Abstract
Major histocompatibility class I–peptide tetramer technology and simian immunodeficiency virus of macaques (SIVmac)-infected rhesus monkeys were used to clarify the distribution of acquired immunodeficiency syndrome virus-specific cytotoxic T lymphocytes (CTL) in secondary lymphoid organs and to assess the relationship between these CTL and the extent of viral replication in the various anatomic compartments. SIVmac Gag epitope-specific CD8+ T cells were evaluated in the spleen, bone marrow, tonsils, thymus, and 5 different lymph node compartments of 4 SIVmac-infected rhesus monkeys. The average percentage of CD8+ T lymphocytes that bound this tetramer in all the different lymph node compartments was similar to that in peripheral blood lymphocytes in individual monkeys. The percentage of CD8+ T cells that bound the tetramer in the thymus was uniformly low in the monkeys. However, the percentage of CD8+ T cells that bound the tetramer in bone marrow and spleen was consistently higher than that seen in lymph nodes and peripheral blood. The phenotypic profile of the tetramer-binding CD8+ T lymphocytes in the different lymphoid compartments was similar, showing a high expression of activation-associated adhesion molecules and a low level expression of naive T-cell–associated molecules. Surprisingly, no correlation was evident between the percentage of tetramer-binding CD8+ T lymphocytes and the magnitude of the cell-associated SIV RNA level in each lymphoid compartment of individual monkeys. These studies suggest that a dynamic process of trafficking may obscure the tendency of CTL to localize in particular regional lymph nodes or that some lymphoid organs may provide milieus that are particularly conducive to CTL expansion.
Introduction
Although it is well established that levels of human immunodeficiency virus (HIV)-1 replication can differ significantly in different anatomic compartments in infected patients, we know little about the associated quantitative local differences in HIV-1–specific immune effector cells. It has long been appreciated that lymph nodes represent one of the main reservoirs for HIV-11 and that substantially more HIV-1 replication takes place in lymph nodes than in peripheral blood lymphocytes (PBL) in infected patients.2 Moreover, the recent demonstration in animal model studies of a bias in lentivirus infection-induced CD4+ T-lymphocyte loss in gut-associated lymphatic tissue suggests that regional differences in HIV-1 replication are likely to occur in secondary lymphoid tissue.3
CD8+ cytotoxic T lymphocytes (CTL) play a major role in containing HIV-1 replication in chronically infected patients. Low virus load and stable clinical status are correlated with potent systemic CTL responses.4,5 In studies in nonhuman primates, the transient elimination of total body CD8+lymphocytes through monoclonal anti-CD8 antibody infusions are associated with periods of high acquired immunodeficiency syndrome (AIDS) virus replication.6 7 Yet, although we appreciate the importance of CTL in containing HIV-1 spread, we know little about the regional trafficking of CTL to anatomic areas of high viral replication and about how the distribution of CTL might reflect local differences in viral replication.
To assess regional anatomic differences in HIV-1–specific CTL distribution, reproducible and quantitative assays are needed to enumerate these cells. Until recently, the detection of virus-specific CTL required the use of cumbersome, nonquantitative functional assays that measured the ability of a cell population to lyse target cells expressing viral antigen. However, it has recently been demonstrated that virus epitope-specific CD8+ CTL can be detected by flow cytometry, measuring the binding of these cells to fluorescent-labeled tetrameric major histocompatibility complex (MHC) class I–peptide complexes.8 This technology provides a powerful quantitative tool for detecting virus-specific CD8+ T lymphocytes in diverse anatomic compartments of an infected person.
The simian immunodeficiency virus of macaques (SIVmac)-infected rhesus monkey develops a disease similarly to HIV-1–induced disease in humans.9,10 This nonhuman primate infection provides an important animal model for the study of the immunopathogenesis of AIDS.11,12 We have made use of a dominant CTL response specific for the SIVmac Gag epitope p11C, C-M in rhesus monkeys expressing the MHC class I molecule Mamu-A*01 to explore the role of CTL in containing SIVmac replication.7 13-17 In the current study, CTL specific for SIVmac have been characterized in various lymphoid compartments of infected, Mamu-A*01+ rhesus monkeys using both Gag peptide-specific functional CTL assays and tetrameric MHC class I–peptide complex staining techniques. Moreover, the association of these CTL with localized SIVmac replication has been assessed.
Materials and methods
Animals and viruses
EDTA-anticoagulated blood samples and lymphocytes from different lymphoid organs were obtained from euthanatized rhesus monkeys (Macaca mulatta) infected with uncloned SIVmac strain 251 for more than 12 months. All rhesus monkeys used in this study wereMamu-A*01+ as determined both by polymerase chain reaction-based MHC class I typing15,16 and by functional CTL assays, as previously described.14 These animals were maintained in accordance with the guidelines of the Committee on Animals for the Harvard Medical School and with the Guide for the Care and Use of Laboratory Animals (National Research Council, National Academic Press, Washington, DC, 1996).
Staining and phenotypic analysis of p11C, C-M–specific CD8+ T lymphocytes
Soluble tetrameric Mamu-A*01/p11C, C-M complex was made as previously described.14 The tetramer was produced by mixing biotinylated Mamu-A*01/p11C, C-M complex with phycoerythrin (PE)-labeled ExtrAvidin (Sigma Chemical, St. Louis, MO) or Alexa 488-labeled NeutrAvidin (Molecular Probes, Eugene, OR) at a 4:1 molar ratio. The monoclonal antibodies (mAbs) used for this study were directly coupled to fluorescein isothiocyanate (FITC), PE-Texas red (ECD), or allophycocyanin (APC). The following mAbs were used: anti-CD8α(Leu2a)-FITC and anti-CD62L (Leu8)-PE (Becton Dickinson, San Jose, CA); anti-CD8αβ(2ST8-5H7)-ECD, anti-CD11a(25.3.1)-PE, anti-CD28(4B10)-PE, anti-CD45RA(2H4)-PE, anti-CD49d(HP2/1)-PE, and anti-HLA-DR(I3)-PE (Coulter Beckman, Miami, FL); and anti-CD95(DX2)-PE (Caltag, Burlingame, CA). The mAb FN18, which recognizes rhesus monkey CD3, a gift from Dr D. M. Neville Jr (National Institutes of Health, Bethesda, MD), was directly coupled to APC. The 3 reagents Alexa 488-coupled tetrameric Mamu-A*01/p11C, C-M complex, anti-CD8αβ-ECD, and anti-rhesus monkey CD3-APC were used either with anti-CD11a-PE, anti-CD28-PE, anti-CD45RA-PE, anti-CD49d-PE, anti-CD62L-PE, anti-CD95-PE, or anti-HLA-DR-PE to perform 4-color flow cytometric analyses. The PE-coupled tetrameric Mamu-A*01/p11C, C-M complex was used with anti-CD8α-FITC in conjunction with anti-CD8αβ-ECD and anti-rhesus monkey CD3-APC. Alexa 488- or PE-coupled tetrameric Mamu-A*01/p11C, C-M complex (0.5 μg) was used in conjunction with the directly labeled mAbs to stain either 100 μL fresh whole blood or 5 × 105 single cells from lymph nodes or 5 × 105 lymphocytes isolated by density-gradient centrifugation over Ficoll–Hypaque after in vitro culture. Samples were analyzed on a Coulter EPICS Elite ESP, as described previously.6 Data presentation was performed using WinMDI software version 2.7 (Joseph Trotter, La Jolla, CA) and Microsoft PowerPoint software version 4.0c (Microsoft, Redmond, WA).
Cytotoxicity assay
Autologous B-LCL were used as target cells and were incubated with 5 μg/mL p11C, C-M (CTPYDINQM), or the negative control peptide p11B (ALSEGCTPYDIN) for 90 minutes during chromium 51Cr labeling. For effector cells, peripheral blood mononuclear cells or single cells isolated from different lymphoid organs of monkeys chronically infected with SIVmac were cultured for 3 days at 4 × 106 cells/mL in the presence of 1 μg/mL peptide p11C, C-M, and then were maintained for another 7 to 11 days in medium supplemented with recombinant human IL-2 (20 U/mL) (provided by Hoffman-La Roche, Nutley, NJ). Lymphocytes cultured according to this protocol were then centrifuged over Ficoll–Hypaque (Ficopaque; Pharmacia Chemical, Piscataway, NJ) and assessed as effector cells in a standard 51Cr release assay using U-bottom microtiter plates containing 104 target cells with effector cells at different effector:target ratios. All wells were established and assayed in duplicate. Plates were incubated in a humidified incubator at 37°C for 4 hours. Specific release was calculated as [(Experimental Release − Spontaneous Release)/(Maximum Release − Spontaneous Release)] × 100. Spontaneous release was less than 20% of maximal release with detergent (1% Triton X-100; Sigma Chemical) in all assays.
Branched DNA quantitation of SIV RNA
SIV RNA was quantitated by a branched DNA (bDNA) signal amplification assay.18 Target probes were designed to hybridize with the pol region of the SIVmac group of virus strains, including SIVmac251, SIVmac239, and SIVmne. SIV RNA was quantified per 106 CD4+ cells by comparison with a standard curve produced by purified, quantified, in vitro transcribed SIVmac239 pol RNA. The lower quantitation limit of this assay was 3,000 SIV RNA equivalents per sample.
In situ hybridization
A 35S-labeled, single-stranded, antisense RNA probe (Lofstrand Laboratories, Gaithersburg, MD) was used. Hybridization was performed on frozen sections, as previously described.19Sections were examined with a microscope equipped with epiluminescent illumination (Axio-phot; Carl Zeiss, Jena, Germany). Cells were considered positive for viral gene expression if the grain count was more than 6 times higher than the background count.
Results
Consistent distribution of tetramer-binding CD8+ T cells in lymph nodes and tonsils of SIVmac-infected rhesus monkeys
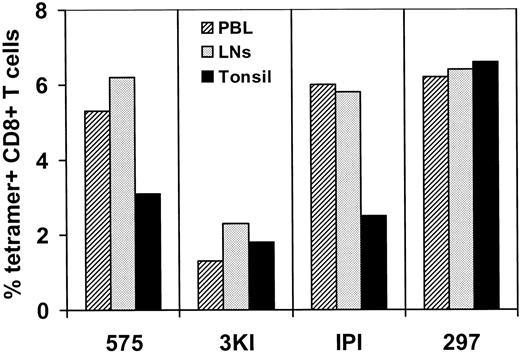
To explore the heterogeneity of distribution of SIV-specific CTL in various lymph node compartments, lymph nodes were sampled simultaneously from different anatomic locations (mandibular, axillary, inguinal, mesenteric, and iliac) in 4 chronically SIVmac-infected rhesus monkeys. Lymphocytes obtained from these nodes were evaluated for virus-specific CTL using tetramer-binding assays. Because the monkeys selected for these studies all shared the MHC class I alleleMamu-A*01, assays were performed to quantitate CD8+ T lymphocytes that recognize the Mamu-A*01-restricted, dominant Gag CTL epitope p11C, C-M (Table1; Figure1). Flow cytometric analyses of these freshly isolated lymph node cells demonstrated similar percentages of CD8+ T lymphocytes binding the tetramer in the anatomically disparate lymph nodes of each monkey. Moreover, as we previously demonstrated in studies of single lymph nodes,15 the average percentage of tetramer-binding CD8+ T cells from the various lymph nodes sampled was remarkably similar to the percentage detected in PBL of the same monkey (Figure 1). The tonsils of these monkeys had variable levels of tetramer-binding CD8+ T lymphocytes. In 2 animals they were comparable to those observed in PBL and lymph node, whereas in the other 2 the levels were lower (Figure 1).
| Monkeys . | PBL . | Lymph nodes . | ||||
|---|---|---|---|---|---|---|
| Mandibular . | Axillary . | Inguinal . | Mesenteric . | Iliac . | ||
| 575 | 5.3* | 15.4 | 4.3 | 3.0 | 2.7 | 5.6 |
| 3KI | 1.3 | 1.3 | 2.3 | 1.1 | 2.8 | 4.0 |
| IPI | 6.0 | 5.6 | 4.5 | 6.4 | 7.7 | 4.6 |
| 297 | 6.2 | 6.3 | 6.2 | 8.6 | 6.2 | 4.9 |
| Monkeys . | PBL . | Lymph nodes . | ||||
|---|---|---|---|---|---|---|
| Mandibular . | Axillary . | Inguinal . | Mesenteric . | Iliac . | ||
| 575 | 5.3* | 15.4 | 4.3 | 3.0 | 2.7 | 5.6 |
| 3KI | 1.3 | 1.3 | 2.3 | 1.1 | 2.8 | 4.0 |
| IPI | 6.0 | 5.6 | 4.5 | 6.4 | 7.7 | 4.6 |
| 297 | 6.2 | 6.3 | 6.2 | 8.6 | 6.2 | 4.9 |
Percent staining by tetrameric Mamu-A*01/p11C, C-M complex on CD8+ T cells. CD8+ T cells are defined by gating on CD8αβ+ CD3+ cells. p11C, C-M represents the 9-amino acid fragment optimal epitope of the SIV Gag 12-amino acid peptide p11C.
Tetrameric Mamu-A*01/p11C, C-M complex binds at comparable percentages of CD8+ T cells from peripheral blood, lymph nodes, and tonsil in SIVmac-infected,
Mamu-A*01+ rhesus monkeys. Simultaneously sampled peripheral blood, lymph nodes, and tonsil from 4 SIVmac-infected,Mamu-A*01+ monkeys (575, 3KI, IPI, 297) were assessed. Lymph node data represent the average of the percentage tetramer-positive CD8+ T cells from 5 different lymph nodes (mandibular, axillary, inguinal, mesenteric, iliac) of each infected animal. Flow cytometric analysis was performed on gated CD8αβ+CD3+ T cells stained with PE-coupled tetrameric Mamu-A*01/p11C, C-M complex.
Tetrameric Mamu-A*01/p11C, C-M complex binds at comparable percentages of CD8+ T cells from peripheral blood, lymph nodes, and tonsil in SIVmac-infected,
Mamu-A*01+ rhesus monkeys. Simultaneously sampled peripheral blood, lymph nodes, and tonsil from 4 SIVmac-infected,Mamu-A*01+ monkeys (575, 3KI, IPI, 297) were assessed. Lymph node data represent the average of the percentage tetramer-positive CD8+ T cells from 5 different lymph nodes (mandibular, axillary, inguinal, mesenteric, iliac) of each infected animal. Flow cytometric analysis was performed on gated CD8αβ+CD3+ T cells stained with PE-coupled tetrameric Mamu-A*01/p11C, C-M complex.
Distribution of tetramer-binding CD8+ T cells in secondary lymphoid compartments of SIVmac-infected rhesus monkeys
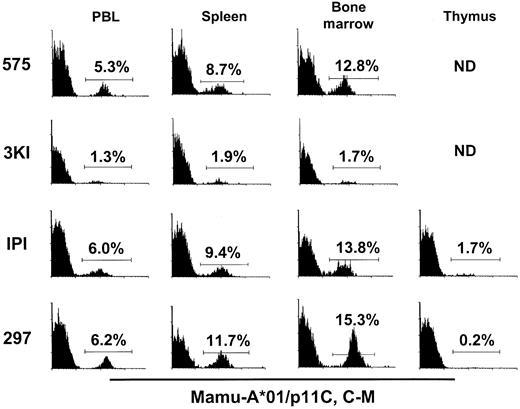
Gag epitope-specific CD8+ T cells were also quantitated in the spleens of these infected monkeys by the tetramer-binding assay. The percentage tetramer-binding CD8+ T cells was consistently greater in the spleen than in lymph nodes and PBL in all 4 monkeys studied (Figure2). For example, the percentage of tetramer-binding CD8+ T cells in the spleen of monkey 297 was 11.7%, whereas these cells constituted 6.2% of CD8+peripheral blood T cells (Figure 2).
Tetrameric Mamu-A*01/p11C, C-M complex binds to a larger percentage of spleen and bone marrow and a smaller percentage of thymic CD8+ T cells than CD8+ T cells from peripheral blood in SIVmac-infected,
Mamu-A*01+ rhesus monkeys. A whole blood specimen (PBL) and a single-cell suspension of spleen, bone marrow, and thymus from 4 SIVmac-infected, Mamu-A*01+ rhesus monkeys (575, 3KI, IPI, 297) were stained with PE-coupled tetrameric Mamu-A*01/p11C, C-M complex and analyzed by flow cytometry with gating on CD8αβ+ CD3+ T cells. ND, not done because of inadequate cell specimens.
Tetrameric Mamu-A*01/p11C, C-M complex binds to a larger percentage of spleen and bone marrow and a smaller percentage of thymic CD8+ T cells than CD8+ T cells from peripheral blood in SIVmac-infected,
Mamu-A*01+ rhesus monkeys. A whole blood specimen (PBL) and a single-cell suspension of spleen, bone marrow, and thymus from 4 SIVmac-infected, Mamu-A*01+ rhesus monkeys (575, 3KI, IPI, 297) were stained with PE-coupled tetrameric Mamu-A*01/p11C, C-M complex and analyzed by flow cytometry with gating on CD8αβ+ CD3+ T cells. ND, not done because of inadequate cell specimens.
Staining of CD8+ T cells from bone marrow and thymus with tetrameric Mamu-A*01/p11C, C-M complex was also assessed (Figure 2). In 3 of the 4 animals studied, the percentage tetramer-binding CD8+ T cells in bone marrow lymphocytes was even greater than that seen in splenic lymphocytes (Figure 2). In fact, 15.3% of CD8+ bone marrow T cells in monkey 297 bound the tetramer, whereas only 6.2% of CD8+ peripheral blood T lymphocytes were tetramer positive (Figure 2). The percentage tetramer-binding CD8+ T cells in the thymus was low in the 2 monkeys evaluated (Figure 2).
The functional p11C, C-M–specific lytic activity in peptide-stimulated lymphocytes was generally comparable to the percentage of tetramer-binding CD8+ T cells detected in these peptide-stimulated lymphocyte populations (Table2). However, the ability of the freshly isolated tetramer-binding CD8+ T lymphocyte populations to expand in vitro after epitope peptide stimulation was variable. For example, tetramer-binding lymphocytes in PBL of monkey 3KI expanded from 1.3% to 61.3% CD8+ T cells, whereas lymphocytes in PBL of monkey 575 only expanded from 5.3% to 18.3% CD8+ T cells (Table 2). Because the assay used to assess functional CTL activity depends on in vitro expansion of effector T-lymphocyte populations, it was not possible to determine the correlation between functional CTL activity and numbers of freshly isolated tetramer-binding CD8+ T lymphocytes in a particular cell population.
| Monkey . | Spleen . | Mesenteric lymph node . | PBL . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fresh . | p11C, C-M*expanded . | Fresh . | p11C, C-M expanded . | Fresh . | p11C, C-M expanded . | ||||
| % tetramer . | % tetramer . | % lysis (5/3/1)† . | % tetramer . | % tetramer . | % lysis (5/3/1) . | % tetramer . | % tetramer . | % lysis (5/3/1) . | |
| 575 | 8.7‡ | 8.32-153 | 29/21/152-153 | 2.7 | 28.8 | 34/25/15 | 5.3 | 18.3 | 14/9/5 |
| 3KI | 3.0 | 49.0 | 55/50/40 | 2.8 | 43.9 | 57/51/40 | 1.3 | 61.3 | 67/67/60 |
| IPI | 9.4 | 49.8 | 55/54/36 | 7.7 | 69.5 | 58/52/46 | 6.0 | 69.5 | 55/53/45 |
| 297 | 11.7 | 19.5 | 18/12/6 | 6.2 | 6.5 | 18/14/7 | 6.2 | 50.3 | 51/42/31 |
| Monkey . | Spleen . | Mesenteric lymph node . | PBL . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fresh . | p11C, C-M*expanded . | Fresh . | p11C, C-M expanded . | Fresh . | p11C, C-M expanded . | ||||
| % tetramer . | % tetramer . | % lysis (5/3/1)† . | % tetramer . | % tetramer . | % lysis (5/3/1) . | % tetramer . | % tetramer . | % lysis (5/3/1) . | |
| 575 | 8.7‡ | 8.32-153 | 29/21/152-153 | 2.7 | 28.8 | 34/25/15 | 5.3 | 18.3 | 14/9/5 |
| 3KI | 3.0 | 49.0 | 55/50/40 | 2.8 | 43.9 | 57/51/40 | 1.3 | 61.3 | 67/67/60 |
| IPI | 9.4 | 49.8 | 55/54/36 | 7.7 | 69.5 | 58/52/46 | 6.0 | 69.5 | 55/53/45 |
| 297 | 11.7 | 19.5 | 18/12/6 | 6.2 | 6.5 | 18/14/7 | 6.2 | 50.3 | 51/42/31 |
p11C, C-M represents the 9-amino acid fragment optimal epitope of the SIV Gag 12-amino acid peptide p11C.
E:T ratios.
Percent staining of tetrameric Mamu-A*01/p11C, C-M complex on CD8+ T cells. CD8+ T cells are defined by gating on CD8αβ+ CD3+ cells.
Percent p11C, C-M-specific lysis was calculated as percent specific release by p11C, C-M-pulsed Mamu-A*01+ target cells minus the percent specific release by control peptide p11B-pulsedMamu-A*01+ target cells.
Phenotypic characterization of tetrameric Mamu-A*01/p11C, C-M-binding CD8+ T cells from different lymphoid organs
We have previously shown that the phenotypic profiles of tetrameric Mamu-A*01/p11C, C-M complex positive T lymphocytes obtained from lymph nodes and PBL are similar.15 We extended this phenotypic analysis to lymphocytes obtained from other lymphoid organs. Monoclonal antibody staining of both CD8+ T cells and CD8+ tetramer-binding T cells in mesenteric lymph node, spleen, bone marrow, tonsil, and thymus of the 4 SIVmac-infectedMamu-A*01+ rhesus monkeys was analyzed using 4-color flow cytometry. Data generated in the analyses of CD8+ T cells and tetramer-binding CD8+ T cells from these 5 anatomic compartments of all 4 monkeys are shown in Tables3 and 4.
Phenotypic characterization of CD8+ T cells in various lymphoid compartments of SIVmac-infected rhesus monkeys
| Cell subset . | % CD8+ T cells staining3-150 . | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 575 . | 3KI . | IPI . | 297 . | |||||||||||||||||
| LNs . | Sp . | BM . | Ton . | Thy . | LNs . | Sp . | BM . | Ton . | Thy . | LNs . | Sp . | BM . | Ton . | Thy . | LNs . | Sp . | BM . | Ton . | Thy . | |
| CD11a+ | 84 | 93 | 81 | 88 | NT3-151 | 94 | 99 | 94 | 97 | NT | 99 | 98 | 98 | 98 | 95 | 36 | 61 | NT | 25 | 97 |
| CD28+ | 51 | 11 | 21 | 23 | NT | 63 | 36 | 29 | 68 | NT | 88 | 75 | 64 | 88 | 85 | 81 | 49 | 66 | 53 | 90 |
| CD45RA+ | 27 | 14 | 11 | NT | NT | 17 | 16 | 13 | 17 | NT | 26 | 20 | 22 | 25 | 1.5 | 55 | 51 | 17 | 84 | 3.3 |
| CD49d+ | 90 | 91 | 93 | NT | NT | 90 | 97 | 93 | 90 | NT | 89 | 91 | 98 | 90 | 97 | 95 | 80 | NT | NT | 98 |
| CD62L+ | 24 | 3.3 | 2.7 | NT | NT | 13 | 4.8 | 5.3 | 12 | NT | 28 | 19 | 11 | 19 | 65 | 57 | 14 | 32 | 37 | 62 |
| CD95+ | 90 | 98 | 98 | NT | NT | 95 | 97 | 98 | 95 | NT | 97 | 99 | 98 | 97 | 87 | 39 | 64 | NT | 28 | 3.1 |
| MHC class II DR+ | 60 | 85 | 58 | NT | NT | 78 | 94 | 90 | 83 | NT | 76 | 87 | 78 | 68 | 30 | 25 | 61 | 54 | 23 | 34 |
| Cell subset . | % CD8+ T cells staining3-150 . | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 575 . | 3KI . | IPI . | 297 . | |||||||||||||||||
| LNs . | Sp . | BM . | Ton . | Thy . | LNs . | Sp . | BM . | Ton . | Thy . | LNs . | Sp . | BM . | Ton . | Thy . | LNs . | Sp . | BM . | Ton . | Thy . | |
| CD11a+ | 84 | 93 | 81 | 88 | NT3-151 | 94 | 99 | 94 | 97 | NT | 99 | 98 | 98 | 98 | 95 | 36 | 61 | NT | 25 | 97 |
| CD28+ | 51 | 11 | 21 | 23 | NT | 63 | 36 | 29 | 68 | NT | 88 | 75 | 64 | 88 | 85 | 81 | 49 | 66 | 53 | 90 |
| CD45RA+ | 27 | 14 | 11 | NT | NT | 17 | 16 | 13 | 17 | NT | 26 | 20 | 22 | 25 | 1.5 | 55 | 51 | 17 | 84 | 3.3 |
| CD49d+ | 90 | 91 | 93 | NT | NT | 90 | 97 | 93 | 90 | NT | 89 | 91 | 98 | 90 | 97 | 95 | 80 | NT | NT | 98 |
| CD62L+ | 24 | 3.3 | 2.7 | NT | NT | 13 | 4.8 | 5.3 | 12 | NT | 28 | 19 | 11 | 19 | 65 | 57 | 14 | 32 | 37 | 62 |
| CD95+ | 90 | 98 | 98 | NT | NT | 95 | 97 | 98 | 95 | NT | 97 | 99 | 98 | 97 | 87 | 39 | 64 | NT | 28 | 3.1 |
| MHC class II DR+ | 60 | 85 | 58 | NT | NT | 78 | 94 | 90 | 83 | NT | 76 | 87 | 78 | 68 | 30 | 25 | 61 | 54 | 23 | 34 |
Values represent the percentages of lymph nodes (LNs) [median of % in lymph nodes that were sampled simultaneously (mandibular, axillary, inguinal, mesenteric, iliac)], spleen (Sp), bone marrow (BM), tonsil (Ton), and thymus (Thy) cells staining for expression of CD11a, CD28, CD45RA, CD49d, CD62L, CD95, and MHC class II-DR on CD8+ T cells. CD8+ T cells are defined by gating on CD8αβ+ CD3+ cells.
NT, not tested.
| Cell subset . | % tetramer+ CD8+ T cells staining4-150 . | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 575 . | 3KI . | IPI . | 297 . | |||||||||||||||||
| LNs . | Sp . | BM . | Ton . | Thy . | LNs . | Sp . | BM . | Ton . | Thy . | LNs . | Sp . | BM . | Ton . | Thy . | LNs . | Sp . | BM . | Ton . | Thy . | |
| CD11a+ | 98 | 99 | 99 | 96 | NT4-151 | 99 | 99 | 100 | 99 | NT | 99 | 99 | 99 | 99 | 96 | 99 | 99 | NT | 98 | 99 |
| CD28+ | 38 | 16 | 12 | 33 | NT | 58 | 38 | 28 | 59 | NT | 80 | 50 | 45 | 70 | 63 | 80 | 58 | 18 | 68 | 90 |
| CD45RA+ | 15 | 10 | 9.5 | NT | NT | 13 | 15 | 14 | 14 | NT | 14 | 8.7 | 5.0 | 6.6 | 5.1 | 15 | 28 | 10 | 25 | 10 |
| CD49d+ | 96 | 97 | 95 | NT | NT | 95 | 97 | 93 | 95 | NT | 97 | 99 | 99 | 98 | 99 | 97 | 95 | NT | NT | 99 |
| CD62L+ | 22 | 5.1 | 5.8 | NT | NT | 11 | 5.1 | 3.4 | 10 | NT | 8.5 | 4.4 | 3.0 | 7.6 | 24 | 14 | 7.7 | 7.2 | 10 | 20 |
| CD95+ | 98 | 99 | 99 | NT | NT | 98 | 99 | 98 | 99 | NT | 99 | 99 | 99 | 99 | 97 | 99 | 97 | NT | 95 | 97 |
| MHC class II DR+ | 83 | 96 | 76 | NT | NT | 91 | 95 | 92 | 95 | NT | 91 | 90 | 79 | 85 | 82 | 87 | 95 | 92 | 92 | 80 |
| Cell subset . | % tetramer+ CD8+ T cells staining4-150 . | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 575 . | 3KI . | IPI . | 297 . | |||||||||||||||||
| LNs . | Sp . | BM . | Ton . | Thy . | LNs . | Sp . | BM . | Ton . | Thy . | LNs . | Sp . | BM . | Ton . | Thy . | LNs . | Sp . | BM . | Ton . | Thy . | |
| CD11a+ | 98 | 99 | 99 | 96 | NT4-151 | 99 | 99 | 100 | 99 | NT | 99 | 99 | 99 | 99 | 96 | 99 | 99 | NT | 98 | 99 |
| CD28+ | 38 | 16 | 12 | 33 | NT | 58 | 38 | 28 | 59 | NT | 80 | 50 | 45 | 70 | 63 | 80 | 58 | 18 | 68 | 90 |
| CD45RA+ | 15 | 10 | 9.5 | NT | NT | 13 | 15 | 14 | 14 | NT | 14 | 8.7 | 5.0 | 6.6 | 5.1 | 15 | 28 | 10 | 25 | 10 |
| CD49d+ | 96 | 97 | 95 | NT | NT | 95 | 97 | 93 | 95 | NT | 97 | 99 | 99 | 98 | 99 | 97 | 95 | NT | NT | 99 |
| CD62L+ | 22 | 5.1 | 5.8 | NT | NT | 11 | 5.1 | 3.4 | 10 | NT | 8.5 | 4.4 | 3.0 | 7.6 | 24 | 14 | 7.7 | 7.2 | 10 | 20 |
| CD95+ | 98 | 99 | 99 | NT | NT | 98 | 99 | 98 | 99 | NT | 99 | 99 | 99 | 99 | 97 | 99 | 97 | NT | 95 | 97 |
| MHC class II DR+ | 83 | 96 | 76 | NT | NT | 91 | 95 | 92 | 95 | NT | 91 | 90 | 79 | 85 | 82 | 87 | 95 | 92 | 92 | 80 |
Values represent the percentages of the Mamu-A*01+/p11C, C-M complex population staining for expression of CD11a, CD28, CD45RA, CD49d, CD62L, CD95, and MHC class II-DR in lymph nodes (LNs) [median of % in lymph nodes that were sampled simultaneously (mandibular, axillary, inguinal, mesenteric, iliac)], spleen (Sp), bone marrow (BM), tonsil (Ton), and thymus (Thy) gated on CD8+ T cells. CD8+ T cells are defined by gating on CD8αβ+ CD3+ cells.
NT, not tested.
Phenotypic profiles of the tetramer-binding CD8+ T lymphocytes were similar to those of the unselected CD8+ T lymphocytes (Tables 3, 4). Moreover, the phenotypic appearance of the tetramer-binding CD8+ T lymphocytes was similar in each sampled lymphoid compartment. Very high and homogeneous expressions of CD11a, CD49d, and CD95 were seen on tetramer-positive CD8+T cells obtained from lymph node, spleen, bone marrow, tonsil, and thymus of all monkeys, indicating that these cells were activated (Table 4). Those tetramer-binding CD8+ T cells were, for the most part, intermediate positive or negative for CD45RA and negative for CD62L expression, typical of memory cells. Heterogeneity was seen in the expression of CD28. A high level of MHC class II-DR expression was observed on these lymphocytes from 3 of 4 animals studied. Only monkey 297 had MHC class II-DR expression on less than 50% of its lymphocytes. The MHC class II-DR expression by tetramer-binding CD8+ T cells in the 4 monkeys was higher than its expression on unselected CD8+ T cells (Tables 3, 4).
Correlation between the percentage tetrameric Mamu-A*01/p11C, C-M complex-binding CD8+ T cells and T-cell–associated viral RNA in lymphocytes from different anatomic locations of infected rhesus monkeys
In view of the importance of virus-specific CTL for containing SIV replication, we sought to determine the association between the percentage of tetrameric Mamu-A*01/p11C, C-M complex-binding CD8+ T cells and the quantity of SIV RNA in the lymphocytes sampled from these different anatomic locations in the infected monkeys. Single-cell suspensions prepared from the sampled tissues were analyzed for SIV viral RNA by bDNA assay and for SIV Gag-specific CTL by tetramer staining. Lymphocytes from 2 animals (3KI, IPI) had detectable SIV RNA copies (range, 0.017 × 106-16 × 106copies/106CD4+ cells); those from the other 2 animals (575, 297) had, for the most part, undetectable SIV RNA (less than 3 × 103copies/106CD4+cells) (Figure 3). In concordance with these bDNA results, SIV RNA was also detected by in situ hybridization only from animals 3KI and IPI (Figure 4). Moreover, these results were consistent with the viral loads measured in the plasma of the monkeys. The 2 animals with detectable SIV RNA in lymphoid tissue and monkeys 3KI and IPI had plasma SIV RNA levels of 4.5 × 106 and 4.0 × 106 copies/mL, respectively. Monkeys 575 and 297, with no detectable SIV RNA in lymphoid tissue, had plasma SIV RNA levels of 1.2 × 105and less than 1.5 × 103 copies/mL, respectively.
Lack of correlation between tetrameric Mamu-A*01/p11C, C-M complex binding CD8+ T cells and SIVmac load in secondary lymphoid organs of SIVmac-infected,
Mamu-A*01+ rhesus monkeys. Simultaneously sampled whole blood (PBL), 5 different lymph nodes (mandibular [LN Md], axillary [LN Ax], inguinal [LN Ig], mesenteric [LN Ms], iliac [LN Ic]), spleen (Sp), bone marrow (BM), tonsil (Ton), and thymus (Thy) from 4 SIVmac-infected, Mamu-A*01+ monkeys (575, 3KI, IPI, 297) were assessed. The percentage of tetramer-binding CD8+ T cells (□) and the SIV RNA copies × 106/106 CD4+ cells (■) from each animal studied are shown. The SIV RNA copies were measured by a bDNA assay. ND, not done because of inadequate cell specimens.
Lack of correlation between tetrameric Mamu-A*01/p11C, C-M complex binding CD8+ T cells and SIVmac load in secondary lymphoid organs of SIVmac-infected,
Mamu-A*01+ rhesus monkeys. Simultaneously sampled whole blood (PBL), 5 different lymph nodes (mandibular [LN Md], axillary [LN Ax], inguinal [LN Ig], mesenteric [LN Ms], iliac [LN Ic]), spleen (Sp), bone marrow (BM), tonsil (Ton), and thymus (Thy) from 4 SIVmac-infected, Mamu-A*01+ monkeys (575, 3KI, IPI, 297) were assessed. The percentage of tetramer-binding CD8+ T cells (□) and the SIV RNA copies × 106/106 CD4+ cells (■) from each animal studied are shown. The SIV RNA copies were measured by a bDNA assay. ND, not done because of inadequate cell specimens.
Detection of SIV RNA by in situ hybridization in secondary lymphoid organs of SIVmac-infected rhesus monkeys.
Sections of spleen from animal 3KI (A) and lymph nodes from animals 575 (B), 297 (C), and IPI (D) are shown. In situ hybridization signals for SIV viral RNA are shown by blue signals.
Detection of SIV RNA by in situ hybridization in secondary lymphoid organs of SIVmac-infected rhesus monkeys.
Sections of spleen from animal 3KI (A) and lymph nodes from animals 575 (B), 297 (C), and IPI (D) are shown. In situ hybridization signals for SIV viral RNA are shown by blue signals.
Surprisingly, there was no apparent correlation between the percentage of tetrameric Mamu-A*01/p11C, C-M complex-binding CD8+ T cells in a lymphoid compartment and the magnitude of the viral load in that compartment of an individual monkey (Figure 3). Furthermore, levels of detectable viral RNA in a monkey were not even correlated with our ability to detect tetramer-binding CD8+ T cells in that same animal.
Discussion
Data from previous studies have indicated that the extent of lentiviral replication in diverse lymphoid compartments is heterogeneous. More viral replication has been reported in lymph node lymphocytes than in PBL.2 Moreover, greater viral replication has been seen in sites in which lymphocytes are activated, such as gut-associated lymphocytes, than in relatively quiescent lymphocytes in the periphery of infected individuals.3 In fact, insufficient data were generated in the current study to confirm or refute these predictions because SIV was detected by RNA assays in lymphoid cells of only 2 of the evaluated monkeys. Nevertheless, consistent with earlier studies, substantially more viral RNA was detected in lymph nodes than in peripheral blood in those 2 animals. Contrary to predictions, however, these 2 monkeys had similar patterns of viral replication in the various lymph nodes that were evaluated. One monkey had high levels and the other had low levels of viral replication in the gut-associated mesenteric lymph nodes. Overall, however, there was considerable homogeneity in the amount of viral replication detected in the various sampled lymph nodes.
With homogeneity seen in local viral replication in distinct lymph node compartments of the monkeys, an associated homogeneity was also seen in the representation of tetramer-positive CD8+CD3+ T cells in these tissues. The representation of tetramer-positive CD8+CD3+lymphocytes in anatomically disparate lymph nodes was remarkably consistent in each individual monkey. This finding suggests that a dynamic process of CTL trafficking may obscure the tendency of CTL to localize to particular regional lymph nodes.
There were, however, consistent differences seen in the representation of tetramer-binding CD8+ T lymphocytes in various secondary lymphoid organs. Larger numbers were seen in spleen than in PBL, and even larger numbers were detected in the bone marrow (Figure 2). These differences did not reflect local variations in SIVmac replication. Rather, these findings suggested that CTL may be preferentially trapped in certain lymphoid compartments or that some lymphoid organs may provide milieus that are particularly conducive to CTL expansion.
We have previously shown that the MHC class I–SIVmac Gag peptide tetramer binding CD8+ T lymphocytes in PBL, lymph nodes, and even in semen of chronically infected rhesus monkeys are phenotypically similar.15 20 These lymphocyte populations all express activation- and memory-associated molecules, suggesting that they are active CTL. The current study indicates that the tetramer-binding CD8+ T lymphocytes in the spleen and bone marrow are also activated memory cells.
There are certainly caveats associated with the current study. The number of evaluated monkeys was small. Moreover, only a single viral epitope-specific CD8+ T lymphocyte population was evaluated. Nevertheless, this study clearly indicates that SIVmac-specific CD8+ T lymphocytes are present in similar frequencies in disparate lymph nodes but that they are present in higher frequencies in spleen and bone marrow. This study demonstrates the usefulness of the tetramer technology to quantitate and compare antigen-specific CD8+ T lymphocytes from different lymphoid organs, and it suggests that there is no correlation between the percentage of tetramer-binding CD8+ T lymphocytes and the magnitude of the cell-associated SIV RNA level in particular lymphoid compartments of individual monkeys.
Acknowledgments
We thank W. Charini and C. Lord for MHC class I typing of the monkeys used in this study, Lisa Franz for assistance in preparation of this manuscript, and Casey Wingfield for SIV RNA analysis.
Supported by National Institutes of Health grants AI 28147 and AI 20729. M.J.K. and J.E.S. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Marcelo J. Kuroda, Division of Viral Pathogenesis, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, RE-102, PO Box 15732, Boston, MA 02215; e-mail:mkuroda@caregroup.harvard.edu.

![Fig. 3. Lack of correlation between tetrameric Mamu-A*01/p11C, C-M complex binding CD8+ T cells and SIVmac load in secondary lymphoid organs of SIVmac-infected,. / Mamu-A*01+ rhesus monkeys. Simultaneously sampled whole blood (PBL), 5 different lymph nodes (mandibular [LN Md], axillary [LN Ax], inguinal [LN Ig], mesenteric [LN Ms], iliac [LN Ic]), spleen (Sp), bone marrow (BM), tonsil (Ton), and thymus (Thy) from 4 SIVmac-infected, Mamu-A*01+ monkeys (575, 3KI, IPI, 297) were assessed. The percentage of tetramer-binding CD8+ T cells (□) and the SIV RNA copies × 106/106 CD4+ cells (■) from each animal studied are shown. The SIV RNA copies were measured by a bDNA assay. ND, not done because of inadequate cell specimens.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/4/10.1182_blood.v96.4.1474/4/m_h81600057003.jpeg?Expires=1764959483&Signature=jekHDaLHWwIjoCYoAPc8k92KilApPUlM4Z0YIkLLhIY65Guh2OXSGtweDVfdM7xvwowSWPCskTE2bHmnRGAhrZTrgLeKTsKSow4qNEwG1p3UqZ-gA2gM4llITJzeWYHQ89qybd6DWfReR2aSYBGyOBp~NFaSN2dq8ae1vXglKyICWpDgbotdIijrT5Ib62bu81zfR-LSZS1v7xiCaBg3WYrk9sufVrs2VF4qwfZyOOLbzHkLJ3-A9lXO7yVN7SJUzvvUaa~VKhDtRhLEk-bHa8v6Z6qDH5xLI1ePpj5VmfUQ7kZ23-40~r8bxFelvELQdcGXThYGmeVgiq5NUFw4eA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal