Abstract
Oncogenic anaplastic lymphoma kinase (ALK) fusion proteins (nucleophosmin–ALK [NPM-ALK] and other variants) are expressed in many cases of anaplastic large-cell lymphoma (ALCL) but are absent from normal tissues. The possibility that ALK proteins are immunogenic was investigated with the use of an immunocytochemical technique to screen plasma from ALK-positive ALCL on transfectants expressing ALK proteins and by an in vitro kinase assay. Circulating antibodies against NPM-ALK protein were present in all ALK-positive ALCL patients (11 out of 11 cases) studied while 10 patients also had antibodies recognizing normal ALK protein. Weak antibodies reactive with NPM-ALK (which may represent anti-NPM autoantibodies) were detected by the in vitro kinase assay in 3 of the 10 control samples (but not by immunocytochemistry). The presence of anti-ALK antibodies may be relevant to the relatively good prognosis of ALK-positive ALCL. The immunocytochemical technique for detecting anti-ALK activity is simple and semiquantative and may provide a means of detecting B-cell responses to other tumor-associated molecules.
Introduction
In recent years, there has been renewed interest in the topic of tumor antigens since the immune recognition of such tumor-associated antigens has opened up the prospect of new therapeutic strategies.1,2 Examples of such tumor-associated antigens recognized by both the cell-mediated and humoral arms of the immune system are Her-2/neu or c-erbB-2, MAGE-1, NY-ESO-1, and immunoglobulin idiotypes.3-9
Anaplastic lymphoma kinase (ALK)–positive anaplastic large-cell lymphoma (ALCL) or “ALKoma” has been recently identified as a distinct lymphoma entity of T- or null-cell origin.10 This tumor is associated with the (2;5) translocation that results in the expression by the neoplastic cells of a hybrid oncogenic tyrosine kinase consisting of the amino terminus of the nuclear protein nucleophosmin (NPM) fused to the intracytoplasmic region of the ALK receptor tyrosine kinase.11,12 Variants of the (2;5) translocation, fusing ALK to partners other than NPM, are present in a minority of ALKomas.10
Although NPM is ubiquitously expressed, its partner protein, ALK, is absent from normal cells (with the exception of scattered cells in the central nervous system).13 There is, therefore, a strong possibility that the NPM-ALK protein would be the target of an immune response, and this might account for the paradox that ALK-positive ALCL is highly aggressive histologically but has a better prognosis than other large-cell lymphomas.14-16 It would also open up the possibility of new therapeutic options in cases of this disease that are refractory to conventional treatment.
The present report describes a simple immunocytochemical assay to study the presence of an antibody response to ALK proteins. These results were confirmed by means of an immunochemical technique based on an in vitro kinase assay.
Study design
Subjects
Plasma was obtained from 11 ALK-positive ALCL patients attending the John Radcliffe Hospital, Oxford, UK (4); the Park Hospital, Slough, UK (1); Milton Keynes General Hospital (1); and the Hematology Institute, Perugia, Italy (5). ALCL was diagnosed according to the REAL classification system. The ALK-positive ALCL patients presented with differing stages of the disease, and plasma samples were obtained at varying times after diagnosis (Table 1).
Clinical details and antibody status of patients with ALK-positive ALCL
| Patient . | Stage . | Time of plasma collection . | Follow-up† . | Plasma reactivity with transfectants* . | In vitro kinase assay (kd) . | ||
|---|---|---|---|---|---|---|---|
| Vector–NPM-ALK . | Vector–ALK . | Vector only . | |||||
| 1 | IIA | Pretreatment | CR (15 mo) | + (high titer) | + | − | 80 |
| 2 | IV | 2 mo posttreatment | PD (12 mo) | + (low titer) | − | − | 80 |
| 3 | IVB | Pretreatment | Died (10 mo) | + (low titer) | + | − | 80 |
| 4 | IVB | Pretreatment | Died (18 mo) | + (low titer) | + | − | 80 |
| 5 | IV | 102 mo posttreatment | CR (120 mo) | + (low titer) | +/− | − | 80 |
| 6 | IIA | Pretreatment | CR (25 mo) | + (high titer) | + | − | 80 |
| 7 | IIIB | Pretreatment | CR (31 mo) | + (high titer) | + | − | 80 |
| 8 | IVB | Pretreatment | PD (9 mo) | + (high titer) | + | − | 80 |
| 9 | IVB | 2 mo posttreatment | CR (13 mo) | + (high titer) | + | − | 80 |
| 10 | IB | Pretreatment | CR (13 mo) | + (high titer) | + | − | 80 |
| 11 | IIA | Pretreatment | CR (8 mo) | + (high titer) | + | − | ND |
| Patient . | Stage . | Time of plasma collection . | Follow-up† . | Plasma reactivity with transfectants* . | In vitro kinase assay (kd) . | ||
|---|---|---|---|---|---|---|---|
| Vector–NPM-ALK . | Vector–ALK . | Vector only . | |||||
| 1 | IIA | Pretreatment | CR (15 mo) | + (high titer) | + | − | 80 |
| 2 | IV | 2 mo posttreatment | PD (12 mo) | + (low titer) | − | − | 80 |
| 3 | IVB | Pretreatment | Died (10 mo) | + (low titer) | + | − | 80 |
| 4 | IVB | Pretreatment | Died (18 mo) | + (low titer) | + | − | 80 |
| 5 | IV | 102 mo posttreatment | CR (120 mo) | + (low titer) | +/− | − | 80 |
| 6 | IIA | Pretreatment | CR (25 mo) | + (high titer) | + | − | 80 |
| 7 | IIIB | Pretreatment | CR (31 mo) | + (high titer) | + | − | 80 |
| 8 | IVB | Pretreatment | PD (9 mo) | + (high titer) | + | − | 80 |
| 9 | IVB | 2 mo posttreatment | CR (13 mo) | + (high titer) | + | − | 80 |
| 10 | IB | Pretreatment | CR (13 mo) | + (high titer) | + | − | 80 |
| 11 | IIA | Pretreatment | CR (8 mo) | + (high titer) | + | − | ND |
Weak nuclear labeling was observed with all plasma samples at 1:100 dilution. This labeling disappeared on further dilution.
Plasma immunoreactivity with transfected cells: low titer, 1:100 dilution or less; high titer, 1:750 to 1:6750.
PD indicates progressive disease; CR, complete remission.
Control plasma samples were collected from 5 normal subjects, 5 carcinoma patients attending the Oncology Clinic at the Churchill Hospital, Oxford, and 3 patients with ALK-negative ALCL attending the Department of Haematology, John Radcliffe Hospital, Oxford (2), and the Department of Internal Medicine, Hospital Merkur, Zagreb, Croatia (1).
Cells and tissue sections
The ALCL-derived t(2;5)–positive SU-DHL-1 cell line and the COS-1 monkey epithelial cell line were cultured, and cytocentrifuge preparations were made as previously described.13
Cryostat sections (6 μm) were cut from fresh tonsil obtained from the Ear, Nose and Throat Department, Radcliffe Infirmary, Oxford. All tissue sections and cytocentrifuge preparations were fixed in acetone for 10 minutes and stored at −70°C.
COS-1 cells were transiently transfected with the use of the diethylaminoethyl (DEAE)–Dextran method17 with one of the following plasmids: pcDNA3-ALK (encoding full-length ALK), pcDNA3-NPM-ALK (encoding NPM-ALK protein), and the pcDNA3 expression vector alone. Cytocentrifuge preparations were made from cells harvested after 72 hours of culture.
Immunostaining and in vitro kinase assays
Cytocentrifuge preparations of transfected COS-1 cells were incubated with plasma (diluted between 1:100 and 1:6750) for 30 minutes, washed in phosphate-buffered saline, and then incubated with horseradish-peroxidase (HRP)–conjugated rabbit antihuman immunoglobulin (Ig)G (DAKO/AS, Glostrup, Denmark). Transfectants labeled with the use of monoclonal anti-ALK13 followed by HRP-conjugated goat antimouse immunoglobulin (DAKO/AS) were used as a positive control.
The in vitro kinase assay was performed as previously described18 but with the use of 5μL aliquots of human plasma to immunoprecipitate proteins from cytocentrifuge preparations of SU-DHL-1 cells or from tonsil sections.
Results and discussion
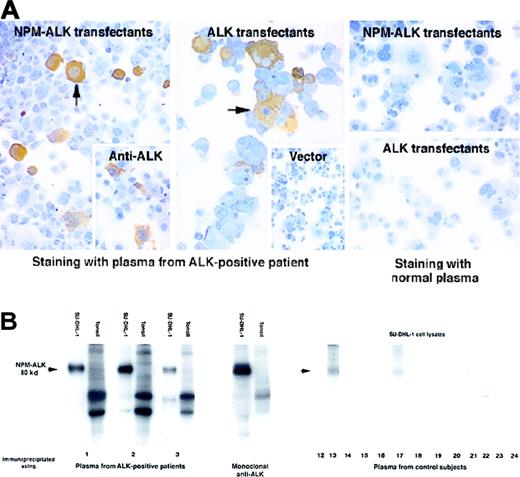
Circulating antibodies recognizing the NPM-ALK kinase protein were detected in all 11 ALCL patients studied, irrespective of their disease status. Antibodies were detected initially by immunostaining COS-1 cells transfected with NPM-ALKcomplementary DNA (cDNA) (Table 1, Figure1A), and their specificity was then confirmed by an in vitro kinase assay of proteins immunoprecipitated from the t(2;5)–positive SU-DHL-1 cell line. Plasma from 10 patients tested immunoprecipitated an autophosphorylated 80-kd protein, a pattern identical to that obtained with a control monoclonal anti-ALK antibody. No proteins of similar size were observed in the immunoprecipitates from lysates of normal tonsil cells (Table 1, Figure 1B).
Detection of antibodies to ALK proteins in patients with ALK-positive ALCL.
(A) Immunoperoxidase labeling of transfected COS-1 cells. Left panel: Plasma from a patient with ALK-positive ALCL (diluted 1:250) strongly labeled COS-1 cells expressing NPM-ALK (arrow). This pattern of labeling is comparable to that obtained with monoclonal anti-ALK (inset). Central panel: Strong staining by this plasma is also seen in COS-1 cells expressing full-length ALK protein. In contrast, no labeling is seen in COS-1 cells that have been transfected with the “empty” pcDNA3 vector (inset). Right panel: No staining is observed when plasma from a control subject is tested on COS-1 cells expressing either NPM-ALK or ALK. (B) Detection of antibodies to NPM-ALK by biochemical analysis. Left panel: Cell protein extracts were immunoprecipitated with plasma from 3 ALK-positive ALCL patients (numbers 1-3) and subjected to an in vitro kinase assay. A strongly autophosphorylated 80-kd protein is observed in immunoprecipitates of the t(2;5)–positive cell line SU-DHL-1 identical in size to that seen in immunoprecipitates prepared with the control monoclonal anti-ALK antibody. No corresponding phosphorylated proteins are present in the tonsil preparations. Comparable results were obtained with the use of plasma from all the other ALK-positive ALCL patients (not shown). Right panel: The control subjects comprise 5 normal patients (12-16), 5 patients with carcinoma (17-21), and 3 patients with ALK-negative ALCL (22-24). A weak 80-kd band (arrowed) is precipitated from SU-DHL-1 lysates by plasma from 3 control subjects (13, 17, and 24), but the other controls are negative.
Detection of antibodies to ALK proteins in patients with ALK-positive ALCL.
(A) Immunoperoxidase labeling of transfected COS-1 cells. Left panel: Plasma from a patient with ALK-positive ALCL (diluted 1:250) strongly labeled COS-1 cells expressing NPM-ALK (arrow). This pattern of labeling is comparable to that obtained with monoclonal anti-ALK (inset). Central panel: Strong staining by this plasma is also seen in COS-1 cells expressing full-length ALK protein. In contrast, no labeling is seen in COS-1 cells that have been transfected with the “empty” pcDNA3 vector (inset). Right panel: No staining is observed when plasma from a control subject is tested on COS-1 cells expressing either NPM-ALK or ALK. (B) Detection of antibodies to NPM-ALK by biochemical analysis. Left panel: Cell protein extracts were immunoprecipitated with plasma from 3 ALK-positive ALCL patients (numbers 1-3) and subjected to an in vitro kinase assay. A strongly autophosphorylated 80-kd protein is observed in immunoprecipitates of the t(2;5)–positive cell line SU-DHL-1 identical in size to that seen in immunoprecipitates prepared with the control monoclonal anti-ALK antibody. No corresponding phosphorylated proteins are present in the tonsil preparations. Comparable results were obtained with the use of plasma from all the other ALK-positive ALCL patients (not shown). Right panel: The control subjects comprise 5 normal patients (12-16), 5 patients with carcinoma (17-21), and 3 patients with ALK-negative ALCL (22-24). A weak 80-kd band (arrowed) is precipitated from SU-DHL-1 lysates by plasma from 3 control subjects (13, 17, and 24), but the other controls are negative.
The antibodies detected by these techniques could potentially recognize epitopes present in either the NPM or the ALK portions of NPM-ALK (or in both moieties). Patients' plasma was therefore tested against transfected COS-1 cells expressing full-length ALK protein (Table 1, Figure 1A), and in 10 cases positive reactivity indicated the presence of antibodies recognizing the ALK moiety of NPM-ALK. In the remaining patient (number 2), it is possible that anti-ALK antibodies were present at too low a titer to be detected by immunostaining. This result could, however, also be explained by the presence of antibodies recognizing only novel epitopes spanning the NPM-ALK junction and/or epitopes present in the amino terminus of NPM (the region of NPM retained in the NPM-ALK protein).
No immunocytochemical evidence was found for circulating antibodies to NPM-ALK or ALK protein in any of the control subjects (Figure 1A). This was confirmed by the kinase assay in 7 of the control subjects (Figure 1B). However, plasma from one normal subject, one carcinoma patient, and one ALK-negative ALCL patient (13, 17, and 24, respectively) recognized a weakly phosphorylated 80-kd protein, corresponding in size to NPM-ALK (Figure 1B). Autoantibodies to normal NPM have been described previously19 and may account for this weak reactivity with NPM-ALK, but it is also possible that antibodies recognizing NPM-ALK were present at levels too low to detect by the immunocytochemical technique.
Controversy surrounds the possible antitumor effect of antibodies to tumor-associated molecules in patients with malignant disease.20,21 There is, however, evidence that boosting antibody responses to tumor-associated antigens is clinically beneficial in melanomas22-24 and B-cell lymphomas.7-9 25 In the present preliminary study, we could draw no conclusions concerning the clinical significance of anti–NPM-ALK antibodies although it was clear that they differed in titer, with a tendency to higher levels in pretreatment blood samples. However, it is of interest that circulating antibodies to NPM-ALK and ALK proteins persisted in a patient (number 5) who had been in complete remission for more than 9 years. Further studies are therefore necessary to establish whether anti–NPM-ALK titers are relevant to clinical prognosis.
In conclusion, the present study describes for the first time a specific immune response to the NPM-ALK fusion kinase in patients with ALK-positive lymphoma. Whether this contributes to the relatively good prognostic outlook of ALK-positive ALCL requires further study, but the present study also indicates that a simple immunocytochemical technique can be used to detect and quantitate the B-cell immune response to a tumor-associated protein.
Acknowledgments
Dr R. Kusec, Department of Internal Medicine, Hospital Merkur, Zagreb, Croatia, and Robin Roberts-Gant, Medical Informatics Unit, John Radcliffe Hospital, Oxford, UK.
Supported by the Leukaemia Research Fund (Grant 9464) and the Italian Association for Cancer Research (A.I.R.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
K. Pulford, Nuffield Department of Clinical Laboratory Sciences, Room 5501, Level 5, John Radcliffe Hospital, Oxford, OX3 9DU; e-mail: karen.pulford@cellular-science.ox.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal