Abstract
Strong evidence exists for an association between high vascular endothelial growth factor (VEGF) levels and poor prognoses in patients with solid tumors and acute leukemia. Using Western blot analysis and solid-phase radioimmunoassay, we measured cellular VEGF levels in B-cell chronic lymphocytic leukemia (CLL) samples from 225 patients and correlated these levels with disease characteristics and prognoses. The median VEGF level in CLL samples was 7.26 times the median level detected in normal peripheral blood mononuclear cells. Patients with lower levels of VEGF protein showed a trend toward shorter survival (P = .07). However, in a subgroup of CLL patients with good prognoses or early-stage disease (Rai stages 0-II, Binet stages A,B; β2-M ≤ 2.8 mg/dL), lower levels of VEGF were associated with shorter survival times. For the entire group of patients, no correlation was found between VEGF levels and β2-M levels or Rai and Binet stage. Most samples from patients with CLL expressed the 43-kd VEGF isoform in addition to the commonly expressed 45-kd isoform. It remains to be seen whether the expression of the 43-kd isoform is responsible for this reversed correlation with outcome.
Vascular endothelial growth factor (VEGF) is a potent mitogen for microvascular and macrovascular endothelial cells derived from arteries, veins, and lymphatics, and overexpression has been correlated with increased angiogenesis.1-4 Because of this characteristic, VEGF has been associated with tumor growth, invasion, and metastasis in solid tumors.5-7 The gene for human VEGF has been localized to chromosome 6p21.3.8 It is a single gene containing 8 exons and 7 introns.9Alternative splicing produces VEGF isoforms with sequences of 121, 165, 189, and 206 amino acids.9 The 165-amino acid isoform (VEGF165) appears to be the most abundantly expressed isoform in most cells.10 VEGF isoforms 121 and 165, which have no or low heparin-binding activity, are secreted by tumor cells.11 VEGF mRNA is markedly up-regulated in many cancer cells and in tumor-infiltrating lymphocytes.10 VEGF was originally cloned from HL60 leukemia cells12 and, more recently, was found to be expressed in other leukemic cell lines,13-15 but the role of angiogenesis and angiogenesis-regulatory molecules has not been investigated extensively in patients with leukemia. Perez–Atayde et al16 found that children with acute lymphoid leukemia (ALL) had increased angiogenesis in the bone marrow and high urinary basic fibroblast growth factor. Fiedler et al14 found VEGF transcription in a high percentage of patients with acute myeloid leukemia (AML). In a series of 99 patients with newly diagnosed AML, we found that low levels of intracellular VEGF protein were associated with higher rates of complete response (CR) rate and survival (P < .05).15 Salven et al reported that high level of serum-VEGF in patients with non-Hodgkin lymphoma correlated with shorter survival times and other poor prognostic features. We studied intracellular VEGF levels in peripheral blood samples from patients with CLL using Western blot and solid-phase radioimmunoassay (RIA).
Study design
Peripheral blood samples were obtained from 225 patients diagnosed with B-cell CLL. Diagnosis was based on morphology, flow cytometry analysis (CD5+, CD19+, CD23+), and molecular evaluation. Mononuclear cells from the peripheral blood of 31 normal persons were used as controls. In 8 patients CD19 and CD3 cells were isolated using magnetic beads and MACS columns (Miltenyi Biotec, Auburn, CA). The sorted cells were confirmed to be more than 90% pure B and T cells using CD20 and CD7, respectively.
Results and discussion
The clinical characteristics of the 225 patients with CLL are summarized in Table 1. All analyzed samples contained at least 70% lymphocytes.
Clinical characteristics (n = 225)
| Characteristic . | No.evaluable . | Median (range) . | Value . | No.patients (%) . |
|---|---|---|---|---|
| Patient age (years) | 225 | 58 (21-87) | <60 | 119 (53) |
| ≥60 | 106 (47) | |||
| Rai stage | 181 | 0-II | 122 (67) | |
| III-IV | 59 (33) | |||
| Binet stage | 178 | A | 82 (46) | |
| B | 50 (28) | |||
| C | 46 (26) | |||
| ≥40 | 109 (50) | |||
| β2-microglobulin (mg/dL) | 175 | 2.8 (1.1-36.6) | <2 | 35 (20) |
| ≥2 | 140 (80) |
| Characteristic . | No.evaluable . | Median (range) . | Value . | No.patients (%) . |
|---|---|---|---|---|
| Patient age (years) | 225 | 58 (21-87) | <60 | 119 (53) |
| ≥60 | 106 (47) | |||
| Rai stage | 181 | 0-II | 122 (67) | |
| III-IV | 59 (33) | |||
| Binet stage | 178 | A | 82 (46) | |
| B | 50 (28) | |||
| C | 46 (26) | |||
| ≥40 | 109 (50) | |||
| β2-microglobulin (mg/dL) | 175 | 2.8 (1.1-36.6) | <2 | 35 (20) |
| ≥2 | 140 (80) |
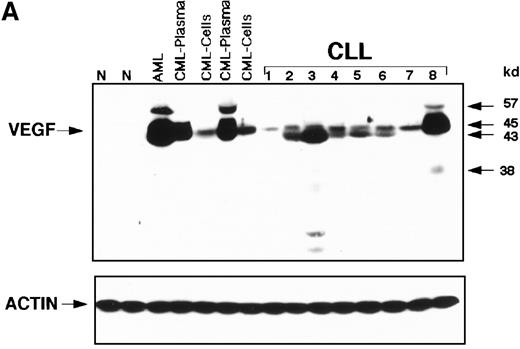
Overexpression of cellular VEGF in CLL samples was demonstrated on Western blots, whereas normal peripheral blood showed no detectable VEGF protein (Figure 1A). Most of the CLL patients expressed mainly the 43-kd VEGF isoform, though low-level expression of the 45-kd isoform was also seen (Figure1A). The patterns of expression in most CLL patients were different from those seen in patients with chronic myeloid leukemia (CML) or acute myeloid leukemia (AML). Some samples expressed trace amounts of the 57-kd and 38-kd isoforms. Solid-phase-RIA was used for quantification. The median number of counts per minute (cpm) in 31 samples of peripheral blood mononuclear cells obtained from normal persons was assigned a value of 1, and levels for the various samples were expressed relative to the control median. By this method, the median value of VEGF for the CLL patients was 7.26 cpm (range, 2.45-17 cpm), indicating significantly higher levels of cellular VEGF in CLL patients than in the normal group (P < .001, Kruskal–Wallis test). All analyzed samples contained more than 70% CD19+/CD5+ cells. To test whether the presence of residual normal cells in the analyzed samples would affect the overall values of VEGF, we sorted CD19+ and CD3+ positive cells from the monocytes and compared VEGF levels of these 3 subpopulations to those obtained from unfractionated samples. Using the Kruskal–Wallis test, we found no significant difference in values obtained from an unfractionated sample and the CD19+ cell population. In contrast, VEGF values of unfractionated cells were significantly different from those obtained from CD3+ cells and monocytes (P = .04 and .02, respectively).
Expression of VEGF protein in CLL and correlation with survival.
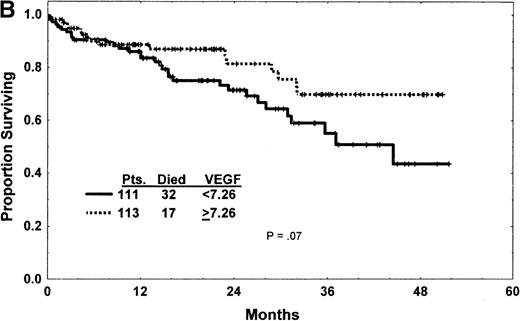
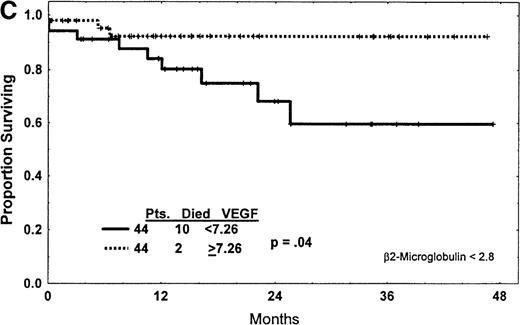
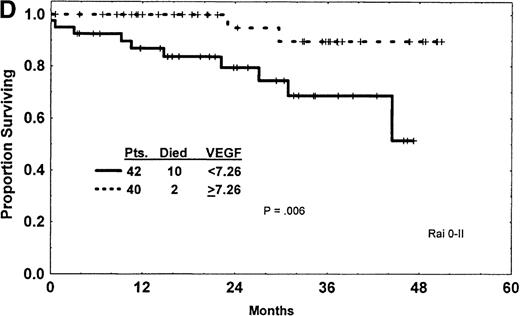
(A) Western blot analysis showed that the patterns of expression in most samples from patients with CLL were different from those seen in plasma and cells from patients with CML and AML. The VEGF protein expressed in CLL samples was mainly the 43-kd and 45-kd isoforms. Some samples expressed trace amounts of the 57-kd and 38-kd isoforms. (B) Patients with VEGF levels above the median had slightly better survival times (P = .07) when all patients with CLL were compared. (C) Higher levels of VEGF reflected better survival times in the subgroup of patients with low β2-M (good prognosis group). (D) High levels of VEGF correlated with better survival times in the subgroup of patients with early disease by Rai stages 0 to II.
Expression of VEGF protein in CLL and correlation with survival.
(A) Western blot analysis showed that the patterns of expression in most samples from patients with CLL were different from those seen in plasma and cells from patients with CML and AML. The VEGF protein expressed in CLL samples was mainly the 43-kd and 45-kd isoforms. Some samples expressed trace amounts of the 57-kd and 38-kd isoforms. (B) Patients with VEGF levels above the median had slightly better survival times (P = .07) when all patients with CLL were compared. (C) Higher levels of VEGF reflected better survival times in the subgroup of patients with low β2-M (good prognosis group). (D) High levels of VEGF correlated with better survival times in the subgroup of patients with early disease by Rai stages 0 to II.
VEGF levels correlated directly with absolute lymphocyte counts (R = −.08; P = .05) and inversely with white blood cell counts (R = −.12; P = .06). Although a correlation was not found between VEGF and other prognostic covariates, including Rai or Binet stages, a trend for a direct correlation was noted for VEGF and β2-microglobulin (R = −.01; P = .15). Analysis of survival revealed better survival times in patients with cellular VEGF levels above the median7.26 than below the median, though this difference did not reach statistical significance (P = .07) (Figure 1B).
The effect of VEGF level on survival was evaluated after dividing patients into groups with high β2-M (poor prognosis) and low β2-M (good prognosis) concentrations. VEGF levels in patients with high β2-M levels did not correlate with survival (P = .51). However, low VEGF levels (less than 7.26) correlated with worse outcomes in patients with low levels of β2-M (P = .04) (Figure 1C).
This was confirmed using Rai and Binet stages; low levels of VEGF correlated with shorter survival times in patients with Rai stages 0 to II disease (Figure 1D) and Binet stages A and B disease (P = .006 for both). No effect of VEGF was noticed for the subgroup of patients with more advanced disease, Rai stage III or IV (P = .69) and Binet stage C (P = .46). Most (192 of 225) of our patients were previously untreated. Analysis of the previously untreated patients showed survival patterns similar to those of the entire group. On comparing VEGF levels with duration of disease, as measured from the time of diagnosis to the time of sample collection and analysis, we found reversed correlation (R = −.119; P= .07). This suggests that VEGF protein expression decreases as CLL progresses.
The exact role of VEGF protein in the growth and proliferation of the B cells of CLL is unclear. Our demonstration of an inverse relationship between VEGF expression and survival time, particularly in the subgroup of patients with early disease, was not expected. Although further study and evaluation of more homogeneously treated patients is necessary, the current data suggest that VEGF levels may help identify patients with early-stage disease who are at high risk for disease progression and death.
Most studies have found a correlation between high VEGF levels and poor survival times in patients with solid tumors.18-22Occasional studies, including studies of pancreatic tumor, squamous cell head and neck carcinoma, and breast cancer, showed no correlation between VEGF expression and survival.7,19,20 We found a correlation between high levels of VEGF and poor prognosis in the bone marrow of patients with AML,15 but the inverse correlation between VEGF and survival in CLL is interesting and requires further investigation. The possibility that different isoforms of VEGF may have different functions must be explored. We are investigating whether the 43-kd isoform is secreted. Of interest, we found no significant increase in vascularity in the bone marrow of patients with CLL.23 In conclusion, VEGF levels in patients with B-cell CLL are high, and they help predict disease behavior in a subgroup of patients with early-stage disease for whom prognostic factors are scarce.
Reprints:Maher Albitar, Department of Hematopathology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Box 72, Houston, TX 77030-4095.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal