Abstract
Site directed mutagenesis (350 variants) of recombinant staphylokinase (SakSTAR), a potent fibrin-selective thrombolytic agent, was undertaken in order to reduce its antigenicity while maintaining its potency. Variants with K35A, (ie, Lys[K] in position 35 substituted with Ala[A]), E65D or E65Q, K74R or K74Q, E80A+D82A, K130T, and K135R displayed increased enzymatic activity or reduced binding of human staphylokinase-specific antibodies. Additive mutagenesis identified 8 variants with intact thrombolytic potencies, which absorbed down to less than a third of SakSTAR-specific antibodies. Intra-arterial administration in 61 patients with peripheral arterial occlusion caused no significant allergic reactions. Median neutralizing antibody titers (with 15 to 85 percentiles), expressed as microgram (μg) compound neutralized per milliliter plasma, were 4.4 (0.3 to 49) for the variants, compared with 12 (4 to 100) in 70 patients given wild-type SakSTAR (P = .002 by Mann-Whitney rank sum test). Overt neutralizing antibody induction (more than 5 μg compound neutralized per milliliter plasma) was observed in 57 of 70 patients (81%) given wild-type SakSTAR, but only in 28 of 60 patients (47%) treated with variants (P < .0001 by Fisher exact test). On the basis of this study, the variant SakSTAR (K35A, E65Q, K74R, D82A, S84A, T90A, E99D, T101S, E108A, K109A, K130T, K135R) (code SY155) has been selected for further clinical development.
Introduction
Thrombolytic therapy of acute myocardial infarction is based on the premise that its proximal cause is thrombosis, triggered by rupture of an atheromatous plaque in a coronary artery. Early and sustained recanalization may prevent cell death, reduce infarct size, preserve organ function, and reduce early and late mortality. Thrombolysis consists of the pharmacologic dissolution of the blood clot, by intravenous infusion of plasminogen activators that activate the fibrinolytic system. Thrombolytic agents that are either approved or under clinical investigation in patients with acute myocardial infarction include streptokinase, recombinant tissue-type plasminogen activator (rt-PA or alteplase), rt-PA derivatives such as reteplase and TNK-rtPA, anisoylated plasminogen-streptokinase activator complex (APSAC or anistreplase), 2-chain urokinase-type plasminogen activator (tcu-PA or urokinase), recombinant single-chain u-PA (scu-PA, pro-u-PA or prourokinase), and recombinant staphylokinase and derivatives.1-3 Staphylokinase (SakSTAR), a 136 amino acid profibrinolytic agent secreted by some strains of Staphylococcus aureus, has recently been shown to hold promise for thrombolytic therapy of acute myocardial infarction.3 It is at least equipotent to rt-PA for coronary artery recanalization, significantly more fibrin-selective and obtainable in high yield by cytosolic expression in Escherichia coli. However, being a heterologous bacterial protein, its administration induces neutralizing antibody formation in a majority of patients.4 5
Wild-type staphylokinase (SakSTAR variant6) contains 3 nonoverlapping immunodominant epitopes, 2 of which can be eliminated by substitution of clusters of 2 or 3 charged amino acids with Ala.7 The combination mutants SakSTAR (K35A, E38A, K74A, E75A, R77A) (variants of wild-type SakSTAR are identified by the substituted amino acids in single letter symbols, followed by their position number in the mature staphylokinase sequence [136 amino acids] and by the substituting amino acids in single letter symbols. Added amino acids are indicated by a “▿”, followed by their position number and their identity in single letter symbols) in which Lys35, Glu38, Lys74, Glu75, and Arg77, and SakSTAR(K74A, E75A, R77A, E80A, D82A) in which Lys74, Glu75, Arg77, Glu80, and Asp82 were substituted with Ala, bound only two thirds of the neutralizing antibodies elicited in patients by treatment with wild-type SakSTAR,7 and induced less antibody formation than wild-type SakSTAR in patients with peripheral arterial occlusion.8 However, their specific activities were only 50% of that of wild-type SakSTAR and they displayed a reduced temperature stability,7 which would be of concern with respect to the clinical use of these compounds. Reversal from Ala to the wild-type residue of one or more of the 7 amino acids of SakSTAR listed above (ie, Lys35, Glu38, Lys74, Glu75, Arg77, Glu80, and Asp82) revealed that SakSTAR(K74A), with a single substitution of Lys74 with Ala, had a high residual specific activity and a slightly reduced temperature stability, but did not absorb 40% of the antibodies induced in patients by treatment with wild-type SakSTAR.9 It induced statistically significantly lower antibody levels when administered to patients with peripheral arterial occlusion,10 11 providing proof of principle that the antigenicity as well as the immunogenicity of SakSTAR can be reduced by site-directed mutagenesis, without reducing its specific activity.
Therefore, the current comprehensive site-directed mutagenesis study was carried out. Approximately 350 plasmids encoding SakSTAR mutants were constructed and expressed in E coli, and the expression products were purified and characterized in terms of specific activity, and absorption of antibodies from plasma of patients treated with wild-type SakSTAR. Eight selected variants were produced on a preparative scale and conditioned for use in vivo. They were found to have unaltered pharmacokinetics and thrombolytic potencies in hamsters and were shown to have a markedly reduced binding of antibodies elicited with wild-type SakSTAR and immunogenicity in patients with peripheral arterial occlusion. On the basis of the current results, the variant SakSTAR(K35A, E65Q, K74R, D82A, S84A, T90A, E99D, T101S, E108A, K109A, K130T, K135R) (code SY155) was selected for further investigation toward clinical use.
Materials and methods
Reagents
Construction, purification, and physicochemical analysis of SakSTAR variants
Construction of plasmids encoding SakSTAR variants.
Plasmid DNA was isolated using the BIO 101 RPM kit (Vista, CA). All other methods used in this study have previously been described.7 13
The plasmids encoding SakSTAR variants were routinely constructed by polymerase chain reaction (PCR) or by the spliced overlap extension polymerase chain reaction (SOE-PCR),14 using Taq polymerase (Boehringer Mannheim, Mannheim, Germany) and available or generated sakSTAR variants as template. After a denaturation step of 3 minutes at 94°C, the fragments were amplified by cycling 30 times (1 to 5 seconds at 94°C, 1 to 5 seconds at 50°C, 10 seconds at 72°C), followed by a final elongation step of 2 minutes at 72°C. For SOE-PCR, 2 fragments were amplified by PCR, the first one starting from the 5′ end of the staphylokinase gene with primer 5′-CAGGAAACAGAATTCAGGAG-3′ to the region to be mutated (forward primer), the second one from the same region (backward primer) to the 3′ end of the staphylokinase gene with primer 5′-CAAAACAGCCAAGCTTCATTCATTCAGC-3′. The forward and backward primers shared an overlap of around 24 base pairs (bp) (primers not shown). The 2 purified fragments were then assembled together in a new PCR reaction with the 5′ and 3′ end primers (see above), under the conditions described above. The final product was purified, digested withEcoRI and HindIII and cloned into the corresponding sites of pMEX.SakB. The sequence of each variant was confirmed by sequencing of the entire sakSTARcoding region.
Analytical purification.
The plasmids encoding SakSTAR variants were expressed from transformedE coli WK6, grown in terrific broth (TB)15medium, and purified as follows. A 2-mL aliquot of an overnight saturated culture in LB medium was used to inoculate a 1-L culture (in a 5-L flask) in TB containing 100 μg/mL ampicillin. The culture was grown with vigorous aeration for 20 hours at 30°C and isopropylthio-β-D-galactoside (IPTG, 200 μmol/L) was added to boost the expression during the last 3 hours of culture. The cells were pelleted by centrifugation, resuspended in 100 mL 0.01 mol/L phosphate buffer, pH between 5.5 and 6.0, and disrupted by sonication at 0°C. The suspension was then centrifuged for 20 minutes at 20 000 rpm and the supernatant was diluted 5-fold with water and subjected to chromatography on a 1.6 × 6 cm column of SP-Sepharose, equilibrated with 0.01 mol/L phosphate buffer, pH 6.0, and eluted with a 1 mol/L NaCl gradient. The fractions containing SakSTAR variant were pooled, solid NaCl was added to a concentration of 2.5 mol/L, and the material was applied on a 1.6 × 8 cm column of phenyl-Sepharose, followed by elution with 0.01 mol/L phosphate buffer, pH 6.0. The SakSTAR containing fractions, localized by SDS-gel electrophoresis, were pooled for further analysis.
Physicochemical analysis.
The methodology used to determine specific activity and temperature stability has previously been described in detail.7
The absorption with SakSTAR variants, of antibodies elicited in patients by treatment with wild-type SakSTAR was quantitated as follows. A pool of plasma samples from 40 patients with acute myocardial infarction, obtained several weeks after treatment with SakSTAR, and that neutralized more than 5 μg SakSTAR per milliliter plasma was used. This plasma pool was diluted (1/30 to 1/200) until their binding to SakSTAR substituted chips in the BIAcore instrument (Biacore, Breda, The Netherlands) amounted to approximately 2000 RU.16 17 From this dilution, a calibration curve for antibody binding was constructed using further serial 2-fold dilutions. The plasma pools were absorbed for 10 minutes with 100 nmol/L of the SakSTAR variants, and residual binding to immobilized SakSTAR was determined. Residual binding was expressed in percentage of unabsorbed plasma, using the calibration curve.
The fibrinolytic and fibrinogenolytic properties of the SakSTAR variants were determined in human plasma in which a125I-fibrin–labeled plasma clot was submerged, as previously described.8
Preparative purification and analysis of SakSTAR variants for use in vivo
Preparative purification.
Eighteen liter cultures (in 1 to 2 liter batches) of 8 selected variants (listed in Table 3) were grown for 20 hours in TB medium,15 supplemented with 100 μg/mL ampicillin and induced with 200 μmol/L IPTG during the last 3 hours. The cells were pelleted, resuspended in 1/10 volume of 0.01 mol/L phosphate buffer, pH between 5.5 and 6.0, disrupted by sonication, and cleared by centrifugation.
The compounds were purified by chromatography at 4°C on a 10 × 7 cm column of SP-Sepharose, equilibrated with 0.01 mol/L phosphate buffer, pH between 5.5 and 6.0, and eluted with a 1 mol/L NaCl gradient (3 column volumes). The fractions containing SakSTAR variant were pooled, solid NaCl was added to a concentration of 2.5 mol/L, and the material was applied to a 10 × 20 cm column of phenyl-Sepharose, followed by stepwise elution with 0.01 mol/L phosphate buffer, pH between 5.5 and 6.0. The materials were then desalted on a 10 × 45 cm column of Sephadex G25, concentrated by application on a 5 × 10 cm column of SP-Sepharose with stepwise elution with 1.0 mol/L NaCl, and finally, gel filtered on a sterilized 6 × 60 cm column of Superdex 75, equilibrated with 0.14 mol/L NaCl, 0.01 mol/L phosphate buffer, pH 7.5. The SakSTAR variant-containing fractions were pooled, the protein concentration adjusted to 1 mg/mL, and the material sterilized by filtration through a 0.22 μm Millipore filter (Millex-GS, Millipore, Molschein, France).
Biologic analysis.
Endotoxin contamination, bacterial sterility, and acute toxicity in mice of the SakSTAR variants were evaluated as previously described.8 Purity of the preparations was evaluated by SDS gel electrophoresis on 10% gels to which 10 μg of compound was applied per lane.
Pharmacokinetics and thrombolytic properties of SakSTAR variants
Pharmacokinetics.
The pharmacokinetic parameters of the disposition of SakSTAR variants from blood were evaluated in groups of 4 hamsters after intravenous bolus injection of 100 μg/kg SakSTAR variant, as previously described.8 SakSTAR-related antigen was assayed using the enzyme-linked immunosorbent assay (ELISA) described elsewhere.8 Pharmacokinetic parameters included: initial half-life (in minutes), t1/2α = ln2/α; and plasma clearance (in mL/min−1), ClP = dose/AUC.18
Thrombolytic properties.
The thrombolytic potency of the SakSTAR variants was evaluated in a pulmonary embolism model in heparinized hamsters, as described previously.8,19 A 125I-fibrin–labeled platelet-poor human plasma clot, corresponding to 50 μL original plasma, was injected into the jugular vein, the thrombolytic agents were infused intravenously over 60 minutes, and lysis was measured 30 minutes after the end of the infusion. Fibrinogen, α2-antiplasmin and staphylokinase-related antigen levels in plasma were determined as described elsewhere.19
Thrombolytic efficacy and antibody induction of SakSTAR variants in patients with peripheral arterial occlusion
The 8 staphylokinase variants (Table 3) were administered intra-arterially in the proximal end of the occlusive thrombus as a bolus of 2 mg, followed by an infusion of 1 mg/h (reduced overnight to 0.5 mg/h) in 61 patients (groups of 5 to 18 patients) with angiographically documented occlusion of a peripheral artery or bypass graft of less than 120 days duration. Patients were studied after giving informed consent, and the protocol was approved by the Human Studies Committee of the University of Leuven. Inclusion and exclusion criteria, conjunctive antithrombotic treatment (including continuous intravenous heparin), and the study protocol were essentially as previously described.8 10
Absorption with SakSTAR variants of antibodies elicited in patients by treatment with SakSTAR variants
Plasma samples obtained 3 to 4 weeks after treatment with SakSTAR variants from patients with peripheral arterial occlusion, in which the staphylokinase neutralizing activity exceeded 3 μg/mL, were used and compared with a plasma pool of 40 immunized patients after treatment with wild-type SakSTAR.
The plasma samples were serially diluted (50- to 400-fold) for the construction of individual calibration curves for antibody binding to each of the SakSTAR variants. The plasma samples were then absorbed for 10 minutes with 250 nmol/L of the SakSTAR variants and residual binding to immobilized SakSTAR or to immobilized SakSTAR variants was determined by biospecific interaction analysis as described elsewhere.7 Residual binding was expressed in percentage of unabsorbed plasma, using the individual calibration curves.
Statistical analysis
Data are expressed as mean values ± SEM or as a median (15 to 85 percentile ranges). Significance levels were determined by paired or unpaired Student t test in case of Gaussian distributions, and by Mann-Whitney rank sum test in case of non-Gaussian distributions. Chi-square analysis and Fisher's exact test were carried out when indicated in the text. Two-tailedP < .05 was considered to indicate statistical significance. Confidence intervals were determined using the Statexact software (Version 3.01, Cytel Software, Cambridge, MA).
Results
Alanine-scanning and selected site-directed mutagenesis of SakSTAR
Sixty-seven plasmids, encoding variants with substitution of a single or 2 adjacent amino acids with Ala, were constructed, expressed, and purified. Together with the 35 charged residue to Ala-substitution variants previously described,7 9 this analysis covers all residues in SakSTAR, except Gly, Ala, and Pro. Eight of the 102 variants could not be obtained in purified form, primarily as a result of low expression levels, 11 variants were inactive, 56 had a reduced specific activity, and 27 had a maintained or increased specific activity. The median yields of purified material from cultures of expressed plasmids were 16 mg/L (10 to 90 percentiles: 4 to 41 mg/L) (data not shown). SDS polyacrylamide gel electrophoresis (SDS-PAGE) consistently showed one main band with Mr≈ 16 000, usually representing more than or equal to 95% of total protein (not shown). Results obtained with selected variants, used for further analysis, are summarized in Table1 (bold type). Substitution of K35, N95, K130, or K135 with Ala yielded variants with specific activities of more than or equal to 200 kU/mg. Substitution of W66, Y73, or K74 with Ala yielded variants with reduced antibody binding from pooled immunized patient plasma by more than or equal to 15%.
Mutagenesis of Lys35, Tyr73, Lys74, Glu80, Asp82, Asn95, Lys130, Lys135, or Lys136, identified by comprehensive Ala-scanning
| Variant . | Spec. Act. (kU/mg) . | Antibody absorption (%) . |
|---|---|---|
| SakSTAR | 120 | 95 |
| SakSTAR(K35A) | 230 | 91 |
| SakSTAR(K35E) | 160 | 95 |
| SakSTAR(K35Q) | 69 | 95 |
| SakSTAR(W66A) | <5 | 85 |
| SakSTAR(Y73A) | <5 | 63 |
| SakSTAR(Y73F) | 31 | 93 |
| SakSTAR(Y73H) | <5 | 76 |
| SakSTAR(Y73L) | <5 | 81 |
| SakSTAR(Y73S) | <5 | 86 |
| SakSTAR(Y73W) | 27 | 73 |
| SakSTAR(K74A) | 69 | 64 |
| SakSTAR(K74E) | <5 | 83 |
| SakSTAR(K74N) | 39 | 63 |
| SakSTAR(K74Q) | 110 | 70 |
| SakSTAR(K74R) | 150 | 75 |
| SakSTAR(E80A,D82A) | 130 | 89 |
| SakSTAR(E80A) | 160 | 94 |
| SakSTAR(D82A) | 160 | 95 |
| SakSTAR(N95A) | 260 | 95 |
| SakSTAR(N95E) | 79 | 95 |
| SakSTAR(N95G) | 160 | 95 |
| SakSTAR(N95K) | 180 | 95 |
| SakSTAR(K130A) | 280 | 92 |
| SakSTAR(K130T) | 290 | 92 |
| SakSTAR(K135A) | 410 | 95 |
| SakSTAR(K135F) | 64 | 95 |
| SakSTAR(K135R) | 230 | 95 |
| SakSTAR(K136A) | 180 | 91 |
| SakSTAR(K136R) | 94 | 95 |
| SakSTAR(K136F) | 88 | 84 |
| SakSTAR(∇137K) | 1600 | 95 |
| SakSTAR(∇137A) | 88 | 95 |
| SakSTAR(K136A,∇137K) | 1800 | 88 |
| Variant . | Spec. Act. (kU/mg) . | Antibody absorption (%) . |
|---|---|---|
| SakSTAR | 120 | 95 |
| SakSTAR(K35A) | 230 | 91 |
| SakSTAR(K35E) | 160 | 95 |
| SakSTAR(K35Q) | 69 | 95 |
| SakSTAR(W66A) | <5 | 85 |
| SakSTAR(Y73A) | <5 | 63 |
| SakSTAR(Y73F) | 31 | 93 |
| SakSTAR(Y73H) | <5 | 76 |
| SakSTAR(Y73L) | <5 | 81 |
| SakSTAR(Y73S) | <5 | 86 |
| SakSTAR(Y73W) | 27 | 73 |
| SakSTAR(K74A) | 69 | 64 |
| SakSTAR(K74E) | <5 | 83 |
| SakSTAR(K74N) | 39 | 63 |
| SakSTAR(K74Q) | 110 | 70 |
| SakSTAR(K74R) | 150 | 75 |
| SakSTAR(E80A,D82A) | 130 | 89 |
| SakSTAR(E80A) | 160 | 94 |
| SakSTAR(D82A) | 160 | 95 |
| SakSTAR(N95A) | 260 | 95 |
| SakSTAR(N95E) | 79 | 95 |
| SakSTAR(N95G) | 160 | 95 |
| SakSTAR(N95K) | 180 | 95 |
| SakSTAR(K130A) | 280 | 92 |
| SakSTAR(K130T) | 290 | 92 |
| SakSTAR(K135A) | 410 | 95 |
| SakSTAR(K135F) | 64 | 95 |
| SakSTAR(K135R) | 230 | 95 |
| SakSTAR(K136A) | 180 | 91 |
| SakSTAR(K136R) | 94 | 95 |
| SakSTAR(K136F) | 88 | 84 |
| SakSTAR(∇137K) | 1600 | 95 |
| SakSTAR(∇137A) | 88 | 95 |
| SakSTAR(K136A,∇137K) | 1800 | 88 |
Specific activity (Spec. Act.) ≥ 100 kU/mg is represented in bold type. Absorption of antibodies (in %) from pooled immunized patient plasma; values ≤ 85% are represented in bold type. Variants of wild-type SakSTAR are represented by the substituted amino acids in single letter symbols, followed by their position number in the mature staphylokinase sequence (136 amino acids) and by the substituting amino acids in single letter symbols. Added amino acids are indicated by ∇, followed by their position number and their identity in single letter symbols.
Substitution of K35 with A, E, or Q and of K136 with A, R, or E revealed that SakSTAR(K35A) and SakSTAR(K136A) had the most interesting properties, substitution of Y73 (and of W66, data not shown) with F, H, L, S, or W did not rescue the marked reduction in specific activity and K74 confirmed its key role in binding of antibodies from immunized patient plasma, the best specific activity/antigenicity ratios being obtained with SakSTAR(K74Q) and SakSTAR(K74R) (Table 1). SakSTAR(E80A, D82A) was preferred over the single residue variants SakSTAR(E80A) or SakSTAR(D82A) because of its somewhat lower reactivity with immunized patient plasma and SakSTAR(N95A) could not be further improved by substitution of N95 with E, G, K, or R. SakSTAR(K130A) was equivalent in terms of specific activity to SakSTAR(K130T) and SakSTAR(K135R) outperformed SakSTAR(K135A), especially in combination variants. Finally, addition of a Lys, but not of an Ala residue at the COOH-terminus, resulted in an apparent increase of the specific activity of 10- to 15-fold (Table 1).
Sequential additive substitution mutagenesis of selected amino acids in the SakSTAR(K130T, K135R), SakSTAR(E80A, D82A, K130T, K135R), and SakSTAR(E65Q, K74Q, K130T, K135R) templates
The SakSTAR(K130T, K135R) variant was taken as a template for additive mutagenesis, because it displayed a high specific activity with a moderate reduction (to 90%) of the absorption of antibodies from immunized patient plasma (Table 2). Addition of either K74R or K74Q to the template decreased the absorption of antibodies from immunized patient plasma to 76% and 62%, respectively. Combination of E65A or E65Q with K74Q in the SakSTAR(K130T, K135R) template resulted in an absorption of antibodies of 55% and 65%, respectively, without markedly reducing the specific activity. Addition of K35A to SakSTAR(E65Q, K74Q, K130T, K135R) reduced the absorption of antibodies to 45%, whereas addition of N95A slightly increased the specific activity.
Sequential additive substitution mutagenesis of SakSTAR(K130T,K135R), SakSTAR(E80A,D82A,K130T,K135R), or SakSTAR(E65Q,K74Q,K130T,K135R) with selected other amino acids
| Variant . | Code . | Spec. Act. (kU/mg) . | Fibrinolytic potency in human plasma (C50 in μg/mL) . | Antibody absorption (%) . |
|---|---|---|---|---|
| SakSTAR | — | 130 | 0.18 | 95 |
| SakSTAR(K130T,K135R) | SY2 | 280 | 0.15 | 89 |
| SakSTAR(K74R,K130T,K135R) | SY4 | 310 | 0.17 | 76 |
| SakSTAR(K74Q,K130T,K135R) | SY41 | 190 | 0.18 | 62 |
| SakSTAR(E65A,K74Q,K130T,K135R) | SY48 | 170 | 0.20 | 55 |
| SakSTAR(E65Q,K74Q,K130T,K135R) | SY49 | 150 | — | 65 |
| SakSTAR(E65Q,K74Q,N95A,K130A,K135R) | SY72 | 220 | — | 58 |
| SakSTAR(K35A,E65Q,K74Q,K130A,K135R) | SY75 | 110 | 0.27 | 45 |
| SakSTAR(E80A,D82A,K130T,K135R) | SY6 | 250 | — | 80 |
| SakSTAR(K74R,E80A,D82A,K130T,K135R) | SY7 | 220 | 0.15 | 72 |
| SakSTAR(K74Q,E80A,D82A,K130T,K135R) | SY15 | 110 | 0.15 | 48 |
| SakSTAR(K35A,K74R,E80A,D82A,K130T,K135R) | SY17 | 160 | 0.19 | 68 |
| SakSTAR(K35A,K74Q,E80A,D82A,K130T,K135R) | SY28 | 120 | 0.18 | 48 |
| SakSTAR(E65D,K74R,E80A,D82A,K130T,K135R) | SY19 | 140 | 0.24 | 57 |
| SakSTAR(E65Q,K74R,E80A,D82A,K130T,K135R) | SY22 | 140 | 0.16 | 60 |
| SakSTAR(E65D,K74Q,E80A,D82A,K130T,K135R) | SY30 | 110 | 0.15 | 42 |
| SakSTAR(E65Q,K74Q,E80A,D82A,K130T,K135R) | SY47 | 120 | 0.16 | 42 |
| SakSTAR(K35A,E65D,K74R,E80A,D82A,K130T,K135R) | SY29 | 190 | 0.11 | 55 |
| SakSTAR(K35A,E65D,K74Q,E80A,D82A,K130T,K135R) | SY46 | 140 | 0.11 | 40 |
| SakSTAR(E65D,K74R,E80A,D82A,K130T,K135R,K136A) | SY35 | 100 | 0.19 | 45 |
| SakSTAR(K35A,E65D,K74R,E80A,D82A,K130T,K135R,K136A) | SY34 | 81 | — | 40 |
| SakSTAR(K35A,E65Q,K74Q,D82A,S84A,T90A,E99D,T101S,E108A,K109A,K130T,K135R) | SY151 | 210 | 0.18 | 34 |
| SakSTAR(K35A,E65Q,K74R,D82A,S84A,T90A,E99D,T101S,E108A,K109A,K130T,K135R) | SY155 | 180 | 0.21 | 36 |
| SakSTAR(E65Q,K74Q,K130T,K135R) | SY49 | 150 | 0.15 | 65 |
| SakSTAR(E65Q,K74Q,D82A,S84A,K130T,K135R) | SY50 | 170 | 0.19 | 45 |
| SakSTAR(E65Q,K74Q,T90A,E99D,T101S,K130A,K135R) | SY98 | 410 | 0.14 | 51 |
| SakSTAR(E65Q,K74Q,E108A,K109A,K130T,K135R) | SY83 | 180 | 0.18 | 50 |
| SakSTAR(E65Q,K74Q,D82A,S84A,E108A,K109A,K130T,K135R) | SY95 | 110 | 0.18 | 41 |
| SakSTAR(E65Q,K74Q,D82A,S84A,E108A,K109A,K130T,K135R,K136A,∇137K) | SY118 | 1500 | 0.15 | 30 |
| SakSTAR(E65Q,K74Q,D82A,S84A,T90A,E99D,T101S,E108A,K109A,K130T,K135R,K136A,∇137K) | SY128 | 2900 | 0.23 | 28 |
| SakSTAR(K35A,E65Q,K74Q,D82A,S84A,T90A,E99D,T101S,E108A,K109A,K130T,K135R,K136A,∇137K) | SY141 | 3700 | 0.19 | 24 |
| SakSTAR(K35A,E65Q,K74R,D82A,S84A,T90A,E99D,T101S,E108A,K109A,K130T,K135R,K136A,∇137K) | SY145 | 5700 | 0.19 | 31 |
| Variant . | Code . | Spec. Act. (kU/mg) . | Fibrinolytic potency in human plasma (C50 in μg/mL) . | Antibody absorption (%) . |
|---|---|---|---|---|
| SakSTAR | — | 130 | 0.18 | 95 |
| SakSTAR(K130T,K135R) | SY2 | 280 | 0.15 | 89 |
| SakSTAR(K74R,K130T,K135R) | SY4 | 310 | 0.17 | 76 |
| SakSTAR(K74Q,K130T,K135R) | SY41 | 190 | 0.18 | 62 |
| SakSTAR(E65A,K74Q,K130T,K135R) | SY48 | 170 | 0.20 | 55 |
| SakSTAR(E65Q,K74Q,K130T,K135R) | SY49 | 150 | — | 65 |
| SakSTAR(E65Q,K74Q,N95A,K130A,K135R) | SY72 | 220 | — | 58 |
| SakSTAR(K35A,E65Q,K74Q,K130A,K135R) | SY75 | 110 | 0.27 | 45 |
| SakSTAR(E80A,D82A,K130T,K135R) | SY6 | 250 | — | 80 |
| SakSTAR(K74R,E80A,D82A,K130T,K135R) | SY7 | 220 | 0.15 | 72 |
| SakSTAR(K74Q,E80A,D82A,K130T,K135R) | SY15 | 110 | 0.15 | 48 |
| SakSTAR(K35A,K74R,E80A,D82A,K130T,K135R) | SY17 | 160 | 0.19 | 68 |
| SakSTAR(K35A,K74Q,E80A,D82A,K130T,K135R) | SY28 | 120 | 0.18 | 48 |
| SakSTAR(E65D,K74R,E80A,D82A,K130T,K135R) | SY19 | 140 | 0.24 | 57 |
| SakSTAR(E65Q,K74R,E80A,D82A,K130T,K135R) | SY22 | 140 | 0.16 | 60 |
| SakSTAR(E65D,K74Q,E80A,D82A,K130T,K135R) | SY30 | 110 | 0.15 | 42 |
| SakSTAR(E65Q,K74Q,E80A,D82A,K130T,K135R) | SY47 | 120 | 0.16 | 42 |
| SakSTAR(K35A,E65D,K74R,E80A,D82A,K130T,K135R) | SY29 | 190 | 0.11 | 55 |
| SakSTAR(K35A,E65D,K74Q,E80A,D82A,K130T,K135R) | SY46 | 140 | 0.11 | 40 |
| SakSTAR(E65D,K74R,E80A,D82A,K130T,K135R,K136A) | SY35 | 100 | 0.19 | 45 |
| SakSTAR(K35A,E65D,K74R,E80A,D82A,K130T,K135R,K136A) | SY34 | 81 | — | 40 |
| SakSTAR(K35A,E65Q,K74Q,D82A,S84A,T90A,E99D,T101S,E108A,K109A,K130T,K135R) | SY151 | 210 | 0.18 | 34 |
| SakSTAR(K35A,E65Q,K74R,D82A,S84A,T90A,E99D,T101S,E108A,K109A,K130T,K135R) | SY155 | 180 | 0.21 | 36 |
| SakSTAR(E65Q,K74Q,K130T,K135R) | SY49 | 150 | 0.15 | 65 |
| SakSTAR(E65Q,K74Q,D82A,S84A,K130T,K135R) | SY50 | 170 | 0.19 | 45 |
| SakSTAR(E65Q,K74Q,T90A,E99D,T101S,K130A,K135R) | SY98 | 410 | 0.14 | 51 |
| SakSTAR(E65Q,K74Q,E108A,K109A,K130T,K135R) | SY83 | 180 | 0.18 | 50 |
| SakSTAR(E65Q,K74Q,D82A,S84A,E108A,K109A,K130T,K135R) | SY95 | 110 | 0.18 | 41 |
| SakSTAR(E65Q,K74Q,D82A,S84A,E108A,K109A,K130T,K135R,K136A,∇137K) | SY118 | 1500 | 0.15 | 30 |
| SakSTAR(E65Q,K74Q,D82A,S84A,T90A,E99D,T101S,E108A,K109A,K130T,K135R,K136A,∇137K) | SY128 | 2900 | 0.23 | 28 |
| SakSTAR(K35A,E65Q,K74Q,D82A,S84A,T90A,E99D,T101S,E108A,K109A,K130T,K135R,K136A,∇137K) | SY141 | 3700 | 0.19 | 24 |
| SakSTAR(K35A,E65Q,K74R,D82A,S84A,T90A,E99D,T101S,E108A,K109A,K130T,K135R,K136A,∇137K) | SY145 | 5700 | 0.19 | 31 |
Specific activity (Spec. Act.) ≥ 100 kU/mg is represented in bold type. Absorption of antibodies (in %) from pooled immunized patient plasma; values ≤ 60% are represented in bold type. C50: concentration (in μg/mL) causing 50% clot lysis in 2 hours.
Addition of E80A+D82A to the SakSTAR(K130T, K135R) template did not affect the specific activity and therefore the SakSTAR(E80A, D82A, K130T, K135R) template was selected for further mutagenesis. Addition of K74R and even more of K74Q reduced the reactivity with immunized patient plasma (Table 2). Substitution of K136 with A reduced the reactivity with human antibodies but also the specific activity. Addition of E65D or E65Q to the SakSTAR(K74Q, E80A, D82A, K130T, K135R) template yielded variants with intact specific activity that only bound less than or equal to 45 of the antibodies of pooled immunized patient plasma. On the basis of this analysis, SakSTAR(K74Q, E80A, D82A, K130T, K135R), (code SY15), SakSTAR(E65D, K74R, E80A, D82A, K130T, K135R), (code SY19), and SakSTAR(K35A, E65D, K74Q, E80A, D82A, K130T, K135R), (code SY46), were selected for further characterization.
Next, the SakSTAR(E65Q, K74Q, K130T, K135R) variant was taken as a template because it displayed a high specific activity with a significant reduction of absorption (to 65%) of antibodies from pooled immunized patient plasma. The intermediate variants that were relevant for the composition of the ultimately selected ones are summarized in Table 2. Addition of K35A, D82A and S84A, of T90A, E99D, and T101S or of E108A and K109A reduced the antibody absorption to around 50%, whereas the combined addition of D82A, S84A, and E108A, K109A reduced it to 41%. Substitution of K136A combined with the addition of a Lys at the COOH terminus (▿137K) increased the specific activity in a purified system but not in a plasma milieu (see below) and further reduced the absorption of antibodies from pooled patient plasma to 30%. Finally, addition of the K35A, and T90A, E99D, T101S substitutions to this template yielded a mutant with intact thrombolytic potency, which only bound 24% of the antibodies of pooled immunized patient plasma. On the basis of this analysis, SakSTAR(E65Q, K74Q, D82A, S84A, E108A, K109A, K130T, K135R, K136A, ▿137K), (code SY118), SakSTAR(K35A, E65Q, K74Q, D82A, S84A, T90A, E99D, T101S, E108A, K109A, K130T, K135R, K136A,▿137K), (code SY141), and SakSTAR(K35A, E65Q, K74R, D82A, S84A, T90A, E99D, T101S, E108A, K109A, K130T, K135R, K136A,▿137K) (code SY145) were selected for further characterization.
Finally, in view of the fact that the COOH extension with a Lys residue dramatically increased the apparent specific activity in a purified system but not the thrombolytic potency in a plasma milieu in vitro or in experimental animals and patients in vivo (see below), 2 additional variants SakSTAR(K35A, E65Q, K74Q, D82A, S84A, T90A, E99D, T101S, E108A, K109A, K130T, K135R) (code SY151) and SakSTAR(K35A, E65Q, K74R, D82A, S84A, T90A, E99D, T101S, E108A, K109A, K130T, K135R) (code SY155) were constructed and characterized. In these variants, the normal COOH terminal was reconstructed. These variants had intact specific activities and bound 34% and 36%, respectively, of SakSTAR-specific antibodies from pooled immunized patient plasma.
Fibrinolytic properties of selected SakSTAR variants toward human plasma in vitro
Dose- and time-dependent lysis of125I-fibrin–labeled human plasma clots submerged in human plasma was obtained with the selected variants (Table 2). Equi-effective concentrations of test compound (causing 50% clot lysis in 2 hours; C50), determined graphically from plots of clot lysis at 2 hours versus the concentration of plasminogen activator (not shown), ranged from 0.11 ± 0.01 to 0.27 ± 0.04 μg/mL at which no significant fibrinogen degradation occurred (not shown). Although the specific activities of the variants with a COOH-terminal Lys extension were up to 50-fold higher than that of wild-type SakSTAR, their fibrinolytic potencies in a human plasma milieu were very similar. The concentrations of compound causing 50% fibrinogen degradation in 2 hours in human plasma in the absence of fibrin were determined graphically from dose-response curves (not shown). These values (mean ± SD of 3 independent experiments) ranged from 14 ± 3.2 to 29 ± 3.1 μg/mL (not shown).
Large-scale purification and conditioning of SakSTAR variants for use in vivo
Of culture volumes of 18 L of SakSTAR variant, 76 to 840 mg of purified material was obtained, with specific activities of 140 to 5900 kU/mg. The endotoxin content ranged between less than 0.1 and 0.3 IU/mg (Table 3). Gel filtration on high-performance liquid chromatography (HPLC) revealed a single main symmetrical peak in the chromatographic range of the column, representing more than 98% of the eluted material (total area under the curve) (not shown). SDS gel electrophoresis of 10 μg samples revealed single main components (not shown). Preparations sterilized by filtration proved to be sterile on 3-day testing as described in “Materials and methods.” Intravenous bolus injection of SakSTAR variants in groups of 5 mice (3 mg/kg body weight) did not provoke any acute reaction or reduced weight gain within 8 days, in comparison with mice given an equal amount of saline (not shown).
Preparative purification of SakSTAR variants fromEscherichia coli cytosolic extracts
| Compound . | Code . | Protein (mg) . | Specific activity (kU/mg) . | Endotoxin (EU/mg) . | Clp (mL · min−1) . | C50 (μg/kg) . |
|---|---|---|---|---|---|---|
| SakSTAR | — | 2400 | 150 | 2.9 | 2.2 | 23 |
| SakSTAR(K74Q,E80A,D82A,K130T,K135R) | SY15 | 840 | 140 | <0.1 | 4.1 | 22 |
| SakSTAR(E65D,K74R,E80A,D82A,K130T,K135R) | SY19 | 800 | 150 | 0.3 | 3.7 | 28 |
| SakSTAR(K35A,E65D,K74Q,E80A,D82A,K130T,K135R) | SY46 | 340 | 140 | <0.1 | 1.6 | 18 |
| SakSTAR(E65Q,K74Q,D82A,S84A,E108A,K109A,K130T, K135R,K136A,∇137K) | SY118 | 690 | 1400 | <0.1 | 0.6 | 18 |
| SakSTAR(K35A,E65Q,K74Q,D82A,S84A,T90A,E99D,T101S, E108A,K109A,K130T,K135R,K136A,∇137K) | SY141 | 110 | 2700 | <0.1 | 1.0 | 19 |
| SakSTAR(K35A,E65Q,K74R,D82A,S84A,T90A,E99D,T101S, E108A,K109A,K130T,K135R,K136A,∇137K) | SY145 | 200 | 5900 | <0.1 | 0.8 | 29 |
| SakSTAR(K35A,E65Q,K74Q,D82A,S84A,T90A,E99D,T101S, E108A,K109A,K130T,K135R) | SY151 | 630 | 170 | 0.1 | 0.6 | 19 |
| SakSTAR(K35A,E65Q,K74R,D82A,S84A,T90A,E99D,T101S, E108A,K109A,K130T,K135R) | SY155 | 76 | 180 | 0.3 | 1.6 | 28 |
| Compound . | Code . | Protein (mg) . | Specific activity (kU/mg) . | Endotoxin (EU/mg) . | Clp (mL · min−1) . | C50 (μg/kg) . |
|---|---|---|---|---|---|---|
| SakSTAR | — | 2400 | 150 | 2.9 | 2.2 | 23 |
| SakSTAR(K74Q,E80A,D82A,K130T,K135R) | SY15 | 840 | 140 | <0.1 | 4.1 | 22 |
| SakSTAR(E65D,K74R,E80A,D82A,K130T,K135R) | SY19 | 800 | 150 | 0.3 | 3.7 | 28 |
| SakSTAR(K35A,E65D,K74Q,E80A,D82A,K130T,K135R) | SY46 | 340 | 140 | <0.1 | 1.6 | 18 |
| SakSTAR(E65Q,K74Q,D82A,S84A,E108A,K109A,K130T, K135R,K136A,∇137K) | SY118 | 690 | 1400 | <0.1 | 0.6 | 18 |
| SakSTAR(K35A,E65Q,K74Q,D82A,S84A,T90A,E99D,T101S, E108A,K109A,K130T,K135R,K136A,∇137K) | SY141 | 110 | 2700 | <0.1 | 1.0 | 19 |
| SakSTAR(K35A,E65Q,K74R,D82A,S84A,T90A,E99D,T101S, E108A,K109A,K130T,K135R,K136A,∇137K) | SY145 | 200 | 5900 | <0.1 | 0.8 | 29 |
| SakSTAR(K35A,E65Q,K74Q,D82A,S84A,T90A,E99D,T101S, E108A,K109A,K130T,K135R) | SY151 | 630 | 170 | 0.1 | 0.6 | 19 |
| SakSTAR(K35A,E65Q,K74R,D82A,S84A,T90A,E99D,T101S, E108A,K109A,K130T,K135R) | SY155 | 76 | 180 | 0.3 | 1.6 | 28 |
Temperature stability of selected SakSTAR variants
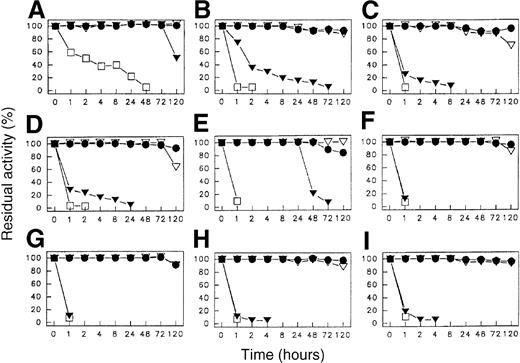
The temperature stability of preparations of the 8 selected SakSTAR variants, dissolved to a concentration of 1.0 mg/mL in 0.15 mol/L NaCl, 0.01 mol/L phosphate buffer, pH 7.5 at various temperatures, is illustrated in Figure 1. At temperatures up to 37°C, all compounds remained fully active for up to at least 5 days. At 56°C and 70°C the variants were less stable than wild-type SakSTAR.
Temperature stability.
Temperature stability of SakSTAR (A); SY15 (B); SY19 (C); SY46 (D); SY118 (E); SY141 (F); SY145 (G); SY151 (H); and SY155 (I). (●): 25°C; (▿): 37°C: (▾): 56°C; (■): 70°C. Code identification in Table 3.
Temperature stability.
Temperature stability of SakSTAR (A); SY15 (B); SY19 (C); SY46 (D); SY118 (E); SY141 (F); SY145 (G); SY151 (H); and SY155 (I). (●): 25°C; (▿): 37°C: (▾): 56°C; (■): 70°C. Code identification in Table 3.
Pharmacokinetic properties of SakSTAR variants after bolus injection in hamsters
The disposition rate of staphylokinase-related antigen from blood after bolus injection of 100 μg/kg of the selected SakSTAR variants in groups of 4 hamsters could adequately be described by a sum of 2 exponential terms by graphical curve peeling (results not shown),18 from which the plasma clearances (Clp) summarized in Table 3 were derived. The clearances of the mutants were not markedly different from those of wild-type SakSTAR. Initial plasma half-lives (t1/2(α)) ranged between 2.0 and 2.8 minutes (not shown) and plasma clearances (Clp) between 0.6 and 4.1 mL/min.
Thrombolytic properties of SakSTAR variants in a hamster pulmonary embolism model
The thrombolytic potencies of SakSTAR and the 8 variants were compared in a hamster pulmonary embolism model. Equipotent doses of SakSTAR variants, causing 50% pulmonary clot lysis ranged from 18 to 29 μg/kg, compared with 23 μg/kg for wild-type SakSTAR (Table3). Fibrinogen and α2-antiplasmin levels did not decrease after infusion of SakSTAR, or its variants, whereas the steady-state staphylokinase-related antigen level in plasma increased proportionally with the infusion rate (not shown).
Comparative antibody induction of SakSTAR and its variants after intra-arterial administration in patients with peripheral arterial occlusion
Groups of 5 to 18 patients with angiographically documented peripheral arterial occlusion (PAO), with an estimated median duration of 7 days and a median length of 14 cm, were treated with one of the 8 SakSTAR variants (61 patients in total) and compared with 80 patients given wild-type SakSTAR. Relevant baseline characteristics of the patient groups and the results of treatment and outcome in the different groups are reported in detail elsewhere.21Intra-arterial infusion, at a mean dose of 11 to 16 mg and a duration of 15 to 22 hours, induced complete recanalization in 55 patients and partial recanalization in 6. Circulating fibrinogen, plasminogen, and α2-antiplasmin levels remained essentially unchanged during infusion of the SakSTAR moieties.
Antibody-related neutralizing activity and corresponding specific IgG were low at baseline and during the first week after the infusion. From the second week on, neutralizing activity levels increased to reach median values (15 to 85 percentiles) at 3 to 4 weeks of 4.4 (0.3-49) μg SakSTAR variant neutralized per milliliter plasma in the 60 patients treated with the respective compounds (median values among compounds ranging from 1.5 to 33 μg/mL) (Table4), compared with the median value of 12 (4.0-100) μg wild-type SakSTAR neutralized per milliliter in 70 patients treated with wild-type SakSTAR (P = .002 for variants against wild-type SakSTAR by Mann-Whitney rank sum test). The levels of specific IgG against the SakSTAR variants increased to median values at 3 to 4 weeks of 270 (32-2600) μg anti-SakSTAR IgG per milliliter plasma in the 60 patients treated with the respective compounds (median values among compounds ranging from 53 to 2000 μg/mL plasma in patients treated with the respective variants), compared with the median value of 380 (81-1850) μg anti-SakSTAR IgG per milliliter plasma in the total group of 70 patients treated with SakSTAR (P = NS) (Table 4).
Immunogenicity of SakSTAR variants in patients with peripheral arterial occlusion
| Compound . | n . | Neutralizing activity . | Specific IgG (μg/mL) . | |
|---|---|---|---|---|
| (μg/mL) . | n with >5 μg/mL . | |||
| SakSTAR | 70 | 12 (4.0-100) | 57 | 380 (81-1850) |
| SY15 | 6 | 9.3 (0.1-19) | 3 | 400 (32-740) |
| SY19 | 18 | 1.5 (0.2-7.0) | 5 | 110 (37-260) |
| SY46 | 6 | 12 (0.9-42) | 3 | 1800 (140-7100) |
| SY118 | 6 | 27 (17-49) | 5 | 2000 (1300-3600) |
| SY141 | 6 | 1.7 (0.1-8.3) | 2 | 53 (4.5-1800) |
| SY145 | 7 | 1.6 (1.2-49) | 2 | 540 (110-790) |
| SY151 | 6 | 33 (3.5-40) | 4 | 1900 (310-3500) |
| SY155 | 5 | 15 (6.6-63) | 4 | 360 (160-1500) |
| Total mutants | 60 | 4.4 (0.3-49) | 28 | 270 (32-2600) |
| Compound . | n . | Neutralizing activity . | Specific IgG (μg/mL) . | |
|---|---|---|---|---|
| (μg/mL) . | n with >5 μg/mL . | |||
| SakSTAR | 70 | 12 (4.0-100) | 57 | 380 (81-1850) |
| SY15 | 6 | 9.3 (0.1-19) | 3 | 400 (32-740) |
| SY19 | 18 | 1.5 (0.2-7.0) | 5 | 110 (37-260) |
| SY46 | 6 | 12 (0.9-42) | 3 | 1800 (140-7100) |
| SY118 | 6 | 27 (17-49) | 5 | 2000 (1300-3600) |
| SY141 | 6 | 1.7 (0.1-8.3) | 2 | 53 (4.5-1800) |
| SY145 | 7 | 1.6 (1.2-49) | 2 | 540 (110-790) |
| SY151 | 6 | 33 (3.5-40) | 4 | 1900 (310-3500) |
| SY155 | 5 | 15 (6.6-63) | 4 | 360 (160-1500) |
| Total mutants | 60 | 4.4 (0.3-49) | 28 | 270 (32-2600) |
Data represent median and 15 to 85 percentile range frequencies or mean ± SEM. Code identification in Table 3.
Absorption with SakSTAR variants of antibodies elicited in patients by treatment with SakSTAR variants
Overt immunization (neutralizing activity at 3 to 4 weeks of more than or equal to 5 μg compound per milliliter plasma) was observed in 81% (57 of 70) patients treated with SakSTAR, and in 47% (28 of 60) patients treated with the 8 selected SakSTAR variants (Table 4). The absorption and binding patterns of the antibodies in 18 patients given SY19, SY141, SY145, SY151, or SY155 were evaluated as described in “Materials and methods” and compared with those of the pooled plasma of 40 immunized patients after treatment with wild-type SakSTAR (Table 5). Wild-type SakSTAR absorbed virtually all the antibodies binding to insolubilized SakSTAR or SakSTAR variants, irrespective of the compound used for treatment, which indicates that no neoantigens were introduced in the variants by substitution mutagenesis. SakSTAR, SY19, SY141, SY145, SY151, or SY155, respectively, absorbed all the antibodies (median value more than 90%) binding to insolubilized SakSTAR, SY19, SY141, SY145, SY151, or SY155, respectively, from plasma of patients treated with SakSTAR, SY19, SY141, SY145, SY151, or SY155, respectively (values indicated with superscript A in Table 5), which supports the validity of the absorption method used. From the pooled plasma of 40 patients treated with SakSTAR, SY19 absorbed 59% of antibodies binding to SakSTAR, SY141 bound 24%, SY145 31%, SY151 34%, and SY155 36%, of SakSTAR-specific antibodies, which confirms the progressive elimination of B-cell epitopes during the sequential additive mutagenesis. Unexpectedly, using plasma from patients treated with SY141 or SY145, these compounds absorbed 85% to 91% of antibodies binding to the respective insolubilized compound, but only 53% and 73% of antibodies binding to SakSTAR (superscript B in Table 5), suggesting that infusion of these variants elicited a significant fraction of antibodies specific for wild-type SakSTAR that were not recognized by the variant used for treatment.
Absorption with SakSTAR variants of antibodies elicited with SakSTAR variants in patients with peripheral arterial occlusion (%)
| Treatment . | n . | Absorbant . | Insolubilized compound . | |||||
|---|---|---|---|---|---|---|---|---|
| WT . | SY19 . | SY141 . | SY145 . | SY151 . | SY155 . | |||
| WT | Pool 40 | WT | +A | + | + | + | + | + |
| SY19 | 59 | + | + | + | + | + | ||
| SY141 | 24 | 45 | + | 66 | 70 | 59 | ||
| SY145 | 31 | 55 | + | + | 91 | 73 | ||
| SY151 | 34 | 54 | 90 | 84 | + | 70 | ||
| SY155 | 36 | 69 | + | + | + | + | ||
| SY19 | 4 | WT | 94 | 92 | + | + | + | + |
| SY19 | 92 | +A | + | + | + | + | ||
| SY141 | 41 | 70 | 90 | 85 | 88 | 86 | ||
| SY145 | 78 | + | + | + | + | + | ||
| SY151 | 80 | + | + | + | + | + | ||
| SY155 | 83 | + | + | + | + | + | ||
| SY141 | 3 | WT | 87 | + | + | 94 | + | + |
| SY19 | 87 | + | + | + | + | + | ||
| SY141 | 53B | 72 | 85A | 80 | 77 | 78 | ||
| SY145 | 83 | 90 | + | 93 | 93 | 94 | ||
| SY151 | 81 | 85 | + | + | + | + | ||
| SY155 | 82 | 92 | + | + | + | + | ||
| SY145 | 3 | WT | + | + | + | 93 | 92 | 92 |
| SY19 | 81 | + | 93 | 90 | 92 | 89 | ||
| SY141 | 66 | 73 | 79 | 76 | 77 | 78 | ||
| SY145 | 73B | 82 | 94 | 91A | 90 | 90 | ||
| SY151 | 71 | 78 | 93 | 88 | 88 | 88 | ||
| SY155 | 78 | 83 | 94 | 93 | 93 | 94 | ||
| SY151 | 4 | WT | + | + | + | + | + | + |
| SY19 | + | + | + | + | + | + | ||
| SY141 | 83 | 83 | 93 | 92 | 93 | 89 | ||
| SY145 | 87 | 87 | + | + | + | 93 | ||
| SY151 | 88 | 91 | + | + | +A | 94 | ||
| SY155 | 87 | 90 | + | + | + | + | ||
| SY155 | 4 | WT | + | + | + | + | + | + |
| SY19 | + | + | + | + | + | + | ||
| SY141 | 84 | 87 | + | 89 | 89 | 90 | ||
| SY145 | 92 | 94 | + | + | + | + | ||
| SY151 | 92 | 94 | + | + | + | + | ||
| SY155 | 94 | + | + | + | + | +A | ||
| Treatment . | n . | Absorbant . | Insolubilized compound . | |||||
|---|---|---|---|---|---|---|---|---|
| WT . | SY19 . | SY141 . | SY145 . | SY151 . | SY155 . | |||
| WT | Pool 40 | WT | +A | + | + | + | + | + |
| SY19 | 59 | + | + | + | + | + | ||
| SY141 | 24 | 45 | + | 66 | 70 | 59 | ||
| SY145 | 31 | 55 | + | + | 91 | 73 | ||
| SY151 | 34 | 54 | 90 | 84 | + | 70 | ||
| SY155 | 36 | 69 | + | + | + | + | ||
| SY19 | 4 | WT | 94 | 92 | + | + | + | + |
| SY19 | 92 | +A | + | + | + | + | ||
| SY141 | 41 | 70 | 90 | 85 | 88 | 86 | ||
| SY145 | 78 | + | + | + | + | + | ||
| SY151 | 80 | + | + | + | + | + | ||
| SY155 | 83 | + | + | + | + | + | ||
| SY141 | 3 | WT | 87 | + | + | 94 | + | + |
| SY19 | 87 | + | + | + | + | + | ||
| SY141 | 53B | 72 | 85A | 80 | 77 | 78 | ||
| SY145 | 83 | 90 | + | 93 | 93 | 94 | ||
| SY151 | 81 | 85 | + | + | + | + | ||
| SY155 | 82 | 92 | + | + | + | + | ||
| SY145 | 3 | WT | + | + | + | 93 | 92 | 92 |
| SY19 | 81 | + | 93 | 90 | 92 | 89 | ||
| SY141 | 66 | 73 | 79 | 76 | 77 | 78 | ||
| SY145 | 73B | 82 | 94 | 91A | 90 | 90 | ||
| SY151 | 71 | 78 | 93 | 88 | 88 | 88 | ||
| SY155 | 78 | 83 | 94 | 93 | 93 | 94 | ||
| SY151 | 4 | WT | + | + | + | + | + | + |
| SY19 | + | + | + | + | + | + | ||
| SY141 | 83 | 83 | 93 | 92 | 93 | 89 | ||
| SY145 | 87 | 87 | + | + | + | 93 | ||
| SY151 | 88 | 91 | + | + | +A | 94 | ||
| SY155 | 87 | 90 | + | + | + | + | ||
| SY155 | 4 | WT | + | + | + | + | + | + |
| SY19 | + | + | + | + | + | + | ||
| SY141 | 84 | 87 | + | 89 | 89 | 90 | ||
| SY145 | 92 | 94 | + | + | + | + | ||
| SY151 | 92 | 94 | + | + | + | + | ||
| SY155 | 94 | + | + | + | + | +A | ||
Results represent mean values of n patients. Absorption ≥ 95% is represented by +. WT: wild-type SakSTAR. Code identification in Table 3. For an explanation of superscripts A and B, see “Absorption with SakSTAR variants of antibodies elicited in patients by treatment with SakSTAR variants.”
Discussion
This study was inititated by the observation that certain “clustered charge-to-alanine” substitution variants of recombinant staphylokinase (SakSTAR variant6) had a reduced reactivity with antibodies induced by treatment with wild-type SakSTAR7,9 and induced less antibodies than wild-type SakSTAR in patients with peripheral arterial occlusion.8 10 In an effort to optimize the specific activity versus antigenicity ratio, a comprehensive mutagenesis study, comprising the construction and expression of approximately 350 plasmids encoding SakSTAR variants, and the purification of the translation products, was undertaken. The SakSTAR variants were characterized in terms of specific activity and absorption of SakSTAR-specific antibodies from the pooled plasma of patients treated with wild-type SakSTAR.
Eight variants of SakSTAR, which emerged from the present site directed mutagenesis program, are characterized by an intact or increased apparent specific activity, maintained fibrinolytic potency and fibrin-selectivity in a human plasma milieu, acceptable although slightly reduced temperature stability, and a markedly reduced reactivity with anti-SakSTAR antibodies in pooled immunized patient plasma. An intriguing observation was that extension of the molecule at the carboxyterminal end with a lysine markedly increased the apparent specific activity measured via plasminogen activation in a purified system but did not affect the fibrinolytic potency in a human plasma milieu in vitro or the thrombolytic potency in a hamster pulmonary embolism model. The cause of this “artifact” is at present unclear. It is tempting to speculate that the COOH-terminal lysine would bind to the lysine binding site of substrate plasminogen and thereby stabilize the Michaelis complex, resulting in an apparently increase affinity.
These 8 selected variants were produced in sufficiently large amounts and conditioned for the evaluation of their pharmacokinetics and thrombolytic potencies in hamsters and of their thrombolytic potency/immunogenicity ratios in patients with peripheral arterial occlusion. Highly purified, sterilized preparations were found to contain low endotoxin levels, to be devoid of acute toxicity in mice after intravenous bolus injection at a dose of 3 mg/kg, and to have pharmacokinetic properties after bolus intravenous injection in hamsters, and thrombolytic potencies in a hamster pulmonary embolism model similar to those of SakSTAR.
Intra-arterial administration of these 8 selected SakSTAR variants as a bolus of 2 mg, followed by an infusion of 1 mg/h in 61 (groups of 5 to 18) patients with angiographically documented occlusion of a peripheral artery or bypass graft, resulted in complete recanalization in 55 patients and partial recanalization in 6, without measurable systemic plasminogen activation as evidenced by unaltered fibrinogen and α2-antiplasmin levels, as reported in detail elsewhere.21 Significant allergic reactions were not observed, but reduced allergenicity of these variants will have to be confirmed in larger groups of patients. After administration of variant SakSTAR, neutralizing antibody titers and specific IgG levels remained low for 1 week. From the second or third week onward, an increase of SakSTAR-neutralizing activity to more than or equal to 5 μg/mL plasma was observed in 57 of 70 patients (81%, 95% confidence interval 72% to 91%) given wild-type SakSTAR, but only in 28 of 60 patients (47%, 95% confidence interval of 35% to 59%) given any of the selected SakSTAR variants (P < .0001 by Fisher exact test). These neutralizing activities were most likely due to specific IgG as demonstrated by the increase of staphylokinase-specific IgG in the absence of increased IgM titers (not shown).
The antibodies induced by treatment with SakSTAR were completely absorbed by SakSTAR but incompletely (down to less than 30%) by the SakSTAR variants, confirming the elimination in these variants of certain epitopes present in wild-type SakSTAR. The antibodies induced by treatment with the SakSTAR variants were completely absorbed by wild-type SakSTAR, indicating that immunization was not due to neoepitopes generated by the amino acid substitutions but to residual epitopes in the variants. One strange and, at present, unexplained observation was that, in some patients, treatment with a SakSTAR variant induced antibodies specifically binding to wild-type SakSTAR but not to the variant used for treatment.
In summary, the current experience illustrates that staphylokinase variants with markedly reduced recognition of antibodies elicited with wild-type SakSTAR and antibody induction but intact thrombolytic potency can be generated. On the basis of this study, variant SakSTAR(K35A, E65Q, K74R, D82A, S84A, T90A, E99D, T101S, E108A, K109A, K130T, K135R) (code SY155) has been selected for further development toward fibrin-selective thrombolytic therapy in patients with thromboembolic disease. This variant was selected over variants SY141 or SY145, notwithstanding the fact that these variants bound fewer antibodies from the immunized patient plasma pool (24% and31%, respectively, versus 36 % with SY155) and induced lower neutralizing antibody titers (median values of 1.6 and 1.7 μg/mL, respectively, versus 15 μg/mL with SY155). This choice was based on the dramatically increased apparent specific activities of SY141 and SY145 in a purified system, with unaltered potency in a plasma milieu and in animal models. It was concluded that this unexplained artifact might indeed hamper the clinical development of these compounds, and that further reduction of the immunogenicity might be better approached via additional elimination of T-cell epitopes.
Supported in part by a sponsored research agreement between the University of Leuven (Leuven Research and Development, VZW) and Thromb-X NV, a spin-off company of the University of Leuven, in which D.C. has an equity interest.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Desire Collen, Center for Transgene Technology and Gene Therapy, University of Leuven, Campus Gasthuisberg O & N, Herestraat 49, B-3000 Leuven, Belgium; e-mail:desire.collen@med.kuleuven.ac.be.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal