Abstract
Rho GTPases control a variety of cellular processes, including actin polymerization, integrin complex formation, cell adhesion, gene transcription, cell cycle progression, and cell proliferation. A patient is described who has recurrent infections and defective neutrophil cellular functions similar to those found in Rac2-deficient mice. Molecular methods were used to clone the expressed Rac2 cDNA from this patient, and a single base pair change (G→A at nucleotide 169) in the coding sequence was identified. This results in an asparagine for aspartic acid mutation at amino acid 57 (D57N), a residue that is involved in nucleotide binding and is conserved in all mammalian Rho GTPases. The cloned cDNA was then introduced into normal bone marrow cells through retrovirus vectors, and neutrophils expressing this mutant exhibited decreased cell movement and production of superoxide in response to fMLP. The expressed recombinant protein was also analyzed biochemically and exhibited defective binding to GTP. Functional studies demonstrated that the D57N mutant behaves in a dominant-negative fashion at the cellular level. The syndrome of Rac2 dysfunction represents a human condition associated with mutation of a Rho GTPase and is another example of human disease associated with abnormalities of small G protein signaling pathways.
Introduction
Circulating blood neutrophils, which make up the initial cellular component of inflammatory infiltrates, interact with endothelium in a multistep process involving selectin-initiated endothelial capture, integrin-mediated adhesion, and chemotaxis-induced diapedesis (reviewed in Lowe and Ward1). The importance of neutrophil movement and function in host defense is demonstrated by genetic defects affecting neutrophil–endothelial interactions (leukocyte adhesion deficiency),2 microbial killing (chronic granulomatous disease),3,4 and movement (neutrophil actin dysfunction).5
To accomplish cellular responses to environmental signals, cells use various signaling pathways linking receptors for extracellular stimuli with effectors in the cytoplasm and nucleus. The Rho family of GTPases, members of the Ras superfamily of small signaling molecules, plays key roles in regulating these responses. Rho GTPases are involved in a wide array of cellular functions, including cytoskeletal (particularly actin) reorganization, integrin complex formation and cell adhesion, gene transcription, cell cycle progression, and cell proliferation.6-8 Rho GTPases, in a fashion similar to Ras, cycle between an active, GTP-bound state and an inactive, GDP-bound state. Proteins that activate these transitions (guanine nucleotide exchange factors and GTPase-activating proteins) and the downstream effector proteins that interact with activated Rho-GTPases have been increasingly identified.9 However, the specific mechanism(s) by which Rho GTPases control a wide range of cellular processes and the relationship between the cellular functions controlled by the same or different Rho GTPases remain(s) largely unknown.
Several distinct members of the Rho family of GTPases have been characterized, including Rho, Rac, and Cdc42. Each member of the family appears to control a distinct function of the actin cytoskeleton.9 In fibroblasts, the activation of Rho leads to the formation of actin–myosin stress fibers and integrin-containing adhesion complexes. Cdc42 activation induces spike-like filopodia rich in F-actin, whereas the activation of Rac is associated with broad lamellipodia.7 The latter 2 morphologic structures are associated with poorly defined integrin complexes.9Interestingly, Rac members of the Rho family have also been implicated in regulation of the NADPH oxidase enzyme complex that generates superoxide in phagocytes.10-15 Although human gene mutations encoding other components of the NADPH oxidase complex (gp91phox, p47phox, p67phox, p40phox) results in absent or reduced superoxide production and chronic granulomatous disease, no patients have yet been recognized with Rac-associated abnormalities in the NADPH oxidase.3 4
Recent work by our laboratory and by other investigators has begun to elucidate the role of Rho GTPases in phagocyte cell function. Rho has been demonstrated to mediate actin polymerization in neutrophils,16-18 and, using dominant negative and constitutively activated mutants, Rac function has been implicated in membrane ruffling, lamellipodia formation, and chemotaxis in a macrophage cell line.19,20 In these same cells, Cdc42 function appears essential for cell polarization mediated by filopodia toward a chemotactic gradient. Using a genetic approach to generate mice deficient in the hematopoietic specific Rho GTPase, Rac2, we demonstrated that Rac2 plays an essential and unique role in neutrophil rolling via l-selectin, F-actin assembly, and chemotaxis in response to several chemoattractants and in superoxide generation in response to some, but not all, agonists.21 22Rac2-deficient mice display a unique phenotype of immunodeficiency consisting of the above cellular defects, leukocytosis, and neutrophilia, but increased susceptibility to fungal infections.
Based on the phenotypic analysis of Rac2-deficient mice, it is possible to predict a human phenotype associated with functional abnormalities in Rac. We now characterize the molecular, biochemical, and biologic defects in a patient with a phenotype similar to that of Rac2-deficient mice by cloning and sequencing the expressed Rac2 mRNA, and demonstrate that the mutation in Rac2 acts in a dominant negative fashion in blood cells. The cDNA mutation is consistent with genomic sequence recently published23 on this same patient.
Patients, materials, and methods
Blood and bone marrow isolation and neutrophil separation
Blood and bone marrow (BM) were obtained after informed consent. The protocol for obtaining these samples was approved by the institutional review boards of the University of Michigan School of Medicine and Indiana University School of Medicine. Peripheral polymorphonuclear leukocytes (PMN) were separated from peripheral venous blood from the patient or from healthy donors by dextran sedimentation, hypotonic lysis of remaining erythrocytes, and separation of neutrophils from mononuclear cells by Ficoll-Paque (Pharmacia, Piscataway, NJ). BM was aspirated from the posterior superior iliac crest of healthy donors. Mononuclear cells were isolated from fresh heparinized BM by centrifugation on Histopaque-1077 (Sigma Diagnostics, St Louis, MO) gradient as described.24 Two million low-density mononuclear cells were plated per well in 6-well tissue culture plates in the presence of human granulocyte colony-stimulating factor (hG-CSF, 100 ng/mL; provided by Amgen, Thousands Oaks, CA), human megakaryocyte growth and differentiation factor (MGDF, 100 ng/mL; Amgen), and human stem cell factor (SCF, 100 ng/mL; Amgen) for 5 days at 37°C in 5% CO2.
Rolling and chemotaxis assays
Neutrophils were isolated from patient or normal control blood using the 1-step Polymorphoprep medium (Gibco-BRL) and procedures recommended by the manufacturer. The rolling adhesion assay was performed as previously described21 with the exception that the current study was limited to 1-wall shear stress (1.26 dynes/cm2) that fell within the range of shear stresses shown to be optimal for initiating and sustainingl-selectin–dependent neutrophil rolling adhesion.25 The bottom surface of the rolling chamber was coated with a 2 μg/mL solution of GlyCAM-1 (a generous gift of Dr S. D. Rosen) as described.21 Tethered and rolling cells were scored and averaged in 10 to 12 different fields of the GlyCAM-1–coated plate in the second minute of the rolling assay. Data represent an average of 2 independent cell preparations and 3 independent plate preparations.
Chemotaxis was evaluated using 2 methods. Neutrophils were suspended in Dulbecco minimum essential medium (DMEM) containing 0.1% bovine serum albumin (Sigma, St Louis, MO) and gradually warmed for 1 hour to 20°C. N-formyl-methionyl-leucyl-phenylalanine (10−7 mol/L) (fMLP; Sigma) and IL-8 (5 × 10−9 mol/L) (Peprotech, Rocky Hill, NJ) were diluted in the same solution and added to the lower wells of 48-well microchambers. Polycarbonate filters of 3 μm pore size (Nuclepore, Pleasanton, CA) were used to separate upper from lower wells. PMN (5 × 104) were added to the upper wells, and the chambers were placed in a humidified incubator at 37°C with 5% CO2 for 30 minutes. The filter was removed, stained, and mounted. The number of neutrophils migrating through the filter was determined by counting 5 random 400× microscope fields. Data are expressed as mean number of cells per field ± SEM.
In addition, PMN chemotaxis in response to fMLP (0-10−5mol/L) was directly observed using a Zigmond chamber (Neuro Probe, Cabin John, MD) as previously described.21 Microscopic images were recorded at 1-minute intervals using a 40× oil-immersion objective lens on an inverted microscope (Nikon Diaphot 300) equipped with DIC optics and epifluorescence optics. Collection of images from a cooled charged-coupled device camera (Pentamax model RTEA 1372K/2; Princeton Instruments, Princeton, NJ) began 10 minutes after the chamber was set up, to allow time for a stable gradient to form. Green fluorescent protein (GFP)-positive cells were identified by epifluorescence using 480-nm excitation and 535-nm emission filters (Chroma Technology, Brattleboro, VT). Metamorph software (Universal Imaging, West Chester, PA) was used to acquire and analyze the images. Because most in vitro differentiated cells, regardless of origin, were nonmotile, analysis was restricted to the cells that exhibited any movement.
Phagocytosis assays
Phagocytosis assays were carried out essentially as previously described.26 27 Antibody-treated erythrocytes (EIgG) were washed 3 times and suspended in the same buffer at 2 to 3 × 109 cells/mL. After incubation, neutrophils were activated with fMLP (100 nmol/L) for 10 minutes at 37°C; EIgG (2 × 106/mL) were added to the activated neutrophils, and incubation was continued for an additional 30 minutes at 37°C. EIgG that were not internalized were lysed with distilled water, returned to isotonicity by the addition of 0.6 mol/L KCl, and fixed with 1% glutaraldehyde. Phagocytosis was quantitated microscopically and expressed as the number of particles ingested per 100 cells (phagocytic index).
Measurement of NADPH oxidase activity and NBT test
Superoxide production was measured in a quantitative kinetic assay based on the reduction of cytochrome C (Sigma) after the stimulation of cells with fMLP alone or with cytochalasin B or phorbol myristate acetate (PMA) alone (all Sigma). PMN were treated with 5 μg/mL cytochalasin B for 3 minutes and then by 400 nmol/L fMLP for 5 minutes, or fMLP alone, or 10 ng/mL PMA alone for 5 minutes. Data were expressed as mean nmol 02−/106PMN/5 minutes ± SEM. The nitroblue tetrazolium (NBT) test for superoxide production was performed on PMN derived from the patient or from healthy donors after expansion with or without transduction in vitro as described.28 The percentage of NBT-positive cells and the intensity of staining were determined by evaluating 100 cells.
Adhesion to fibrinogen
Neutrophils were activated with 10−7 mol/L fMLP for 5 minutes at 37°C, then washed with and resuspended in Dulbecco phosphate-buffered saline containing 5 mmol/L glucose. Plates (24 well) were coated with fibrinogen (50 μg/well) (Chromogenix, Milan, Italy) for 2 hours at 37°C, washed with Dulbecco phosphate-buffered saline, and blocked with 1% gelatin (Norland Products, New Brunswick, NJ) for 1 hour at 22°C. Plates were again washed, then neutrophils (1 × 105/well) were added to plates at appropriate times to yield the durations specified. Plates were incubated at 22°C. Neutrophils that had not adhered were washed away, and adherent cells were fixed in 1% glutaraldehyde. The number of adherent neutrophils was determined by counting 5 random 200× microscopic fields.
Cloning and sequencing of Rac2 cDNA
Total RNA was isolated from expanded BM cells of healthy donors and the patient using TRIZOL reagent as described by the manufacturer (Life Technologies, Rockville, MD). First-strand cDNA synthesis was carried out using Superscript II RNase H− reverse transcriptase (Life Technologies) and oligo (dT)16 as primer (Perkin Elmer, Foster City, CA). Polymerase chain reaction (PCR) was performed using primers spanning the entire human Rac2 coding region (5′ CCGGAATTCATGCAGGCCATCAAGTGTGTGGTG 3′ and 5′ CCGCTCGAGCTAGAGGAGGCTGCAGGCGCGCTT C 3′). The 597-bp PCR products were cloned into pCR4-TOPO vector using the procedures recommended by the manufacturer (Invitrogen, Carlsbad, CA). Individual colonies were then picked and sequenced with T3 and T7 primers. Thirteen clones from the patient and 7 from the normal sample were successfully sequenced.
Construction of retroviral vectors, transfections, and infection of target cells
An improved murine stem cell virus (MSCV)-based bi-cistronic retroviral vector, MIEG3, was constructed and used in this study. MIEG3 gives brighter EGFP fluorescence than the parental MSCV and MIG vectors,29 which allows direct visualization of the EGFP-positive cells under a fluorescent microscope. To construct MIEG3, the RSGFP in the MIG retroviral vector was replaced with the EGFP of pEGFP-C1 (Clontech Laboratories, Palo Alto, CA), yielding MIEG vector (Hanenberg H, D.A.W., unpublished data). Subsequently, theBglII-NcoI fragment in MIEG containing the disabled internal ribosome entry site (IRES) was replaced withBamHI-NcoI fragment of the pIRES2-EGFP (Clontech Laboratories), yielding MIEG3 vector. This last maneuver restores the encephalomyocarditis virus (EMCV) IRES element to its original EMCV viral configuration, resulting in more efficient translation of EGFP.
Flag epitope tag was introduced into the N-terminal region of the murine wild-type (WT) Rac2 cDNA using a PCR-based technique. The primers used in this study were as follows: 5′ CGGAATTCACCATGGACTACAAAGACGATGACGACAAGCAGGCCA-TCATTGTGTGGTGGTGGGTGATG 3′ and 5′ GCTCGAGCCTAGAGCAGGCTGCAGG GGCGCTTCTGCTG 3′. Similarly, the Kozak consensus translation initiation sequence was incorporated into the normal human Rac2 cDNA and the mutant (D57N) Rac2 cDNA derived from the patient to maximize the translation of the expressed Rac2 genes. The following primers were used: 5′ CCGGAATTCCACCATGCAGGCCATCAAGTG TGTGGTG 3′ and 5′ CCGCTCGAGCTAGAGGAGGCTGCAGGCGCGCTT C 3′. Amplified fragments were cloned into pPCR-Script SK plasmid (Stratagene, La Jolla, CA) and sequenced multiple times. The sequence-confirmed, Flag-tagged murine Rac2, normal human Rac2, and human mutant D57N Rac2 inserts were digested with EcoRI and XhoI and were cloned into the same sites of MIEG3 (see below) yielding MIEG3FR2, HR2WT, and HR2MU vectors, respectively.
Viral supernatant collected from the transfected Phoenix-Ampho packaging cell line (obtained from American Type Culture Collection, Manassas, VA) was used to infect the GP+E86 packaging cell line.30 The GFP-expressing cells with high-fluorescence intensity after 2 consecutive infections were selected by fluorescence-activated cell sorter (FACS). The retroviral titer was determined by FACS analysis using a method similar to that reported recently.31 The titer of the stable MIEG3 and MIEG3FR2 E86 clone viral supernatant was approximately 2.5 × 104cfu/mL and 1.4 × 105 cfu/mL, respectively.
Transient retroviral supernatant derived from Phoenix-Ampho (titers of approximately 5 × 105/mL) was also used to directly infect human mononuclear cells using the procedures described previously.24 Infected cells were cultured with 100 ng/mL MGDF, 100 ng/mL hG-CSF, and 100 ng/mL SCF for 5 days. Transduced cells were analyzed for GFP expression by FACScan (Becton Dickinson, Mountain View, CA) 48 hours after the second infection.
Flow cytometric analysis, Western analysis, and cell sorting
After the initial 5 days of expansion in 3 cytokines (see above), the transduced cells were further expanded 10 to 14 days in G-CSF, SCF (as above), and recombinant human IL-3 (100 ng/mL; Peprotech) to enhance myeloid differentiation. Before functional assays, cells were analyzed for transduction by flow analysis of GFP, and the differentiation of the cells was evaluated by expression of human CD13 on the cell surfaces using a FACScan (Becton Dickinson) after staining the cells with phycoerythrin antihuman CD13 (Pharmingen, San Diego, CA). The GFP-positive cells were isolated by FACS (FACStar Plus; Becton Dickinson) under sterile conditions. Reanalysis of the GFP-sorted cells showed purity of greater than 90%. NIH/3T3 cells were infected twice with the MIEG3, HR2MU, HR2WT, and MIEG3FR2 retroviral supernatants. GFP-positive cells were sorted using FACS. After culturing in DMEM for several days, a portion of the cells was analyzed by FLOW for GFP expression, and the remaining cells were used for Western blot analysis using a 1:5000 dilution of anti-Rac2 antibody (kindly supplied by Gary Bokoch, Scripps Institute, La Jolla, CA). The Western blot was performed as previously described.32
Cell shape changes
MIEG3, HR2WT, and HR2MU-infected NIH3T3 cells were seeded on gelatinized glass coverslips. Several days after seeding, the cells were serum starved for 20 hours. The cells were then treated with 5 ng/mL platelet-derived growth factor (Peprotech) or mock treated for 8 minutes and fixed in 3.7% paraformaldehyde for 20 minutes at room temperature. Subsequently, the cells were stained with rhodamine–phalloidin as previously described.33
Expression, purification, and guanine nucleotide binding of recombinant proteins
The cDNA of human D57N mutant Rac2 or WT human Rac2 was cloned into the bacterial expression vector pET-28a (Novagen, Milwaukee, WI) at the EcoRI and XhoI cloning sites. Both WT and D57N Rac2 were expressed in Escherichia coli as a fusion protein with His6 tag. Purification of these proteins was carried out according to the manufacturer's instructions. The purified recombinant proteins were then separated on an SDS-PAGE gel to confirm the purity (greater than 90%) of the expressed proteins.3H-GDP (10 μmol/L) and 35S-γGTP (1 μmol/L) (Amersham/Pharmacia Biotech, Piscataway, NJ) were loaded onto the recombinant GTPases (1-2 μg) in the presence of 20 mmol/L Tris-HCl (pH 7.6), 100 mmol/L NaCl, 2 mmol/L EDTA, and 1 mmol/L dithiothreitol for 20 minutes at room temperature. Reactions were terminated by the addition of MgCl2 to a final concentration of 10 mmol/L, and the mixture was placed on ice. Binding was quantitated by vacuum filtration, as described.34
Results
Case history
The patient was a 1-year-old boy who had multiple recurrent, life-threatening infections characterized by leukocytosis and notable for the absence of pus in the inflamed tissues. A detailed clinical description of the child's history before transplantation will be presented elsewhere (A. Kurchubasche et al, manuscript submitted).
A phagocyte defect was suspected and found by the original treating physicians on the basis of neutrophilia, absence of pus in the wounds, and improved healing in response to granulocyte transfusions of an umbilical infection. Evaluation of neutrophil function at the University of Michigan confirmed the original impression of the referring physicians. Neutrophil functional data revealed that the patient's neutrophils responded normally to PMA in terms of the respiratory burst. In addition, the presence and density of CD11b, CD11c, and CD18 were normal. New data indicated that the expression of P-selectin and CD62L (data not shown) were also normal. Other neutrophil functions are described below.
The patient was referred to the University of Michigan Pediatric Bone Marrow Transplantation Program for allogeneic bone marrow transplantation from his HLA-identical older brother. A detailed workup of neutrophil function was completed before transplantation (see below). The patient was conditioned with antithymocyte globulin 90 mg/kg, intravenous busulfan 16 mg/kg, and cyclophosphamide 200 mg/kg. Transplantation was complicated by severe veno-occlusive disease of the liver, necessitating serial paracentesis and prolonged mechanical ventilation. Neutrophils became engrafted on day 11. The only infection-related complication was an infected arterial line that grewStenotrophomonas maltophilia and Enterobacter cloacae, which responded well to intravenous antibiotic therapy. The patient made a full recovery and is currently thriving at home 4 months after undergoing bone marrow transplantation. He has had no infections since hospital discharge, and he requires no medications. His white blood cell count and differential are normal, and he is well engrafted for red blood cells and platelets, though mild thrombocytopenia persists. Immunologic studies confirm normal T- and B-cell subsets and normal immunoglobulin levels. His bone marrow demonstrates 100% donor cells by a variable number of tandem repeat analyses.
Neutrophil function assays
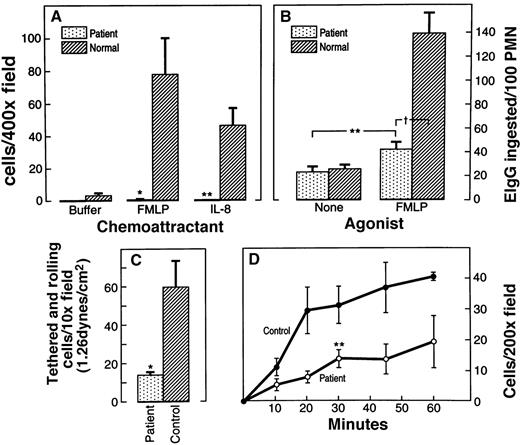
The patient had markedly impaired chemotaxis in response to fMLP and IL-8 in modified Boyden chamber analysis (Figure1A). Patient neutrophils migrating in response to 100 nmol/L fMLP and 5 mmol/L IL-8 were significantly reduced (0.5 ± 0.1 and 0.3 ± 0.1 cells/400× field, respectively) compared to PMN obtained from a healthy donor (77.3 ± 22.1 and 45.5 ± 10.9, respectively; mean ± SEM; n = 5). The lack of movement was confirmed using direct observation of PMN movement in a Zigmond chamber in the presence of a gradient of fMLP (10−5 mol/L) (see below). Phagocytosis was also impaired, but not as severely as chemotaxis. Phagocytosis of erythrocytes opsonized with IgG (EIgG) (Figure 1B) by patient neutrophils stimulated with 100 nmol/L fMLP was 42 ± 6.0 EIgG/100 PMN, significantly higher (P < .05) than that for unstimulated neutrophils but lower (P < .001) than the 138.5 ± 17.5 EIgG ingested per 100 PMN by normal fMLP-stimulated controls. Thus, fMLP-stimulated phagocytosis was only slightly increased in patient cells, whereas it was increased 6-fold in control cells. Because a striking characteristic of neutrophils derived from Rac2-deficient mice previously described was defective rolling by l-selectin, the ability of patient neutrophils to tether and subsequently roll on the l-selectin ligand, GlyCAM-1, was analyzed (Figure 1C). At 1.26 dynes/cm2, the patient neutrophils demonstrated an approximately 5-fold reduction in the number of cells that tethered and rolled on GlyCAM-1. Adhesion of fMLP-stimulated patient PMN to fibrinogen-coated plates over 10 to 60 minutes tended to be reduced compared to normal controls (Figure 1D), but this reduction reached significance only at 30 minutes. In summary, the cellular phenotype of the patient was remarkably similar to that of the actin-based neutrophil cellular defects described in the Rac2-deficient mouse.21
Actin-based function of patient neutrophils compared to normal neutrophils.
(A) Chemotaxis in a modified Boyden chamber in response to fMLP and IL-8. The number of neutrophils migrating through the filter was determined by counting 5 random 400× microscope fields. Data are expressed as mean number of cells per field ± SEM. *P = .01; **P = .05. (B) Phagocytosis of IgG-coated erythrocytes unstimulated and in response to fMLP. **P = .05, fMLP vs no agonist (patient);†P = .001 patient vs normal. (C) Neutrophil rolling on the l-selectin ligand, GlyCAM-1. Tethered and rolling cells were scored and averaged in 10 to 12 different fields of the GlyCAM-1–coated plate in the second minute of the rolling assay. Data shown represent the average number of tethered cells, ± SEM, observed using 2 independently isolated cell preparations and 2 independently prepared GlyCAM-1–substituted chamber surfaces. *P = .01. (D) Time course of neutrophil adhesion to fibrinogen. The number of adherent neutrophils was determined by counting 5 random 200× microscope fields. **P = .05 only at the 30-minute time point; P > .05 (NS) all other points.
Actin-based function of patient neutrophils compared to normal neutrophils.
(A) Chemotaxis in a modified Boyden chamber in response to fMLP and IL-8. The number of neutrophils migrating through the filter was determined by counting 5 random 400× microscope fields. Data are expressed as mean number of cells per field ± SEM. *P = .01; **P = .05. (B) Phagocytosis of IgG-coated erythrocytes unstimulated and in response to fMLP. **P = .05, fMLP vs no agonist (patient);†P = .001 patient vs normal. (C) Neutrophil rolling on the l-selectin ligand, GlyCAM-1. Tethered and rolling cells were scored and averaged in 10 to 12 different fields of the GlyCAM-1–coated plate in the second minute of the rolling assay. Data shown represent the average number of tethered cells, ± SEM, observed using 2 independently isolated cell preparations and 2 independently prepared GlyCAM-1–substituted chamber surfaces. *P = .01. (D) Time course of neutrophil adhesion to fibrinogen. The number of adherent neutrophils was determined by counting 5 random 200× microscope fields. **P = .05 only at the 30-minute time point; P > .05 (NS) all other points.
Superoxide production
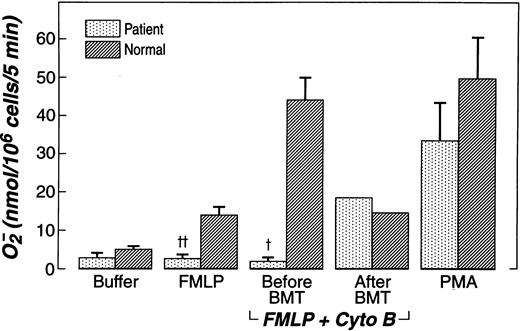
Rac1, Rac2, or both appear to be essential for superoxide production in cell-free systems, and neutrophil NADPH oxidase function is impaired,10-12 but not completely deficient, in neutrophils derived from Rac2-deficient mice.21 To assess NADPH oxidase function in neutrophils from patients and normal controls, cells were stimulated with fMLP and PMA, and superoxide production was measured (Figure 2). Without prior treatment with cytochalasin b, fMLP-elicited O2− production by patient neutrophils was significantly reduced compared to that of normal neutrophils. After treatment with cytochalasin b, fMLP still failed to activate O2− production in the patient's neutrophils, which produced only 1.7 ± 1.2 nmol superoxide/106 cells per 5 minutes. In contrast, as expected, a more than 3-fold increase in O2− production was seen in neutrophils from healthy donors, which produced 43.7 ± 5.8 nmol superoxide/106 cells per 5 minutes (mean ± SEM, n = 5). However, analogous to Rac2-deficient murine neutrophils, PMA (10 ng/mL) stimulation of the patient's neutrophils yielded normal production of superoxide. Also as expected, after successful bone marrow transplantation, O2− production was equivalent on concurrently analyzed patient and normal neutrophils (Figure2).
Superoxide generation of patient neutrophils compared to normal neutrophils.
Assays performed before and after bone marrow transplantation (BMT). PMN were treated with 5 μg/mL cytochalasin B for 3 minutes, then by 400 nmol/L fMLP for 5 minutes, or fMLP alone, or 10 ng/mL PMA alone for 5 minutes. Data were expressed as mean nmol 02−/106 neutrophils/5 minutes ± SEM. †P = .001;‡P = .005. NS, PMA patient vs normal; fMLP + cytochalasin B after BMT.
Superoxide generation of patient neutrophils compared to normal neutrophils.
Assays performed before and after bone marrow transplantation (BMT). PMN were treated with 5 μg/mL cytochalasin B for 3 minutes, then by 400 nmol/L fMLP for 5 minutes, or fMLP alone, or 10 ng/mL PMA alone for 5 minutes. Data were expressed as mean nmol 02−/106 neutrophils/5 minutes ± SEM. †P = .001;‡P = .005. NS, PMA patient vs normal; fMLP + cytochalasin B after BMT.
Sequencing of the Rac2 cDNA and identification of a D57N point mutation in the patient
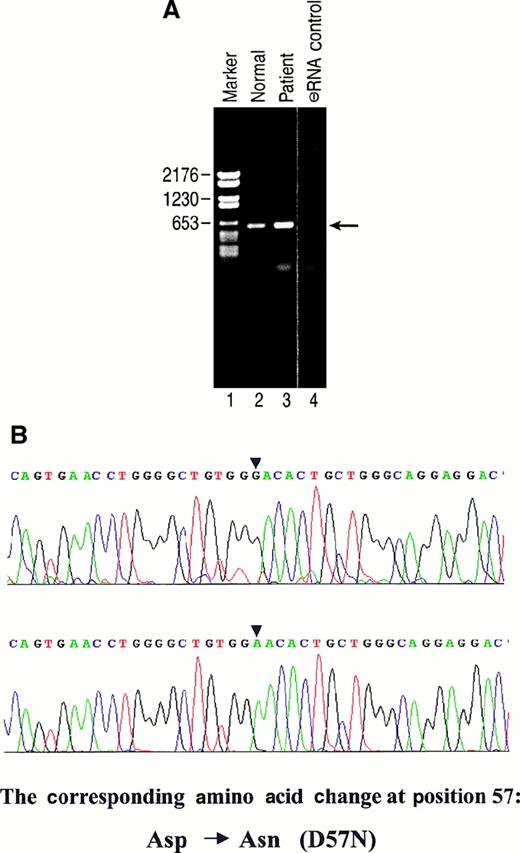
Low-density bone marrow (LDBM) cells obtained from the patient before transplantation or from healthy donors were expanded in multiple cytokines and used to prepare RNA for RT-PCR. PCR primers were designed that flanked the entire Rac2 coding sequence. As seen in Figure 3A, the predicted Rac2 PCR product of 597 bp was obtained from the patient's cells and normal BM cells. PCR products from both were cloned and sequenced. As seen in Figure 3B, multiple sequenced cDNAs from the patient demonstrated a G→A base pair change at nucleotide 169. Among 13 individual clones sequenced from samples from the patient, 8 were found to have the G→A mutation, whereas the remaining 5 were wild type. All 7 clones sequenced from the normal sample were found to contain the WT sequence. In addition, we found another single base-pair change (C→T) at nucleotide position 477 compared with the GenBank human Rac2 sequence; this mutation does not change the coding amino acid sequence, and this mutation was present in all clones sequenced (patient and healthy donor). Northern blot analysis of total cellular RNA from the patient demonstrated nearly normal levels of Rac2 message (data not shown). The predicted amino acid change encoded by the mutant cDNA sequence was a substitution of asparagine for aspartic acid at position 57 (D57N). This mutation occurs in a GTP-binding domain that is highly conserved in all Rho GTPases and in the Ras superfamily.35 Normal levels of mRNA and normal sequences in half the cloned cDNA from the patient suggested that the mutant acted in a dominant-negative fashion at the cellular level.
Expression of mutant Rac 2 in patient bone marrow-derived cells.
(A) RT-PCR performed as described in “Materials and methods” and PCR product separated on agarose gel. Lane 1, marker. Lane 2, PCR product derived from healthy donor. Lane 3, PCR product derived from patient. Lane 4, control; no RNA. Location of predicted 597-bp PCR product is shown at arrow. (B) Partial nucleotide sequence of normal vs mutant cDNA derived from patient RT-PCR product. Upper panel: sequence derived from cDNA product of healthy donor (A, lane 2). Lower panel: sequence derived from cDNA of patient. Location of G→A base-pair change is noted in the sequence on the upper and lower panels by arrowheads. Predicted amino acid change is at position 57.
Expression of mutant Rac 2 in patient bone marrow-derived cells.
(A) RT-PCR performed as described in “Materials and methods” and PCR product separated on agarose gel. Lane 1, marker. Lane 2, PCR product derived from healthy donor. Lane 3, PCR product derived from patient. Lane 4, control; no RNA. Location of predicted 597-bp PCR product is shown at arrow. (B) Partial nucleotide sequence of normal vs mutant cDNA derived from patient RT-PCR product. Upper panel: sequence derived from cDNA product of healthy donor (A, lane 2). Lower panel: sequence derived from cDNA of patient. Location of G→A base-pair change is noted in the sequence on the upper and lower panels by arrowheads. Predicted amino acid change is at position 57.
Construction of retrovirus expressing WT and mutant D57N Rac2 cDNA and functional analysis
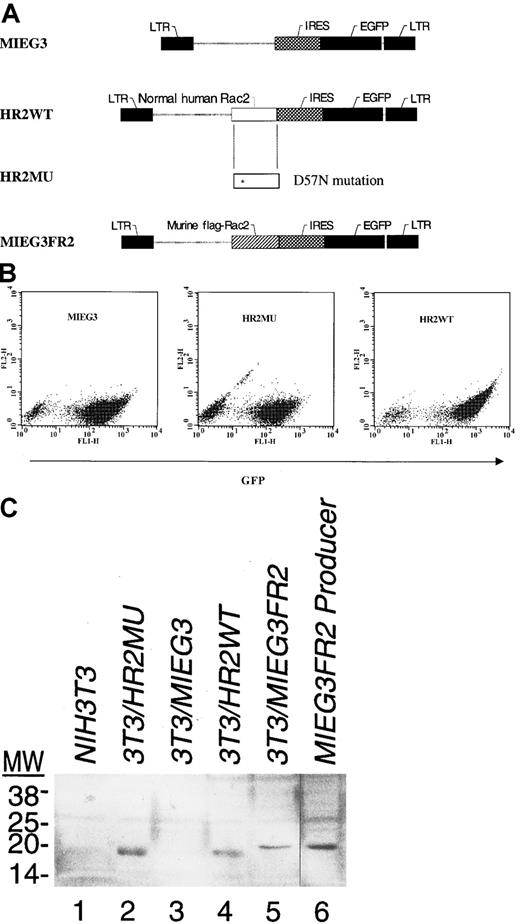
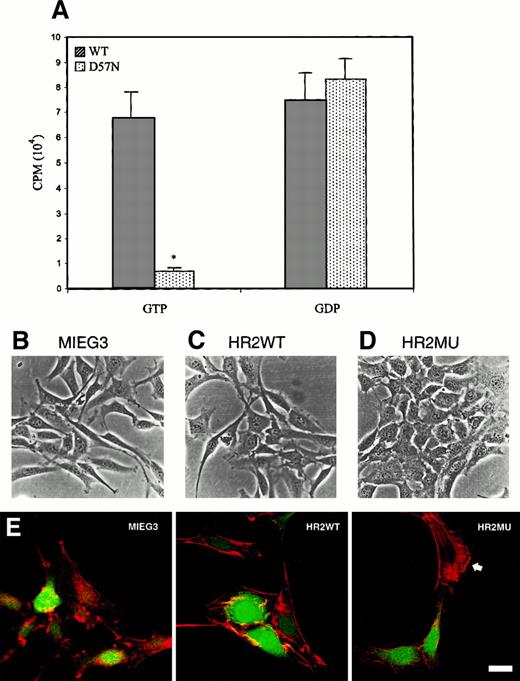
To study the cellular phenotype of the patient and the mutant D57N cDNA in more detail, recombinant retrovirus vectors expressing either the WT (both murine and human) and mutant Rac2 cDNA were constructed (Figure 4A). The retrovirus backbone, MIEG3, is derived from the MSCV retrovirus29 with modifications to improve the expression of GFP. The use of an internal ribosome re-entry site (IRES) in a bi-cistronic vector allows estimates of expression of both GFP and inserted upstream sequences (in this case, Rac2) in a limited number of cells by the intensity of GFP using Flow analysis. To determine that HR2WT and HR2MU both encode Rac2 protein, NIH/3T3 cells, which lack endogenous Rac2 expression, were infected and then sorted for GFP-positive cells (Figure 4B,C). As seen in Figure 4B, most NIH/3T3 cells were GFP positive (the percentages of GFP-positive cells for MIEG3, HR2MU, and HR2WT were 94%, 84%, and 96%, respectively). GFP-sorted NIH/3T3 cells shown in Figure 4B were used for Western blot analysis. As seen in Figure 4C, Western blot analysis confirmed the expression of vector-encoded mutant Rac2 (lane 2) and WT Rac2 (lane 4) and of Flag-tagged murine Rac2 (lane 5) in infected and GFP-sorted cells. WT Flag-tagged murine Rac2 protein is also present, as expected, in the MIEG3FR2 producer cell line. A minor band, likely representing cross-hybridization with Rac1 (see Roberts et al21) is present in uninfected NIH/3T3 cells (lane 1) and 3T3 cells infected with MIEG2 vector (lane 3). NIH/3T3 cells expressing D57N grew more slowly than cells expressing either WT Rac2 or GFP alone (see below).
Retroviral-mediated transduction of normal and patient cells with Rac2-containing vectors.
(A) Recombinant retroviral vectors constructed as described in “Materials and methods” were based on MIEG3, a modified MSCV vector, and contain either the WT Rac2 sequence (HR2WT) or the D57N mutant cDNA derived from the patient (HR2MU). Also shown is the vector expressing the Flag-tagged WT murine Rac2 (MIEG3FR2). LTR, long terminal repeat; IRES, internal ribosome entry site; EGFP, enhanced green fluorescent protein. (B) Flow analysis of NIH/3T3 cells infected with respective retrovirus vectors and analyzed for expression of GFP; horizontal axis shows GFP expression. (C) Western blot analysis of transduced and sorted NIH/3T3 cells for Rac2 protein. Expression of the Rac2 protein is identified in NIH/3T3 cells infected with HR2MU (patient mutant cDNA, lane 2), HR2WT (WT cDNA, lane 4), MIEG3FR2 (murine Flag-tagged Rac2, lane 5), and the viral producer cell expressing MIEG3FR2 virus (lane 6). No expression is seen in NIH/3T3 cells or NIH/3T3 cells infected with the virus expressing only EGFP (lane 1,3). The size of the murine Rac2 protein is slightly higher because of the Flag tag. The faint cross-hybridizing bands seen in lanes 1 and 3 are caused by cross-hybridization with Rac1.21
Retroviral-mediated transduction of normal and patient cells with Rac2-containing vectors.
(A) Recombinant retroviral vectors constructed as described in “Materials and methods” were based on MIEG3, a modified MSCV vector, and contain either the WT Rac2 sequence (HR2WT) or the D57N mutant cDNA derived from the patient (HR2MU). Also shown is the vector expressing the Flag-tagged WT murine Rac2 (MIEG3FR2). LTR, long terminal repeat; IRES, internal ribosome entry site; EGFP, enhanced green fluorescent protein. (B) Flow analysis of NIH/3T3 cells infected with respective retrovirus vectors and analyzed for expression of GFP; horizontal axis shows GFP expression. (C) Western blot analysis of transduced and sorted NIH/3T3 cells for Rac2 protein. Expression of the Rac2 protein is identified in NIH/3T3 cells infected with HR2MU (patient mutant cDNA, lane 2), HR2WT (WT cDNA, lane 4), MIEG3FR2 (murine Flag-tagged Rac2, lane 5), and the viral producer cell expressing MIEG3FR2 virus (lane 6). No expression is seen in NIH/3T3 cells or NIH/3T3 cells infected with the virus expressing only EGFP (lane 1,3). The size of the murine Rac2 protein is slightly higher because of the Flag tag. The faint cross-hybridizing bands seen in lanes 1 and 3 are caused by cross-hybridization with Rac1.21
We found no differences between the functions of murine and human WT Rac2 (data not shown), which was consistent with the high degree of homology of the coding sequences between species. Therefore, in initial studies, either the murine WT or the human Rac2 mutant retroviruses were used to transduce cells. Patient LDBM cells were transduced with empty vector (MIEG3) or with the WT murine or human cDNA (MIEG3FR2 or HR2WT) and sorted for GFP-positive cells after in vitro differentiation into granulocytic cells. As control, normal LDBM cells were transduced with the empty vector. Neutrophil NADPH oxidase activity was assessed using the NBT test, which detects superoxide production in individual cells. Compared to the empty vector, expression of WT Rac2 in patient cells derived from transduced LDBM cells failed to increase the percentage of NBT-positive cells in response to fMLP (4% NBT positive), though, as previously noted, the patient cells showed similar levels of NBT-positive cells in response to PMA (52% NBT positive) (Table 1). In contrast, normal cells transduced with the control retrovirus expressing only GFP demonstrated NBT-positive cells after stimulation with either PMA (91%) or fMLP (55%).
Neutrophil NADPH oxidase activity after transduction of normal and patient bone marrow cells
| Agonist . | Normal BM MIEG3 (%)* . | Patient BM MIEG3 (%) . | Patient BM MIEG3FR2 (%) . |
|---|---|---|---|
| PMA | 91 | 62 | 52 |
| fMLP | 55 | 1 | 4 |
| No stimulant | 20 | 0 | 1 |
| Agonist . | Normal BM MIEG3 (%)* . | Patient BM MIEG3 (%) . | Patient BM MIEG3FR2 (%) . |
|---|---|---|---|
| PMA | 91 | 62 | 52 |
| fMLP | 55 | 1 | 4 |
| No stimulant | 20 | 0 | 1 |
NADPH oxidase activity was monitored in individual neutrophils using the NBT test, as described in “Materials and methods.”
Percentage NBT positive.
To demonstrate more precisely at the cellular level the dominant-negative nature of the D57N mutation, normal human LDBM (or umbilical cord blood) cells were infected with either the WT and the D57N-expressing retrovirus, and the cells were differentiated in vitro into mature neutrophils. Between 2% and 4% of primary bone marrow cells were transduced by the empty retrovirus vector (MIEG3), the WT Rac2 cDNA-containing vector (MIEG3FR2), or the D57N mutant (HR2MU) (data not shown). After transduction and differentiation for 14 days in culture, more than 95% of cells displayed a myeloid phenotype, as assessed by staining with CD13. Transduced GFP-positive cells were sorted to purity by FACS and analyzed for cell movement and superoxide generation by NBT test. After sorting, more than 90% of the cells were found to be CD13 and GFP double positive. As seen in Table2, either PMA or fMLP activates superoxide production in normal human cells transduced with either the vector expressing GFP alone (MIEG3) or WT Rac2 (MIEG3FR2). In contrast, normal human cells expressing the D57N mutant (HR2MU) have few NBT-positive cells in response to fMLP but are able to generate superoxide in response to PMA in a fashion similar to normal. This phenotype (PMA-, but not fMLP-, induced superoxide production) mimics that of the patient's cells and provides evidence of the dominant-negative nature of the D57N mutant at the cellular level.
Neutrophil NADPH oxidase activity after transduction of normal bone marrow cells with Rac2 mutant retrovirus
| Agonist . | MIEG3 (%) . | MIEG3FR2 (%)* . | HR2MU (%) . |
|---|---|---|---|
| PMA | 60 | 68 | 63 |
| fMLP | 24 | 28 | 5 |
| No stimulus | 1 | 1 | 0 |
| Agonist . | MIEG3 (%) . | MIEG3FR2 (%)* . | HR2MU (%) . |
|---|---|---|---|
| PMA | 60 | 68 | 63 |
| fMLP | 24 | 28 | 5 |
| No stimulus | 1 | 1 | 0 |
NADPH oxidase activity was monitored in individual neutrophils using the NBT test. Shown are data from 1 of 3 experiments with similar results.
Percentage NBT positive.
Effects of D57N on movement of normal transduced myeloid cells
To generate adequate numbers of transduced and expanded cells for movement studies, we used umbilical cord blood cells, which have a higher transduction efficiency (20%-40%) than bone marrow cells. Chemotaxis analyzed by modified Boyden chamber demonstrated that GFP+/CD13+ cells expressing either MIEG3 or HR2WT (human WT Rac2) were able to move in response to fMLP (Table3). The expression of WT Rac2 by retrovirus appeared to enhance movement in response to fMLP (Table 3). In contrast, expression of the D57N mutant cDNA significantly impaired movement of these cells in response to fMLP compared with the expression of WT Rac2 (11% ± 4% mutant vs 100% ± 19% WT,P < .001, and impaired movement when compared with normal cells at 11% ± 4% vs 65% ± 14%, P < .01).
Chemotaxis of cord blood-derived neutrophils transduced with WT or mutant Rac2
Cells/low power field ± SD of samples performed in triplicate by modified Boyden chamber assay. Statistical analysis was performed using a one-way ANOVA and Tukey-Kramer post-test for multiple comparisons.
P < .05 HR2WT vs MIEG3.
P < .001 HR2MU vs HR2WT.
P < .01 HR2MU vs MIEG3.
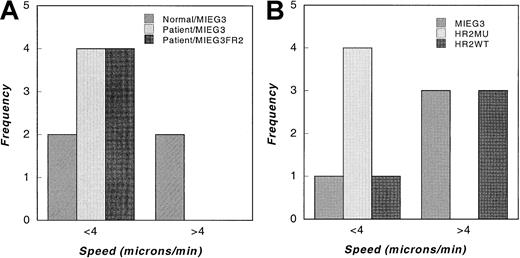
To assess the speed of movement of the patient cells, we analyzed responses to fMLP in a videomicroscopy chamber (Figure5A). Compared to normal neutrophils in which 50% of the cells moved at more than 4 μm/min, patient neutrophils uniformly moved slowly. Wild-type neutrophils were observed to move at rates of up to 13 μm/min, whereas the maximum speed of patient neutrophils recorded was 3.1 μm/min. The expression of WT Rac2 failed to increase the speed of patient neutrophil movement (patient Rac2-EGFP). We next analyzed the effect of WT Rac2 expression vs D57N Rac2 mutant expression on the speed of movement of normal neutrophils derived from transduced bone marrow cells (Figure 5B). GFP-positive cells expressing either the retrovirus containing EGFP alone or WT Rac2 demonstrated fast movement (more than 4 μm/min) in an fMLP gradient, as analyzed by videomicroscopy. In contrast, expression of the D57N mutant increased the number of neutrophils that demonstrated slow movement (less than 4 μm/min), and none of these mutant Rac2-GFP–positive cells were seen moving at a fast speed. Thus, the differential response of both the NADPH oxidase to PMA and the fMLP agonist and the movement of mutant neutrophils in response to fMLP was recapitulated in normal cells by expressing the patient's mutant cDNA. The data demonstrate that expression of the patient's mutant allele is associated with abnormal movement and superoxide production, even in the presence of normal Rac2 protein, and they confirm the dominant-negative nature of this mutation.
Motility of normal and mutant cells expressing Rac proteins analyzed by videomicroscopy.
Transduced patient and normal myeloid cells were allowed to adhere to glass coverslips before they were loaded into a Zigmond chamber with a 0 to 10−5 mol/L chemotactic gradient of fMLP. The speed of GFP-positive cells, identified using epifluorescence, was analyzed from video frames digitized at 1-minute intervals. (A) Histogram of speeds of control transduced normal and patient cells (normal/MIEG3, patient/MIEG3), and patient cells transduced with WT murine Rac2 (patient/MIEG3FR2). Data shown are from 1 of 2 experiments with similar results. (B) Histogram of speeds of normal cells expressing EGFP alone (MIEG3), D57N mutant Rac2 (HR2MU), or WT Rac2 (HR2WT). Data shown represent 1 of 3 experiments with similar results.
Motility of normal and mutant cells expressing Rac proteins analyzed by videomicroscopy.
Transduced patient and normal myeloid cells were allowed to adhere to glass coverslips before they were loaded into a Zigmond chamber with a 0 to 10−5 mol/L chemotactic gradient of fMLP. The speed of GFP-positive cells, identified using epifluorescence, was analyzed from video frames digitized at 1-minute intervals. (A) Histogram of speeds of control transduced normal and patient cells (normal/MIEG3, patient/MIEG3), and patient cells transduced with WT murine Rac2 (patient/MIEG3FR2). Data shown are from 1 of 2 experiments with similar results. (B) Histogram of speeds of normal cells expressing EGFP alone (MIEG3), D57N mutant Rac2 (HR2MU), or WT Rac2 (HR2WT). Data shown represent 1 of 3 experiments with similar results.
D57N mutant fails to bind GTP and acts as a dominant negative for Rac1 and Rac2
To determine the mechanism of the D57N mutation at the biochemical level, GTP- and GDP-binding assays were performed. As seen in Figure6A, compared to recombinant WT Rac2 the D57N mutant protein failed to bind GTP. Binding to GDP appeared normal. These data are consistent with the location of the mutation in the Rac sequence and the biochemical abnormalities previously described for the analogous Ras D57 and Rho D59 position mutations.35Because this sequence and function are highly conserved throughout all members of the Rho GTPase family, and because Rac1 continues to be expressed in myeloid and lymphoid cells, we hypothesized that the D57N phenotype may involve affects on both Rac1 and Rac2 in these lineages. To test the hypothesis that D57N Rac2 can have dominant-negative effects on Rac1, we expressed the D57N mutant in NIH/3T3, which expresses Rac1 but not Rac2. Expression was confirmed by Western blot (Figure 4C). As noted previously, NIH/3T3 cells expressing D57N Rac2 grew more slowly than NIH/3T3 cells expressing either WT Rac2 or GFP only, showing a 2-fold reduction at 72 hours after transduction and a 7-fold reduction 5 days after transduction (T.W., D.A.W., unpublished data). Expression of D57N, but not WT Rac2, significantly altered the morphology of NIH/3T3 cells, generating a cuboidal, epithelial-like shape characterized by loss of cytoplasmic extension (Figure 6B-D), and greatly reduced membrane ruffling in response to platelet-derived growth factor stimulation, as assessed by rhodamine-phalloidin staining (Figure 6E). Morphologic and growth characteristics of these cells closely mimics those of fibroblasts transfected with dominant-negative Rac1 (17N) constructs.36 37 Because Rac2 is not expressed in these cells, these data support the conclusion that the D57N mutant can act in a dominant-negative fashion to interfere with Rac1 and Rac2 function.
GTP and GDP binding to normal and D57N recombinant Rac protein and dominant-negative effects of D57N on morphology of NIH/3T3 cells.
(A) WT and D57N Rac2 protein was expressed in E coli and then assayed for binding to 3H-GDP or35S-γGTP as described in “Materials and methods.” Data presented are from 3 experiments (mean ± SEM) P< .001 vs WT. (B-D) Photomicrographs of GFP-positive NIH/3T3 cells after infection with retrovirus vectors expressing only GFP (MIEG3) (B), WT Rac2 (HR2WT) (C), or D57N mutant Rac2 (HR2MU) (D). (E) Fluorescent photomicrographs of the same cells in B-D, stained with rhodamine–phalloidin. Note the extensive ruffling in cells transfected with MIEG or HR2WT and in the untransfected (cell staining only at arrowhead in HR2MU panel). In contrast, note the complete absence of ruffling in 2 cells shown transduced with HR2MU (green nuclei). Bar represents 20 μm.
GTP and GDP binding to normal and D57N recombinant Rac protein and dominant-negative effects of D57N on morphology of NIH/3T3 cells.
(A) WT and D57N Rac2 protein was expressed in E coli and then assayed for binding to 3H-GDP or35S-γGTP as described in “Materials and methods.” Data presented are from 3 experiments (mean ± SEM) P< .001 vs WT. (B-D) Photomicrographs of GFP-positive NIH/3T3 cells after infection with retrovirus vectors expressing only GFP (MIEG3) (B), WT Rac2 (HR2WT) (C), or D57N mutant Rac2 (HR2MU) (D). (E) Fluorescent photomicrographs of the same cells in B-D, stained with rhodamine–phalloidin. Note the extensive ruffling in cells transfected with MIEG or HR2WT and in the untransfected (cell staining only at arrowhead in HR2MU panel). In contrast, note the complete absence of ruffling in 2 cells shown transduced with HR2MU (green nuclei). Bar represents 20 μm.
Discussion
Cellular immunity mediated by phagocytic cells is an important component of host defense. Several genetic causes of neutrophil dysfunction have been characterized at both the molecular and the cellular levels. The child described by Ambruso et al23 and further characterized by us displayed cellular abnormalities that overlapped previously described syndromes of chronic granulomatous disease,3,4 leukocyte adhesion deficiency,2and actin dysfunction,5 and he shared a phenotype that closely mimicked that of a mouse mutant deficient in the Rho GTPase, Rac2. Previously, another child with recurrent infections who shared many of the phenotypic abnormalities demonstrated in Rac2-deficient mice was also identified.38 The important characteristics of Rac2 dysfunction in mice and humans appear to include leukocytosis, impaired neutrophil chemotaxis in vitro and in vivo, impaired capture and rolling via the l-selectin ligand, GlyCAM-1, but not via p-selectin mild to moderate deficiency in adhesion, spreading and phagocytosis of neutrophils, and reduced generation of superoxide in response to some, but not all, agonists.
The mammalian Rho GTPase family is made up of at least 7 distinct protein subfamilies—Rho, Rac, Cdc42, RhoD, RhoG, RhoE, and TC10.6 These proteins act as switches to control processes by cycling between active (GTP-bound) and inactive (GDP-bound) states. Members of each family have distinct effects in cells. The studies outlined by Ambruso et al23 and by us and in the mice deficient in Rac2 demonstrate the effects of Rac2 in phagocytic cells. These effects appear to relate mainly to abnormalities of actin function, and the extent of the phenotype in these cells is intriguing in light of the continued expression of highly related Rac1, which is 92% identical to Rac2, in these cells.39 This is particularly striking in murine neutrophils, in which Rac1 makes up approximately 50% of the expressed Rho GTPase (M.C.D., unpublished data). In the mast cells derived from Rac2-deficient mice, we recently demonstrated significant abnormalities not only in actin-based cellular functions, but also in cell proliferation and cell survival in spite of increased levels of Rac1 expression in Rac2-deficient cells.40 Taken together, these data suggest that though some functions of Rac1 and Rac2 may overlap, Rac2 is responsible for distinct cellular processes in some hematopoietic cells.
The mutation in the patient described here is located in the GTP-binding region of Rac. Aspartate at position 57 (in Ras, 59 in Rho) is invariant and is part of the G-3 region loop that is highly conserved in all mammalian Rho GTPases. The aspartate is thought to bind catalytic Mg2+ by a water molecule and, ultimately, by the γ-phosphate of GTP. Several mutants of the Ras GTPase at position 57, including D57A, D57N, and D57Y, have been characterized at the biochemical and, to a lesser extent, cellular levels41-45(and reviewed in Bourne et al46). Dominant-negative mutants of Ras demonstrate decreased GTP binding activity with resultant decreased turnover rates, resulting in increased binding to GEF. Indeed, we have demonstrated in vitro that D57N Rac2 is capable of tight binding to GEF but has lost the catalytic GEF responsiveness, thus making D57N a bona fide dominant-negative mutant (Y.Z., unpublished data). The severe phenotype of the patient described here may be explained, in part, by the observation that the mutation clearly affects the function of other Rho GTPases because we demonstrate that expression in NIH/3T3 cells, which express Rac1 but not Rac2, leads to the characteristic phenotypic changes seen in these cells with dominant-negative mutants of Rac1.36 37 Thus, though our data in Rac2−/− cells strongly suggest that Rac2 mediates nonoverlapping functions in some cells, the dominant-negative nature of the Rac2 mutation in this patient and in much previous work also demonstrates some overlapping functions.
The data derived from murine Rac2−/−neutrophils21 and from our patient provide direct genetic evidence of the critical role of Rac2 in neutrophil function in vivo, including chemotaxis, rolling on endothelium and phagocytosis. Although Rac2 is not essential to superoxide formation, clearly there is agonist-specific deficiency in oxidase function. It is possible that additional patients with neutrophil dysfunction may be identified with abnormalities in Rac2, and, as noted by Hall,6understanding the mechanisms that control actin organization has implications for fully understanding pathologic conditions in humans.
Acknowledgments
We thank Dr J. Panepinto (Rhode Island Hospital) for referring this patient. We thank Dr Dirk Roos and members of our laboratory for helpful discussions and Eva Meunier and Sharon Smoot for assistance in preparation of the manuscript.
Supported by National Institutes of Health grants R01 AI-20065 (L.B.), R01 HL-45635 (M.C.D.), GM60523 (Y.Z.), and 1P01CA71932 (J.B.L.), and by the University of Michigan Clinical Research Center. The Wells Center for Pediatric Research is a Center of Excellence in Molecular Hematology (NIDDK P50DK49218).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David A. Williams, Department of Pediatrics, Wells Center for Pediatric Research, Howard Hughes Medical Institute, 1044 West Walnut St, Rm 402, Indianapolis, IN 46202-5225; e-mail: dwilliam@iupui.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal