Abstract
On stimulation by strong agonists, platelets release the contents of 3 storage compartments in 2 apparent waves of exocytosis. The first wave is the release of α- and dense core granule contents and the second is the release of lysosomal contents. Using a streptolysin O-permeabilized platelet exocytosis assay, we show that hexosaminidase release is stimulated by either Ca++ or by GTP-γ-S. This release step retains the same temporal separation from serotonin release as seen in intact platelets. This assay system was also used to dissect the molecular mechanisms of lysosome exocytosis. Lysosome release requires adenosine triphosphate and the general membrane fusion protein, N-ethylmaleimide sensitive factor. Uniquely, 2 syntaxin t-SNAREs, syntaxin 2 and 4, which localize to granules and open canalicular membranes, together with the general target membrane SNAP receptor (t-SNARE) protein SNAP-23 appear to make up the heterodimeric t-SNAREs required for lysosome exocytosis. These studies further show that regardless of stimuli (Ca++or GTP-γ-S) serotonin and hexosaminidase release requires the same membrane fusion machinery.
Introduction
Platelets are small discoid cell fragments that play essential roles in primary hemostasis. They respond to vascular lesions, in part, by secreting small molecules and proteins from 3 intracellular stores: dense core, α-granules, and lysosomes. On encountering vascular damage, platelet surface receptors bind to agonists, such as collagen and von Willibrand factor, which initiate a cascade of signaling events leading to an increase in intracellular calcium and activation of several GTP-binding proteins and protein kinases.1,2 In one description of the events initially following stimulation, platelet granules are centralized by a rearrangement of the cytoskeleton (reviewed in Blockmans et al2). Once in the middle of the cell, the granules fuse with each other and with a specialized region of the plasma membrane known as the open canalicular system (OCS), resulting in the release of granular stores. Exocytosis in response to strong agonists (ie, thrombin) occurs in 2 waves.3,4 The first wave releases small molecules (eg, ADP, serotonin, Ca++) from the dense core granules and proteins (eg, platelet factor 4, von Willibrand factor, β-thromboglobulin) from the α-granules. In the second wave, lysosomal enzymes such as cathepsin, β-hexosaminidase, and heparitinase are released. These hydrolases may serve to complete the activation of platelets trapped in a forming clot5 and perhaps ultimately serve to remodel the site of damage. Although lysosome release can be easily measured in vitro and has been demonstrated in vivo,6 the physiologic significance has been controversial (discussed in Polasek7).
Lysosomes are generally considered degradative compartments not readily associated with secretion; however, extracellular release of lysosomal enzymes does occur in various cell types.8-10 This is particularly seen in hematopoietic cells such as macrophages,11 platelets,12 and cytotoxic T lymphocytes,13 which appear to have so-called secretory lysosomes.10 In some of these cell types, release of lysosomal enzymes is stimulated by transient increases in intracellular calcium analogous to regulated exocytosis events in specialized secretory cells such as neurons.14 Despite the recognition that lysosomal enzyme release may be ubiquitous, little mechanistic information is available.
A growing body of data supports the concept that distinct membrane proteins from the transport vesicle (or secretory granule) and target membrane (ie, plasma membrane) are, at least in part, responsible for the fusion of the 2 lipid bilayers.15-17 As originally stated,18 the SNARE hypothesis proposed that a vesicle membrane protein from the synaptobrevin/VAMP family (v-SNARE) specifically binds to a heterodimeric complex (t-SNAREs) in the target membrane made up of one member of the syntaxin family and one from the SNAP-23/25 family. The resulting heterotrimeric, intermembrane, complex is minimally required for membrane fusion of defined liposomes.19 Recent reports from our laboratory20-23 and others24-26 have begun to unravel the molecular mechanisms of platelet exocytosis. Initial reports have demonstrated the presence of specific v- and t-SNAREs as well as general accessory proteins (p115/TAP, SNAPs, and NSF) and specific regulatory proteins (Rabs and Munc18s).21,22,24-27 These studies have specifically addressed the roles of these proteins in dense core20,24and α-granule secretion.23-25 In this manuscript, we characterized some of the molecular interactions involved in lysosomal enzyme secretion from blood platelets. Functional studies show that lysosomal release is dependent on the general fusion protein NSF and the t-SNARE, SNAP-23. Interestingly, hexosaminidase secretion is inhibited by 2 different antisyntaxin antibodies (antisyntaxin 2 or 4). This suggests that unlike dense core granule release, which is facilitated by one heterodimeric t-SNARE containing SNAP-23 and syntaxin 2,20 lysosome release requires 2 different heterodimers (SNAP-23/syntaxin 2 and SNAP-23/syntaxin 4).
Materials and methods
Antibodies and reagents
Polyclonal anti–SNAP-23, antisyntaxin 2, and 7 antibodies were generated by immunizing rabbits with recombinant human SNAP-23, syntaxin 2, or 7 proteins. Antisyntaxin 2 and 7 antibodies were affinity purified using the appropriate recombinant protein and had no detectable cross-reactivity in platelets as previously demonstrated.20 Anti–SNAP-23 antibody was affinity purified and was shown to have no significant cross-reactivity to SNAP-25.28 Anti-NSF monoclonal antibody2E5from mouse ascites was purified on protein G Sepharose (Sigma Chemical, St Louis, MO). The antisyntaxin 4 antibody (clone 49) was a monoclonal purchased from Transduction Laboratories (Lexington, KY). This antibody had no cross-reactivity with either syntaxin 2 or 7 when tested by Western blotting.20 When the antibodies were used in in vitro assays, they were dialyzed at 4°C against buffer A (see below) before addition. Reduced streptolysin O (SLO) was purchased from Murex (Dartford, UK). The SNAP-23 C-terminal peptide (ANARAKKLIDS) was a generous gift from Dr David Castle (University of Virginia, Charlottesville, VA). Apyrase VII, heparin, prostaglandin I2, P-nitrophenyl-N-acetyl-β-D-glucosaminide, and GTP-γ-S were purchased from Sigma. [1,2-3H(N)]-hydroxytryptamine ([3H]5-HT) was purchase from NEN (Boston, MA). All other chemicals were of reagent grade. Chinese hamster ovary NSF, the cytoplasmic domains of human syntaxin 2, 4, 7, and SNAP-23-encoding DNAs were inserted into the pQE-9 vector (Qiagen, Chatsworth, CA). Recombinant His6-tagged proteins were prepared from Escherchia coli and purified by Ni2+-NTA affinity chromatography.29
Permeabilization of platelets with SLO and assay of [3H]5-HT and hexosaminidase release
Platelets were initially labeled with [3H]5-HT and prepared as previously described.20 Fifty microliters (107-108 cells) of platelets in buffer A (120 mmol/L sodium glutamate, 5 mmol/L potassium glutamate, 20 mmol/L HEPES/NaOH, pH 7.4, 2.5 mmol/L EDTA, 2.5 mmol/L EGTA, 3.15 mmol/L MgCl2, and 1 mmol/L DTT) were mixed with 50 μL of buffer A, containing 8 mmol/L ATP, 1.6 U/mL SLO at room temperature for 5 minutes. The reactions were further incubated at 4°C for 30 minutes with either the antibodies, the peptides, or the recombinant proteins to be tested. As discussed previously,20 the 4°C step is required to limit the activity of the SLO, thereby maintaining the integrity of the intraplatelet granules, whereas reagents diffuse into the permeabilized cells. The samples were then warmed to 25°C for 5 minutes. CaCl2 or GTP-γ-S was added to give the desired final concentration and the reactions were incubated at 25°C for 5 minutes. The reactions were stopped by placing the samples on ice for 4 minutes, followed by centrifugation at 13 000g for 1 minute. The supernatants were collected and assayed as below. [3H]5-HT release was measured by scintillation counting. The hexosaminidase was measured as described by Holmsen et al30: the 5 mL citrate-phosphate buffer pH 4.5 and 2.5 mL of 10 mmol/L substrate (P-nitrophenyl-N-acetyl-β-D-glucosaminide) were mixed and aliquoted (100 μL) into 96-well plates and 5 μL of each reaction supernatant was added. After incubation at 37°C for 18 hours, 60 μL of 0.08 N NaOH was added to stop the hexoaminidase reaction. The absorbance at 405 nm was read in a Titertek Multiscan Plus enzyme-linked immunosorbent assay (ELISA) plate reader (Labsystems, Finland). In these assays, the no enzyme background (OD405 = 0.040) was subtracted. Using these assay conditions, we did not detect any significant differences in the extent of release of dense core granules20 or lysosomes (49 ± 3% versus 48 ± 1.2% n = 3) between freshly prepared and freshly banked platelets. For this reason, freshly banked platelets were used in all assays reported here.
Immunoelectron microscopy
Resting platelets were prepared in the presence of PGI2 and apyrase.20 After apyrase treatment, platelets were harvested and resuspended in Hepes Tyrodes plus 0.35% bovine serum albumin (BSA). An equal volume of 8% paraformaldehyde/0.6% glutaraldehyde in Hepes Tyrodes was added to the platelets, which were incubated at 25°C for 45 minutes. The platelets were subsequently pelleted and resuspended in 4% paraformaldehyde/0.3% glutaraldehyde in 0.1 mol/L Sorenson's phosphate buffer for 1 hour at 25°C. The platelets were washed in 0.1 mol/L Sorenson's buffer 3 times for 10 minutes and dehydrated as follows: 50% ethyl alcohol 2 times 10 minutes and 70% ethyl alcohol 2 times 10 minutes. Platelets were infiltrated with a 1:1 mixture of LR White resin and 70% ethyl alcohol for 10 minutes, followed by a second 10 minutes infiltration with a 2:1 mixture of LR White and 70% ethyl alcohol. Finally, they were infiltrated with 2 changes of pure LR White for 30 minutes each change. Samples were embedded in LR White resin in gelatin capsules and polymerized at 50°C for 24 hours. Samples were sectioned using a Reichert-Jung UltracutE microtome (Vienna, Austria) and mounted on nickel grids. For immunostaining, grids were etched with 10% H2O2 for 30 minutes, then blocked in 5% normal goat serum for 30 minutes. Grids were floated on drops of antibodies diluted in 1% normal goat serum plus 0.3% TX-100 in 0.1 mol/L Sorenson's buffer overnight at 4°C. Grids were rinsed 3 times in 0.5 mol/L Tris containing 0.05% PEG and then floated on colloidal gold conjugated goat antirabbit secondary antibodies diluted in 1% normal goat serum in 0.1 mol/L Sorenson's buffer for 1 hour. Sections were exposed to 1% glutaraldehyde (EM Grade) in 0.1 mol/L Sorenson's for 15 minutes and counterstained with uranyl acetate and lead citrate. Platelets were viewed on a Hitachi H-7000 transmission electron microscope (Tokyo, Japan).
Results
Stimulation of lysosome release by Ca++
Several groups have shown that dense core granule and lysosome secretion from permeabilized platelets are responsive to 10 μmol/L of free Ca++.31-33 To test the validity of our in vitro assay, we used increasing concentrations of Ca++ to stimulate platelet exocytosis. Hexosaminidase release was stimulated at 1 μmol/L and reached a maximal (48% of total cellular) at 10 μmol/L Ca++. The extent of release then declined when the Ca++ was raised to 100 μmol/L and beyond (data not shown). This response to free Ca++ is identical to our previous reports for dense core and α-granule release.20,23 When similar titration experiments were performed with intact platelets, no significant secretion was detected, consistent with previous reports.31,33,34 The Ca++-stimulated release was dependent on ATP because the inclusion of apyrase completely eliminates stimulated release. We next analyzed the kinetics of platelet secretion in our permeabilized cell assay system by measuring a time course of Ca++-stimulated dense core granule and lysosome release. SLO-permeabilized platelets were stimulated with 10 μmol/L Ca++ and the reactions were stopped at the increasing time points by chilling the samples on ice.31 Although it is clear that chilling the reactions will not stop all the events occurring in the activated cells, it does stop membrane fusion and therefore secretion (for example, see lane 10 of Figure 4). Both dense core granules and lysosomes apparently start to release immediately after the addition of 10 μmol/L Ca++ because no lag was detected using our assay system. The half time for 100% secretion of [3H]-5HT was shorter than 10 seconds and the half time for 100% secretion of hexosaminidase was about 20 seconds.
NSF is involved in lysosome secretion. There is an emerging consensus that NSF and SNAPs are required for most heterotypic membrane trafficking events and some homotypic fusion events.35 In previous reports,20,23,24 it was shown that NSF and α-SNAP are required for dense core and α-granule secretion. To further confirm that NSF is required for lysosome release, we performed an antibody inhibition experiment as shown in Figure1. The monoclonal antibody 2E5, which is a known inhibitor of NSF function,36 was added to SLO permeabilized platelets at increasing concentrations and incubated on ice for 30 minutes. After rewarming to room temperature, the platelets were stimulated with 10 μmol/L Ca++ and secretion of hexosaminidase was measured (Figure 1A). The 2E5 inhibited 100% of the maximal dense core granule secretion20 (Figure 4) but only approximately 70% of the maximal lysosome secretion at 80 μg/mL (Figure 1A). The inhibitory effects of 2E5 on lysosome secretion could be partially reversed by the addition of recombinant NSF (1 mg/mL), demonstrating the specificity of the 2E5 antibody. A nonspecific mouse immunoglobulin when added to the assay was without effect. Recombinant NSF by itself only slightly increased dense core granule release20 and had little effect on lysosome secretion (Figure 1B), perhaps because it does not enter the permeabilized platelet due its size (approximately 500 kd). This is consistent with a lack of inhibition by a dominant-negative form of NSF, D1E-Q37 (data not shown). The inhibitory effect of 2E5 on lysosome secretion is not as complete as it is on dense core granule release. The lack of complete inhibition is not due to the background differences between [3H]5-HT and hexosaminidase assay though this difference could reflect a difference in the sensitivity of the [3H]5-HT and hexosaminidase measurements.
Anti-NSF antibody inhibits lysosome secretion.
Panel A: SLO-permeabilized platelets were incubated with increasing amounts of 2E5 (monoclonal antibody against NSF, 0-0.32 mg/mL) and of a nonspecific mouse IgG (0-0.32 mg/mL). The Ca++-stimulated release of hexosaminidase was measured and normalized to the control (no addition of 2E5), which was set at 100%. Panel B: SLO permeabilized platelets were incubated on ice for 30 minutes with buffer (control), with 80 μg/mL 2E5, with 80 μg/mL that had been preincubated with 0.75 mg/mL recombinant NSF, and with 0.75 mg/mL recombinant NSF alone. Platelets were activated with 10 μmol/L Ca++ and the release of hexosaminidase was measured and normalized to the control group.
Anti-NSF antibody inhibits lysosome secretion.
Panel A: SLO-permeabilized platelets were incubated with increasing amounts of 2E5 (monoclonal antibody against NSF, 0-0.32 mg/mL) and of a nonspecific mouse IgG (0-0.32 mg/mL). The Ca++-stimulated release of hexosaminidase was measured and normalized to the control (no addition of 2E5), which was set at 100%. Panel B: SLO permeabilized platelets were incubated on ice for 30 minutes with buffer (control), with 80 μg/mL 2E5, with 80 μg/mL that had been preincubated with 0.75 mg/mL recombinant NSF, and with 0.75 mg/mL recombinant NSF alone. Platelets were activated with 10 μmol/L Ca++ and the release of hexosaminidase was measured and normalized to the control group.
SNAP-23 is involved in lysosome secretion. Previous work has shown a role for the t-SNARE, SNAP-23, in dense core and α-granule release.20,23,25 In this study, we sought to determine whether SNAP-23 was also involved in lysosome secretion. Increasing amounts of anti–SNAP-23 antibody (ab23) were added to the in vitro exocytosis assay. Both dense core granule20 and lysosome release are inhibited by 20 μg/mL of anti–SNAP-23 antibody (Figure 2A). The fact that this inhibitory effect can be reversed by the addition of recombinant SNAP-23 (Figure 2B) and that nonspecific IgG had no effect demonstrates the specificity of the inhibition (Figure3A). The recombinant, full-length SNAP-23 had no effect on secretion, but the C-terminal peptide, which inhibited approximately 40% of the dense core release,20 inhibited approximately 30% of lysosome secretion (Figure 2B). Combining this data with previous reports,20,23,25 SNAP-23 is likely to be a general factor, like NSF and SNAP, required for all 3 granules. Interestingly, in each case the inhibition of hexosaminidase release using the same reagents is not as extensive as that seen for [3H]5-HT release.20
Anti–SNAP-23 antibody inhibits lysosome secretion.
Panel A: SLO-permeabilized platelets were incubated with increasing amounts of a polyclonal antibody against SNAP-23 (ab23, 0-38 μg/mL). The release of hexosaminidase was measured as before and normalized to the control (no addition of ab23). Panel B: SLO-permeabilized platelets were incubated on ice for 30 minutes with buffer (control), 20 μg/mL with ab23, with 20 μg/mL that had been preincubated with 0.5 mg/mL recombinant SNAP-23, with 0.5 mg/mL recombinant SNAP-23 alone, and with 0.15 mg/mL C-terminal peptide of SNAP-23. Platelets were activated with 10 μmol/L Ca++ and the release of hexosaminidase was measured and normalized to the control group.
Anti–SNAP-23 antibody inhibits lysosome secretion.
Panel A: SLO-permeabilized platelets were incubated with increasing amounts of a polyclonal antibody against SNAP-23 (ab23, 0-38 μg/mL). The release of hexosaminidase was measured as before and normalized to the control (no addition of ab23). Panel B: SLO-permeabilized platelets were incubated on ice for 30 minutes with buffer (control), 20 μg/mL with ab23, with 20 μg/mL that had been preincubated with 0.5 mg/mL recombinant SNAP-23, with 0.5 mg/mL recombinant SNAP-23 alone, and with 0.15 mg/mL C-terminal peptide of SNAP-23. Platelets were activated with 10 μmol/L Ca++ and the release of hexosaminidase was measured and normalized to the control group.
Antisyntaxin 2 and 4 antibodies inhibit lysosome secretion.
Panel A: SLO-permeabilized platelets were incubated with increasing amounts of antibodies against syntaxin 2 (pp2), 4, 7, and a nonspecific rabbit IgG (0-0.24 mg/mL). The release of hexosaminidase was measured and normalized to the control (buffer alone). Panel B: SLO permeabilized platelets were incubated on ice for 30 minutes with buffer (control), or with 30 μg/mL antisyntaxin 2 antibody (pp2) or 50 μg/mL antisyntaxin 4 antibody alone or after preincubation with either recombinant syntaxin 2 (200 μg/mL) or syntaxin 4 (200 μg/mL) as indicated. Permeabilized platelets were also incubated on ice with the recombinant syntaxins (2 and 4), preimmune rabbit IgG, or a combination of antisyntaxin 2 and 4 antibodies (30 μg/mL pp2 and 50 μg/mL antisyntaxin 4). Platelets were activated with 10 μmol/L Ca++ and the release of hexosaminidase was measured and normalized to the control group.
Antisyntaxin 2 and 4 antibodies inhibit lysosome secretion.
Panel A: SLO-permeabilized platelets were incubated with increasing amounts of antibodies against syntaxin 2 (pp2), 4, 7, and a nonspecific rabbit IgG (0-0.24 mg/mL). The release of hexosaminidase was measured and normalized to the control (buffer alone). Panel B: SLO permeabilized platelets were incubated on ice for 30 minutes with buffer (control), or with 30 μg/mL antisyntaxin 2 antibody (pp2) or 50 μg/mL antisyntaxin 4 antibody alone or after preincubation with either recombinant syntaxin 2 (200 μg/mL) or syntaxin 4 (200 μg/mL) as indicated. Permeabilized platelets were also incubated on ice with the recombinant syntaxins (2 and 4), preimmune rabbit IgG, or a combination of antisyntaxin 2 and 4 antibodies (30 μg/mL pp2 and 50 μg/mL antisyntaxin 4). Platelets were activated with 10 μmol/L Ca++ and the release of hexosaminidase was measured and normalized to the control group.
Syntaxin 2 and 4 are both required for lysosome secretion.Three syntaxins have been found in platelets: syntaxin 2, 4, and 7.20,22 Syntaxin 2 has been shown to be required for dense core granule release20 and it appears that both syntaxin 2 and 4 are required for α-granule release.23,25 To identify which syntaxins are involved in lysosome secretion, antibodies against syntaxin 2 (pp2), 4 (clone 49), and 720 were used in the platelet secretion assay. Antisyntaxin 2 and 4 antibodies both inhibited lysosome release and no inhibition was observed with either antisyntaxin 7 or rabbit IgG (Figure 3A). Competition with recombinant proteins confirmed the specificity of the inhibition by the polyclonal antibody pp2 and the monoclonal antisyntaxin 4 antibody (Figure 3B). Neither recombinant syntaxin 2 nor 4 affected lysosome secretion by themselves but they could reverse the inhibitory effect of their respective antibody (Figure 3B). The inhibitory effect of each antibody was not reversed by a similar amount of an inappropriate recombinant syntaxin. Interestingly, when both antibodies were added together (Figure 3B), there was no increase in the level of inhibition. These data imply that, although syntaxin 2 appears solely involved in dense core granules release,20 both syntaxin 2 and 4 are involved in lysosome secretion.
GTP-γ-S- and Ca++-stimulated secretion use the SNARE exocytosis machinery. It has been shown that GTP-γ-S can stimulate release of intracellular stores from permeabilized platelets.31-33 Under our assay conditions, GTP-γ-S can stimulate secretion from both dense core granules and lysosomes at 25 μmol/L and is maximal at 100 μmol/L in the absence of free Ca++ (pCa < 9). We next performed a time-course experiment in which permeabilized platelets were stimulated with 100 μmol/L of GTP-γ-S and the release reactions were stopped at different time points by chilling the samples on ice. Both GTP-γ-S–stimulated dense core granule and lysosome release are slower than Ca++-stimulated secretion (see above), which appears to be caused by a 100-second lag before the start of the dense core granule release and a 300-second lag before lysosome secretion. We next examined the effect that GTP-γ-S and Ca++ have together on platelet exocytosis. For these experiments, permeabilized platelets were incubated for differing times with 100 μmol/L GTP-γ-S before stimulation for 4 minutes with 0, 0.1, 1, and 10 μmol/L free Ca++. GTP-γ-S pretreatment increased the sensitivity for Ca++ stimulation but did not increase the overall extent of release that occurred on stimulation with 10 μmol/L Ca++. This effect is similar for both dense core granule and lysosome release confirming that GTP-γ-S and Ca++ are synergistic but only when Ca++ concentrations are lower than 1 μmol/L.32 These data taken together demonstrate that our assay system faithfully mimics previous observations using these stimuli on permeabilized platelets.31-33
Previous reports31-33 have suggested that the relationship between Ca++ and GTP-γ-S is indicative of parallel stimulation pathways that can lead to platelet exocytosis. If there are multiple pathways to stimulate granule release, then it is important to establish that the NSF/SNAP/SNARE machinery is used regardless of stimuli. To test this, permeabilized platelets were preincubated with various reagents to determine whether the GTP-γ-S stimulation pathway uses the SNARE machinery. In Figure 4, antibodies to SNAP-23, NSF, and syntaxin 2 and 4 inhibited secretion from GTP-γ-S–stimulated lysosome release. Nonspecific immunoglobulins and antibodies to syntaxin 7 were without effect. Syntaxin 4 antibodies did not inhibit Ca++-stimulated release of [3H]5-HT20 nor did they affect GTP-γ-S–stimulated release of [3H]5-HT. The GTP-γ-S–stimulated secretion of [3H]5-HT was dependent on ATP and temperature as shown by inclusion of apyrase,38or incubation at 4°C, which both eliminated secretion. Interestingly, there was some release of hexosaminidase that was resistant to apyrase treatment and in all cases the extent of inhibition of dense core release was greater than that for lysosome release. What these data indicate is that the secretory pathway stimulated by GTP-γ-S goes through the same SNARE machinery, as does Ca++-stimulated secretion.
The SNARE machinery is required for GTP-γ-S stimulated secretion.
[3H]5-HT–labeled and SLO-permeabilized platelets were first incubated with antibodies against NSF (0.15 mg/mL, 2E5), SNAP-23 (20 μg/mL), ab23 syntaxin 2 (60 μg/mL, pp2), syntaxin 7 (60 μg/mL antisyntaxin 7) and rabbit IgG (60 μg/mL) and mouse IgG (200 μg/mL). The platelets were then activated with 100 μmol/L GTP-γ-S and the release of [3H]5-HT and hexosaminidase was measured and normalized to control (buffer alone). The reactions were also performed by activating the platelet with GTP-γ-S on ice (Ice) or under ATP depletion condition (30 μg/mL apyrase; -ATP). It should be noted that the antibody inhibition results in a below background (no stimulus control) level of [3H]5-HT release hence the negative numbers for those samples.
The SNARE machinery is required for GTP-γ-S stimulated secretion.
[3H]5-HT–labeled and SLO-permeabilized platelets were first incubated with antibodies against NSF (0.15 mg/mL, 2E5), SNAP-23 (20 μg/mL), ab23 syntaxin 2 (60 μg/mL, pp2), syntaxin 7 (60 μg/mL antisyntaxin 7) and rabbit IgG (60 μg/mL) and mouse IgG (200 μg/mL). The platelets were then activated with 100 μmol/L GTP-γ-S and the release of [3H]5-HT and hexosaminidase was measured and normalized to control (buffer alone). The reactions were also performed by activating the platelet with GTP-γ-S on ice (Ice) or under ATP depletion condition (30 μg/mL apyrase; -ATP). It should be noted that the antibody inhibition results in a below background (no stimulus control) level of [3H]5-HT release hence the negative numbers for those samples.
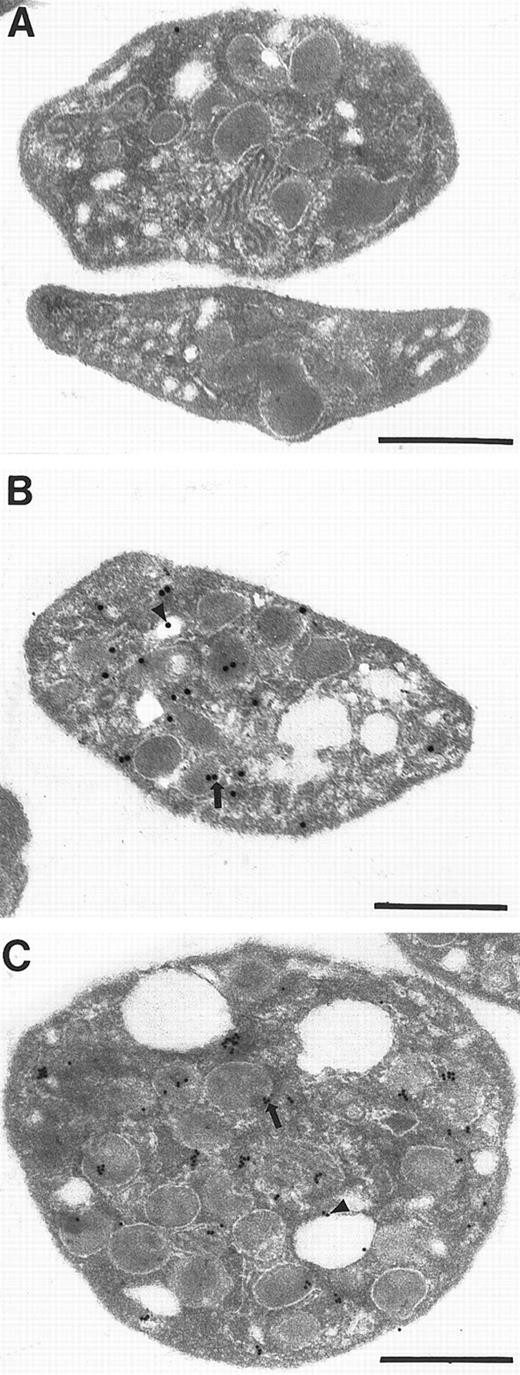
Syntaxin 2 and syntaxin 4 localize to granules and open canalicular system
Because both syntaxins appear to be involved in α-granule and lysosome release, we next examined the subcellular localization syntaxin 2 and 4 in platelets. Initially, we used immunoflourescence microscopy. Fixed and permeabilized platelets were incubated with antisyntaxin 2 or 4 antibodies, followed by the addition of FITC-conjugated antirabbit antibodies. The immunolocalization of syntaxin 2 and 4 in resting platelets consists of a punctate intracellular staining pattern for both antigens (data not shown). The punctate intracellular staining pattern obtained in this experiment indicates that syntaxin 2 and 4 could be localized to the OCS, which would appear as an intracellular compartment by this technique, to platelet granules, or to both. In this experiment, no staining was seen in the absence of primary antibodies or when the primary antibodies were preabsorbed with the appropriate recombinant syntaxin protein. Because of the limited resolution of platelet subcellular compartments by light level microscopy, we turned to immunoelectron microscopy to discriminate between these possibilities. Platelets were fixed, dehydrated, and embedded in LR White resin. After thin-sectioning, platelet-containing grids were subjected to immunolabeling with antisyntaxin 2 or 4 primary antibodies and colloidal gold-conjugated secondary antibodies, followed by counterstaining with uranyl acetate and lead citrate. On examination of these grids, we found syntaxin 2 and 4 associated with what are most likely α-granules and lysosomes. These granules contain opaque interiors whose boundaries can be distinguished by their halo-like appearance (Figure5B,C). At this level of analysis, it is difficult to distinguish between the 2 types of compartments and dense core granule morphology is generally lost during the fixation conditions that were used. We also observed gold labeling on the OCS, the vacuole-like structures seen throughout the platelet cytoplasm (Figure 5B,C). No staining was seen in the absence of primary antibodies, when the primary antibodies were preabsorbed with the appropriate recombinant syntaxin protein, or when preimmune immunolgobulin was used as primary antibody (Figure 5A). The grain distribution data were quantified by counting the grains that were localized to granules, the OCS, and the plasma membrane (PM). As shown in Table 1, 17% and 30% of the antisyntaxin 2 labeling is associated with the OCS and granules, respectively, whereas 15% is found on the PM. Similarly, 33% of the antisyntaxin 4 labeling was present over the OCS with 25% on the granules. Only 6% of the gold grains were associated with the PM. Care was taken to assign grains to the proper category. If the locale of the grain could not be definitively determined, it was designated as “other”; therefore our distribution probably represents an underestimation of the specific localization of syntaxin 2 and 4. The fact that 58% of antisyntaxin 4 labeling and 47% of antisyntaxin 2 labeling localizes to granules and the OCS implicates these proteins as players in trafficking between these 2 membrane compartments.
Syntaxin 2 and syntaxin 4 localize to granules and OCS
. For immunoelectron microscopy, platelets were fixed, dehydrated in a series of ethyl alcohols, and embedded in LR White resin. Samples were thin sectioned, incubated with primary antisyntaxin antibodies (panel B for syntaxin 2 and C for syntaxin 4) or a preimmune immunoglobulin fraction (panel A). The grids were then incubated with gold-conjugated goat antirabbit secondary antibodies 20 nm particle for syntaxin 2, 10 nm particle for syntaxin 4, and counterstained with uranyl acetate and lead citrate. Arrows indicate labeling of the granule. Arrowheads refer to gold particles found on the OCS. Bars equal 0.5 μm.
Syntaxin 2 and syntaxin 4 localize to granules and OCS
. For immunoelectron microscopy, platelets were fixed, dehydrated in a series of ethyl alcohols, and embedded in LR White resin. Samples were thin sectioned, incubated with primary antisyntaxin antibodies (panel B for syntaxin 2 and C for syntaxin 4) or a preimmune immunoglobulin fraction (panel A). The grids were then incubated with gold-conjugated goat antirabbit secondary antibodies 20 nm particle for syntaxin 2, 10 nm particle for syntaxin 4, and counterstained with uranyl acetate and lead citrate. Arrows indicate labeling of the granule. Arrowheads refer to gold particles found on the OCS. Bars equal 0.5 μm.
Immunolocalization of syntaxin 2 and 4 in resting platelets
| Antibody . | Total grains counted* . | Plasma membrane (%) . | OCS (%) . | Granule (%) . | Other (%)† . |
|---|---|---|---|---|---|
| Syntaxin 2 | 1074 | 15 | 17 | 30 | 38 |
| Syntaxin 4 | 1163 | 6 | 33 | 25 | 35 |
| Antibody . | Total grains counted* . | Plasma membrane (%) . | OCS (%) . | Granule (%) . | Other (%)† . |
|---|---|---|---|---|---|
| Syntaxin 2 | 1074 | 15 | 17 | 30 | 38 |
| Syntaxin 4 | 1163 | 6 | 33 | 25 | 35 |
The number represents the percentage of grains associated with the indicated structure.
This category represents structures that were not definitively identifiable.
Discussion
In this manuscript, we have reconstructed the stimulus-driven release of the lysosomal enzyme, hexosaminidase, from permeabilized platelets and shown that this exocytosis process requires NSF and SNAREs. Ca++ initiates a rapid release from dense core granules and lysosomes at different rates. GTP-γ-S stimulates exocytosis from the 2 stores after differing lag times but once initiated, release of hexosaminidase and 5-HT appears to occur at comparable rates. GTP-γ-S does seem to be synergistic with Ca++ but only when Ca++ concentrations are low (less than 1 μmol/L). As concluded previously,32GTP-γ-S seems to increase the Ca++ affinity of the platelet release machinery. From these experiments, it is clear that our assay system reflects platelet responses reported by others, but it is difficult to conclude whether Ca++ and GTP-γ-S stimulate exocytosis through the same or through differing pathways. However, regardless of stimuli, lysosome release requires the SNARE machinery, namely, NSF, SNAP-23 and syntaxin 2 and 4.
Several groups have demonstrated that Ca++ and GTP-γ-S can stimulate exocytosis in permeabilized platelets.31-33Two key findings that are reiterated in our studies are that GTP-γ-S can stimulate release at low Ca++ (pCa > 9) and that GTP-γ-S–stimulated release is slower than Ca++-induced release. The delay in GTP-γ-S–induced exocytosis is perhaps due to the dilution of an important cytosolic factor on permeabilization. Such a dilution apparently has only a limited effect on the Ca++-sensor mechanism although Ca++-stimulated exocytosis does require cytosol. Alternatively, the delay may be caused by the need for a GDP–GTP-γ-S exchange to activate a G protein, which is not needed on Ca++ stimulation. The difference in release kinetics suggests that either the Ca++-responsive step(s) is more proximal to exocytosis or that the 2 types of stimuli induce exocytosis through different but potentially parallel pathways. Parallel stimulation pathways do occur in platelets; an example is the activation of myosin that occurs either through a calmodulin/Ca++-stimulated myosin light chain kinase or Rho/GTP activation of Rho kinase that inactivates myosin phosphatase.39 Regardless of the pathway used, exocytosis from platelets uses the same SNARE machinery. Shown in this manuscript, Ca++ or GTP-γ-S–stimulated exocytosis from lysosomes and dense core granules requires ATP and is inhibited by anti-NSF antibodies as well as anti–SNAP-23 and syntaxin 2 antibodies. In addition, antisyntaxin 4 antibodies also inhibit hexosaminidase release indicating that, regardless of stimuli, lysosome exocytosis requires 2 different syntaxins.
In an earlier manuscript,20 we reported that syntaxin 2 but not 4 was involved in dense core granule release. Flaumenhaft et al25 reported that syntaxin 4 was involved in α-granule release. We have subsequently shown a role for both syntaxin 2 and 4 in α-granule release.23 Here we report that both syntaxin t-SNAREs are required for lysosome release. These findings could be an indication that lysosome and α-granule release require 2 distinct membrane fusion events: a homotypic granule–granule fusion step and a heterotypic granule–OCS fusion step. Indeed, this would be consistent with our immunoelectron microscopy localizations of the 2 syntaxins, which are on granules and OCS (Figure 5 and Table 1). However, when examining the antibody inhibited permeabilized platelets by electron microscopy, no homotypic fusion intermediates are detected when exocytosis is inhibited by either antibody.23Alternatively, the requirement for the 2 syntaxins could be an indication of a lack of SNARE specificity for this exocytosis event. It is clear from studies of viral fusion proteins40,41 that an accumulation of several fusion complexes is required for bilayer fusion. Assuming this were true for SNARE complexes, then perhaps lysosome–OCS fusion becomes a “numbers game” that does not rely on specific docking. Because the granules and lysosomes are apparently forced to the center of the platelet after stimulation and are therefore in close proximity to their target membrane (the OCS), there is perhaps no need for specificity in lysosome–OCS docking. All that is required is to get enough SNARE complexes arranged together to fuse the membranes. In this manner, antibodies to either syntaxin would be inhibitory because it would reduce the number of transmembrane SNARE complexes that could be formed. Although this appears to be true for lysosome release and for α-granule release,23 it is not true for dense core granule release, which is solely driven by syntaxin 2. Unfortunately, this paradox will not be fully resolved until the v-SNAREs required for these exocytosis events are identified and localized. It is clear that at least one v-SNARE is involved in α-granule release25 and VAMP-321 is present in platelets; but it is not clear which molecules facilitate which events.
Acknowledgments
We thank Dr David Castle for his generous gift of the SNAP-23 peptide. We thank the staff of Central Kentucky Blood Center for their assistance, the members of the Whiteheart laboratory for their helpful discussions, and Mary Gail Engle and Richard Watson for their assistance with the electron microscopy studies. We especially thank Ms Ping He for her excellent technical assistance.
Supported by grant number HL56652 from the National Heart, Lung, and Blood Institute of the National Institutes of Health to S.W.W.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
S. W. Whiteheart, Department of Biochemistry, University of Kentucky College of Medicine, 800 Rose St, Lexington, KY 40536; e-mail: whitehe@pop.uky.edu.




![Fig. 4. The SNARE machinery is required for GTP-γ-S stimulated secretion. / [3H]5-HT–labeled and SLO-permeabilized platelets were first incubated with antibodies against NSF (0.15 mg/mL, 2E5), SNAP-23 (20 μg/mL), ab23 syntaxin 2 (60 μg/mL, pp2), syntaxin 7 (60 μg/mL antisyntaxin 7) and rabbit IgG (60 μg/mL) and mouse IgG (200 μg/mL). The platelets were then activated with 100 μmol/L GTP-γ-S and the release of [3H]5-HT and hexosaminidase was measured and normalized to control (buffer alone). The reactions were also performed by activating the platelet with GTP-γ-S on ice (Ice) or under ATP depletion condition (30 μg/mL apyrase; -ATP). It should be noted that the antibody inhibition results in a below background (no stimulus control) level of [3H]5-HT release hence the negative numbers for those samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/5/10.1182_blood.v96.5.1782/5/m_h81700117004.jpeg?Expires=1765940248&Signature=e00icZewM~c~-ePcYpSXaH~jnJDSz~VITc04y29z1du0QDX7k0cmzEUfiW6mv4RdmRHazlDTSJJfLJeALleoVGZyFK1ZUB6dSRpamju5zEug08HboqbJUj8h1DUc4JqNxm7-A091FVjEWs3hlR45liB9DcSHBMDVngyZ7FtisXj57Fb2Toj1VoGmTDyrbg~1CTSpZyfp2feHCGKj4uEc6zbl~ArdnOwQnPvHF9MCQoF4StiT58yz2noqCyTBZCubSMxFJoZgl7IAFR3rBVoIBBSXrrzMLJ3tMR43Rj1Helcem4rxySWsOWQJQGBrmG1JH1meItZxVEbBClow8O3E5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal