propeptides differ markedly in their ability to recruit vWF into the storage pathway. Human AgII is capable of interacting with mature canine vWF as an intracellular chaperone, resulting in the storage of both proteins. Canine AgII, though able to conduct canine vWF into the storage compartment, is deficient in this interaction with human vWF, which is not stored. Structural similarities between human and canine propeptides allow them to direct the folding and multimerization of vWF protein from the opposite species. The portions of vWF involved in this interaction with AgII are obviously also relatively well conserved. Human and canine AgII also appear to be equally competent in interactions with cellular storage pathway components, transporting themselves into storage granules. That the propeptides from 2 phylogenetically distant species are able to navigate the storage pathway efficiently in cells from yet a third species (AtT-20 murine cells) argues for a relatively well-conserved mechanism of vWF synthesis, processing, and storage.
Supported by National Institutes of Health training grant HL-07209 (S.L.H), National Institutes of Health grants HL-44612 and HL-33721 (R.R.M.), the Clinical Research Center of the Medical College of Wisconsin (M01 RR00058), and the Wiener Foundation (New York, NY).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert R. Montgomery, Department of Pediatrics, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: bob@bcsew.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal