Abstract
Exocytosis of perforin, subsequent binding of perforin to the target cell membrane, and formation of lytic pores form an important pathway involved in the induction of tumor cell death by cytotoxic effector cells. Here we describe a novel escape mechanism employed by tumor cells to protect themselves from granule-mediated cell death: We were able to demonstrate that the resistance of the human leukemia cell line ML-2 to natural killer (NK)-cell–mediated killing is not caused by impaired NK-cell activation but by resistance against effector molecules contained in the granules of cytotoxic cells. No resistance was observed against other pore-forming agents like complement and streptolysin O. By using the NK-susceptible leukemia cell line K562, we could show that the induction of cell death by cytotoxic granules can be blocked completely by anti-perforin antibodies, indicating that perforin is essentially involved in this process. Flow cytometric data revealed that an impaired binding of perforin on the tumor cell membrane is mainly responsible for target cell resistance, because perforin turned out to bind well on K562 cells but is not able to attach to the surface of ML-2 cells. After impaired binding of perforin was identified as a potential mechanism of tumor cell resistance, leukemia cells from 6 patients with acute myeloid leukemia (AML) were examined. As predicted, AML cells that failed to bind perforin on their surface demonstrated complete resistance toward NK-cell–mediated cytotoxicity. Thus, perforin resistance could represent an important tumor escape mechanism that should be considered when cytotoxic effector cells are used for cellular immunotherapy.
Cellular immunotherapy using autologous or allogeneic effector cells that recognize tumor-specific surface structures is a promising new treatment strategy for patients with various types of cancer.1 The potential of this approach was first demonstrated in the context of allogeneic stem cell transplantation in which experimental and clinical findings clearly indicated that two types of effector cells, that is, cytotoxic T cells and natural killer (NK) cells, were able to destroy otherwise resistant leukemia cells.2,3 Currently, a number of clinical studies have been initiated to exploit this so-called graft-versus-tumor effect. Recent experience confirms earlier findings that the infusion of allogeneic lymphocytes is not equally effective in different diseases and different patients with identical diagnoses.4 So far, it is not easily possible to predict which patients will benefit from cellular immunotherapy.
There is some evidence that the in vitro resistance of tumor cells against donor NK cells is of predictive value for patients with chronic myelocytic leukemia,5 whereas in other leukemias such correlations have not been described. Possible reasons for lack of target cell lysis are (1) failure of tumor cell recognition and (2) resistance of the tumor against cytotoxic effector mechanisms. With regard to NK cells, a large body of data has been gathered concerning mechanisms of tumor cell recognition and effector cell activation.6-9 In this paper, we have focused on the efferent part of the killing process.
It is now clear that both T cells and NK cells can use different pathways to kill their targets. The “secretory pathway” (ie, the exocytosis of granules containing perforin and granzymes10-12) and the utilization of members of the tumor necrosis factor (TNF) receptor family (Fas, TNF, and TNF-related apoptosis inducing ligand [TRAIL]) to induce apoptosis13-15 are the two best-known mechanisms of target cell killing. However, studies on tumor surveillance in perforin-deficient mice suggest that perforin-induced target cell lysis is crucial for the successful elimination of tumor cells, whereas the Fas pathway seems less important.16-18 If these observations also apply to the human situation, the elimination of residual tumor cells after treatment of patients with various forms of cellular immunotherapy depends on the integrity of the degranulation pathway. However, little is known about factors influencing the susceptibility of malignant cells with regard to perforin-mediated cell death. Perforin, as a pore-forming protein, binds to the target cell membrane in the presence of calcium,11,19-21 leading to loss of osmotic stability and influx of granule-associated proteins that are able to induce apoptosis.12 So far, resistance against perforin-mediated lysis has been described and studied systematically only in cytotoxic effector cells. Cytotoxic T lymphocytes (CTLs) and NK cells are resistant to cytolysis by other killer cells,22 isolated granules,23,24 or purified perforin.25 Inhibition of pore formation in the plasma membrane,26 possibly caused by a reduced binding of perforin to the surface of CTL and NK cells,27 28 has been proposed to explain this phenomenon.
We asked whether perforin resistance might also serve as an escape mechanism that protects tumor cells from destruction through the immune system.
Materials and methods
Reagents
RPMI supplemented with 4 mmol/L l-glutamine (Sigma, Munich, Germany), 100 U/mL streptomycin (Sigma), 100 U/mL penicillin (Sigma), and 10% fetal calf serum (FCS; Kraeber, Wedel, Germany) was used as cell culture medium. 3,3′-Dioctadecyloxacarbocyanine (DiO; Sigma) was dissolved in dimethyl sulfoxide (DMSO; Sigma), to give a 3 mmol/L DiO solution. Propidium iodide (PI; Sigma) was dissolved in RPMI 1640 to yield a 0.1 mg/mL PI solution. Streptolysin O and complement (both from Sigma) were stored as stock solutions with 25 000 U/mL (streptolysin O) and 33 CH50 U/mL (complement) and diluted in phosphate-buffered saline (PBS). The detergens NP-40 (Sigma) was diluted in PBS to yield a 0.1% stock solution.
Antibodies
For flow cytometry analysis, we used a fluorescein isothiocyanate (FITC)-labeled antibody against perforin (clone δG9; specific for a 70 kd protein in NK lysates, as determined by Western blot analysis [data not shown]; Hoelzel Diagnostica, Cologne, Germany) and an FITC-labeled isotype-antibody (Pharmingen, Hamburg, Germany) as negative control. Perforin-mediated lysis was blocked with the use of a nonlabeled perforin antibody (clone δG9; Hoelzel Diagnostica) or a nonlabeled isotype control antibody (Pharmingen) in various concentrations.
Target cells
The cell lines K562 and ML-2 were obtained from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany) and were maintained in RPMI 1640 with 10% FCS at 37°C in 5% CO2. Fresh leukemia blasts (percentage of blasts in all cases > 90%) from patients with acute myeloid leukemia (AML) were obtained from the Department of Internal Medicine II, University of Kiel, Kiel, Germany, after isolation on a Ficoll-Paque gradient and were stored in FCS with 10% DMSO at −140°C. The tumor cells of the 6 patients were named AML-1 to AML-6 and belonged to the following subtypes of AML: M2 (AML-2, AML-4, AML-5), M4 (AML-1, AML-3), and MDS/RAEB-T (AML-6). The analysis of the expression of the molecules MHC-I, CD50, CD54, and CD58 on the surface of the tumor cells with labeled antibodies by flow cytometry did not reveal any significant differences between NK-resistant and NK-sensitive tumor cells (data not shown).
Effector cells
Peripheral blood mononuclear cells were isolated from buffy coats on a Ficoll-Paque gradient. NK cells were obtained by immunomagnetic separation, using anti-CD56 antibodies coupled to magnetic beads (CD56 MultiSort Kit; Miltenyi Biotec, Bergisch-Gladbach, Germany). CD56+ cells (purity: greater than 90% CD56+, less than 5% CD3+) were resuspended in RPMI 1640 with 10% FCS at a concentration of 1 × 106 cells/mL.
Preparation of NK lysates
Lysates from NK cells were prepared as described previously.29 Briefly, the supernatant from 1 × 107 NK cells was collected after 3 freeze–thaw cycles in 200 μL PBS with 2 mmol/L EDTA and 1 mmol/L MgCl2. Cytolytic activity of the lysate was tested in a hemolytic assay against red blood cells.
Hemolytic assay for perforin activity
A 2% erythrocyte suspension (100 μL) in Tris-buffered saline containing 5 mmol/L CaCl2 was incubated with 30 μg total protein of the NK lysates for 30 minutes at 37°C. Then the supernatant was harvested and analyzed for hemoglobin concentration at 412 nm in a photometer.29
Assay for NK-mediated cytotoxicity
NK-cell–mediated cytotoxicity was analyzed as described previously.30 Briefly, target cells were stained with DiO for 20 minutes at 37°C. After washing the target cells with PBS, NK cells and target cells (ratios: 40:1 and 20:1) were coincubated for 0, 1, 2, 4, or 8 hours in RPMI 1640. Dead cells were counterstained by PI and analyzed by flow cytometry.
Assay for granule-mediated cytotoxicity
A volume of 20 μL of a 1 × 106 cells/mL target cell suspension was incubated with various concentrations of NK lysates for 4 hours at 37°C. Dead cells were stained with 150 μL of the PI solution and analyzed by flow cytometry.
Flow cytometric analysis of NK-cell–mediated lysis
DiO-labeled target cells emit a green fluorescence and PI-stained target cells emit a red fluorescence. A flow cytometer (FACScan, Becton Dickinson, Heidelberg) was used to assess 2 parameter flow histograms. Events of 1 × 104 per sample were acquired. A sample containing only target cells and PI was used to measure spontaneous cell death. The specific lysis of the target cells was determined by using the formula
Detection of perforin on the cell surface
Binding of perforin to the cell surface was examined with the use of a FITC-labeled antiperforin antibody. A volume of 20 μL of a 5 × 106 cells/mL tumor cell suspension was incubated with 100 μg total protein of the NK lysates for 4 hours at 37°C (cells incubated without NK lysates, 10 U/mL complement or 0.02% NP-40 were used as negative control). The cells were then stained with 80 μL of the FITC-labeled perforin antibody (antibody dilution, 1:50 in PBS + 2% human serum albumin [HSA]) or 20 μL of the FITC-labeled isotype control antibody for 60 minutes at 4°C. After washing the cells once with PBS + 2% HSA, they were resuspended in 100 μL PBS and assayed by flow cytometry (FSC versus FL1 dot plots) or counterstained by PI and then assayed by flow cytometry (FL1 versus FL2 dot plots).
Blocking of perforin-mediated lysis
An amount of 30 μg (hemolysis) or 100 μg (lysis of tumor cells) total protein of NK lysates was incubated with various concentrations of a nonlabeled perforin antibody or a nonlabeled isotype antibody as negative control for 10 minutes at room temperature. These pretreated lysates were used in hemolytic assays against red blood cells or in lytic assays against K562 cells as described above.
Lysis of tumor cells with streptolysin O and complement
A volume of 100 μL of a 2 × 106 cells/mL target cell suspension was incubated with 10 U/mL of complement or 5 U/μL of streptolysin O for 30 minutes at 37°C. Dead cells were stained with 150 μL of the PI solution and analyzed by flow cytometry.
Cold target inhibition assay
K562 target cells were stained with DiO for 20 minutes at 37°C. After washing the target cells with PBS, NK cells and DiO-labeled K562 cells (ratio, 20:1) were coincubated with various concentrations of nonlabeled K562 or ML-2 cells in RPMI 1640. After an incubation time of 4 hours, the dead cells were stained with PI and analyzed by flow cytometry.
Results
Killing of tumor cell lines by NK cells
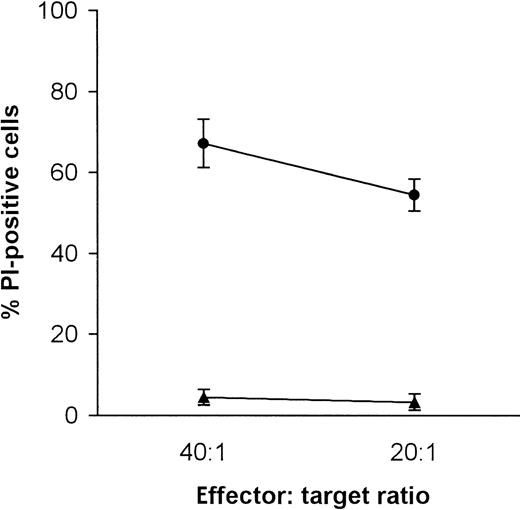
NK-sensitive K562 cells were compared with ML-2 cells, a cell line derived from a human AML. As shown in Figure1, with effector-to-target ratios of 40:1 and 20:1, ML-2 cells were not killed by allogeneic CD56+effector cells, whereas killing of K562 was always in the range of 60%.
Killing of K562 and ML-2 cells by NK cells.
K562 cells, •; ML-2 cells, ▴. Target cells were stained with DiO and coincubated with NK cells (e:t 40:1 and 20:1) for 4 hours at 37°C in 5% CO2. Dead cells were stained with PI and analyzed by flow cytometry.
Killing of K562 and ML-2 cells by NK cells.
K562 cells, •; ML-2 cells, ▴. Target cells were stained with DiO and coincubated with NK cells (e:t 40:1 and 20:1) for 4 hours at 37°C in 5% CO2. Dead cells were stained with PI and analyzed by flow cytometry.
Cold target inhibition assay
Because a reduced killing of ML-2 cells could have been caused by a difference in recognition of K562 and ML-2 cells by the NK cells, we examined whether ML-2 cells bind NK cells to the same extent as K562 cells do. Cold target inhibition assays were performed in which NK cells and DiO-labeled K562 cells were coincubated with nonlabeled K562 and ML-2 cells to inhibit NK-cell–mediated lysis by effector-target conjugate formation. No significant difference in the inhibition of NK-cell-mediated target cell death by nonlabeled K562 or ML-2 cells was observed (Figure 2), indicating that the resistance of ML-2 cells is not due to impaired NK-cell binding. These data suggest that missing cell death of ML-2 cells is caused by a resistance of these cells to cytotoxic effector molecules.
Cold target inhibition assay of the killing of K562 cells by K562 and ML-2 cells.
NK cells and DiO-labeled K562 cells (ratio 20:1) were coincubated with nonlabeled K562 or ML-2 cells in various concentrations. After an incubation time of 4 hours, the dead cells were stained with PI and analyzed by flow cytometry.
Cold target inhibition assay of the killing of K562 cells by K562 and ML-2 cells.
NK cells and DiO-labeled K562 cells (ratio 20:1) were coincubated with nonlabeled K562 or ML-2 cells in various concentrations. After an incubation time of 4 hours, the dead cells were stained with PI and analyzed by flow cytometry.
Killing of tumor cells by NK lysates
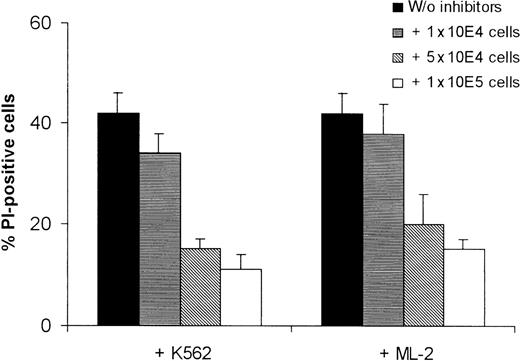
To test the hypothesis that the resistance against cytotoxic effector molecules is responsible for the inability of NK cells to destroy ML-2 leukemia cells, lysates from human NK cells were tested in different concentrations for their capacity to induce killing of K562 and ML-2 cells. In contrast to K562 target cells, ML-2 cells proved to be resistant to killing by NK lysates, even at high concentrations (Figure 3A).
Killing of K562 and ML-2 cells by NK lysates.
(A) Killing of tumor cells by NK lysates. Target cells were incubated with 0.3 μg, 1 μg, 30 μg, or 100 μg total protein of the NK lysates for 4 hours at 37°C in 5% CO2. Dead cells were stained with PI and analyzed by flow cytometry. (B) Kinetics of the tumor cell lysis. Target cells were incubated with 100 μg total protein of the NK lysates for 0, 1, 2, 4, or 8 hours at 37°C in 5% CO2. Dead cells were stained with PI and analyzed by flow cytometry.
Killing of K562 and ML-2 cells by NK lysates.
(A) Killing of tumor cells by NK lysates. Target cells were incubated with 0.3 μg, 1 μg, 30 μg, or 100 μg total protein of the NK lysates for 4 hours at 37°C in 5% CO2. Dead cells were stained with PI and analyzed by flow cytometry. (B) Kinetics of the tumor cell lysis. Target cells were incubated with 100 μg total protein of the NK lysates for 0, 1, 2, 4, or 8 hours at 37°C in 5% CO2. Dead cells were stained with PI and analyzed by flow cytometry.
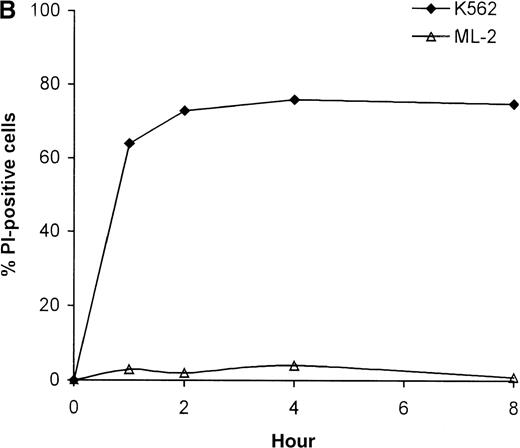
To investigate whether the resistance of ML-2 cells to NK-mediated killing can be overcome by increased incubation times, the time course of tumor cell death was evaluated after treatment of K562 and ML-2 cells, using high concentrations of NK lysates. Most of the K562 cells were killed with the killing being complete within 1 hour—a time course characteristic for perforin-mediated cell death. As depicted in Figure 3B, even after 8 hours of coincubation of ML-2 cells with NK-cell lysates no significant target cell killing was observed.
Involvement of perforin in killing of tumor cells
To identify particular molecules involved in tumor cell killing by the granule preparation, the NK lysates were treated with blocking antibodies. As shown in Figure 4, pretreatment of NK-cell–derived lysates with increasing doses of an antibody directed against perforin reduces the lysis of erythrocytes and K562 cells to baseline levels, thus indicating that the NK-mediated cell death is completely perforin-dependent.
Inhibition of granule-mediated killing with an antiperforin antibody.
(A) Dose-dependent inhibition of hemolysis; 30 μg total protein of the NK lysates were incubated for 10 minutes with different concentrations of the antiperforin antibody and then 100 μL of a 2% erythrocyte suspension was added. After incubation for 30 minutes at 37°C, the supernatant was harvested and analyzed for hemoglobin in a photometer at 412 nm. (B) Inhibition of granule-mediated killing of K562 cells; 100 μg total protein of the NK lysates were incubated for 10 minutes with PBS, an isotype control antibody (100 μg/mL), or the antiperforin antibody (100 μg/mL) and then 20 μL of K562 cells with 2 × 106 cells/mL were added. After incubation for 4 hours at 37°C, the dead cells were stained with PI and analyzed by flow cytometry.
Inhibition of granule-mediated killing with an antiperforin antibody.
(A) Dose-dependent inhibition of hemolysis; 30 μg total protein of the NK lysates were incubated for 10 minutes with different concentrations of the antiperforin antibody and then 100 μL of a 2% erythrocyte suspension was added. After incubation for 30 minutes at 37°C, the supernatant was harvested and analyzed for hemoglobin in a photometer at 412 nm. (B) Inhibition of granule-mediated killing of K562 cells; 100 μg total protein of the NK lysates were incubated for 10 minutes with PBS, an isotype control antibody (100 μg/mL), or the antiperforin antibody (100 μg/mL) and then 20 μL of K562 cells with 2 × 106 cells/mL were added. After incubation for 4 hours at 37°C, the dead cells were stained with PI and analyzed by flow cytometry.
Killing of K562 and ML-2 cells by streptolysin O and complement
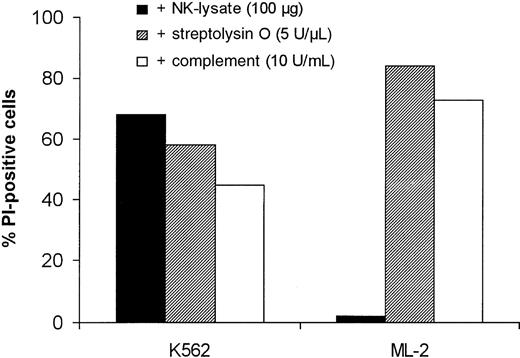
To investigate whether the resistance of ML-2 cells against perforin-mediated cell death also extends to other pore-forming proteins, K562 and ML-2 cells were treated with streptolysin O (5 U/μL) and complement (10 U/mL) for 30 minutes. Figure5 shows that ML-2 cells are killed easily by streptolysin O and complement, suggesting that ML-2 cells possess a perforin-specific defense mechanism against cytotoxic effector cells.
Killing of K562 and ML-2 cells by NK lysates, streptolysin O, and complement.
K562 and ML-2 cells were incubated with 100 μg total protein of the NK lysates, 5 U/μL streptolysin O, or 10 U/mL complement for 30 minutes at 37°C. Dead cells were stained with 150 μL of the PI solution and analyzed by flow cytometry.
Killing of K562 and ML-2 cells by NK lysates, streptolysin O, and complement.
K562 and ML-2 cells were incubated with 100 μg total protein of the NK lysates, 5 U/μL streptolysin O, or 10 U/mL complement for 30 minutes at 37°C. Dead cells were stained with 150 μL of the PI solution and analyzed by flow cytometry.
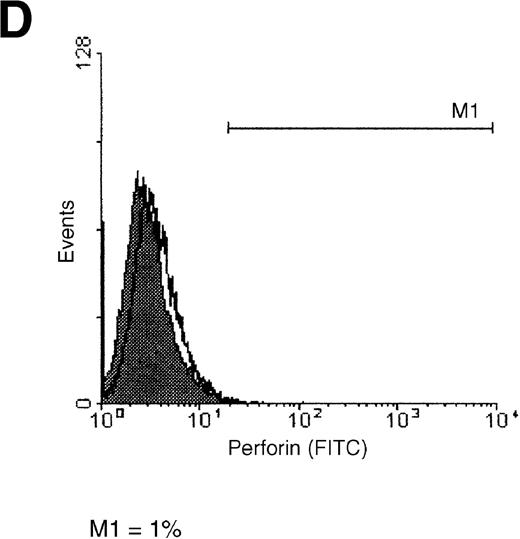
Detection of perforin on the surface of tumor cells
A reduced binding of perforin on the cell surface is known to be one mechanism by which CTLs and NK cells protect themselves from perforin-mediated killing. Therefore, we compared the attachment of perforin to the surface of K562 and ML-2 cells (Figure6). Binding of perforin to the tumor cell membrane was examined with the use of a FITC-labeled anti-perforin antibody. Whereas the majority (51%) of K562 cells showed a strong binding of perforin (Figure 6A), only a minimal proportion (3%) of ML-2 cells stained positive for perforin (Figure 6B). These data, which were confirmed in several experiments, clearly indicate that tumor cells can escape from cell-mediated cytotoxicity by hindering the binding of perforin to their surface.
Perforin binding on the surface of K562 and ML-2 cells.
Target cells were incubated for 4 hours with 30 μL PBS or with 100 μg total protein of the NK lysates. After 1 hour of incubation with a FITC-labeled antiperforin antibody or an FITC-labeled isotype antibody, cells were analyzed by flow cytometry. (A) K562 cells + perforin antibody, (B) ML-2 cells + perforin antibody, (C) K562 cells + isotype antibody, and (D) ML-2 cells + isotype antibody. Open histograms = cells with PBS, shaded histograms = cells with NK lysates. M1 indicates perforin-positive cells.
Perforin binding on the surface of K562 and ML-2 cells.
Target cells were incubated for 4 hours with 30 μL PBS or with 100 μg total protein of the NK lysates. After 1 hour of incubation with a FITC-labeled antiperforin antibody or an FITC-labeled isotype antibody, cells were analyzed by flow cytometry. (A) K562 cells + perforin antibody, (B) ML-2 cells + perforin antibody, (C) K562 cells + isotype antibody, and (D) ML-2 cells + isotype antibody. Open histograms = cells with PBS, shaded histograms = cells with NK lysates. M1 indicates perforin-positive cells.
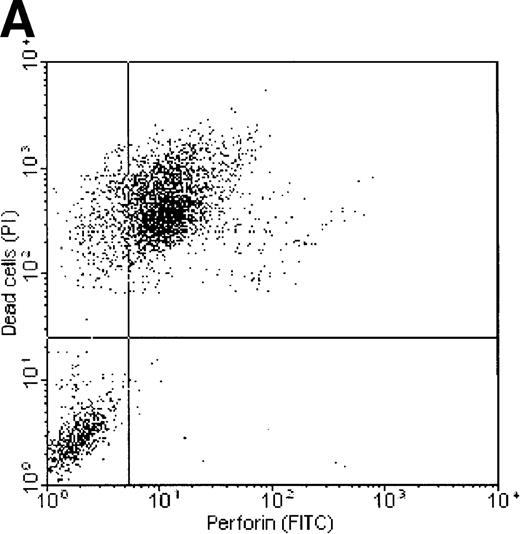
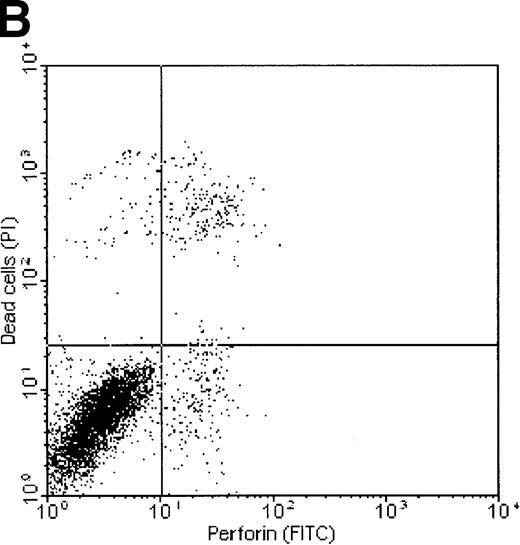
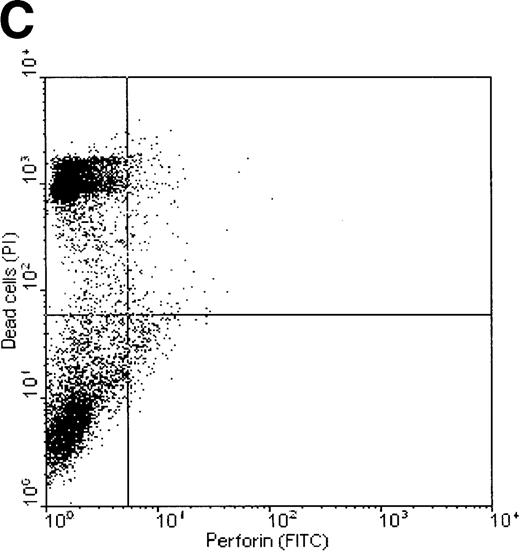
Correlation between perforin binding and killing of tumor cells
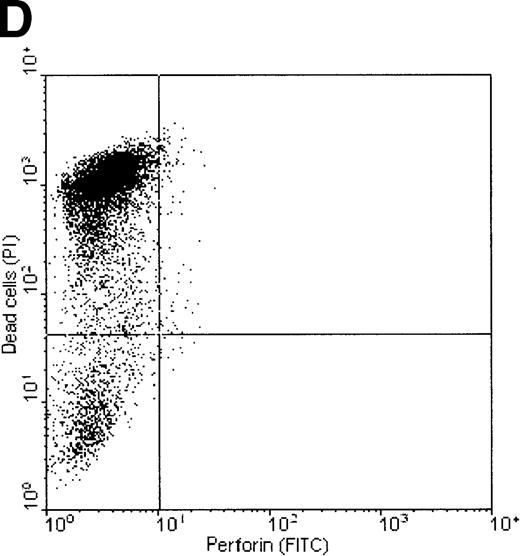
Dual-fluorescence analysis was performed to investigate whether binding of perforin to the tumor cell surface was associated with subsequent death of the target cell. FL1 (perforin) versus FL2 (PI) dot plots of K562 and ML-2 cells, shown in Figure7, indicate that most of the tumor cells staining positive for perforin were also PI+, that is, were dead (consequently, most of the dead cells show binding of perforin on their surface). ML-2 cells showed no binding of perforin and were thus not killed by NK lysates. These observations confirm that binding of perforin is mandatory for target cell death. To exclude the possibility of an unspecific adsorption of the perforin antibody by dead cells, K562 cells killed by complement (Figure 7C) or the detergens NP-40 (Figure 7D) were stained by PI and labeled with the perforin antibody. Although more than 50% of the cells were PI+, none of these cells stained positive for perforin.
Correlation of perforin binding and subsequent cell death of K562 and ML-2 cells.
The cells were incubated for 4 hours with 100 μg total protein of the NK lysates, 0.02% NP-40, or 10 U/mL complement. After staining dead cells with PI and perforin on the cell surface with a FITC-labeled perforin antibody, cells were analyzed in FL1 (FITC) versus FL2 (PI) dot plots by flow cytometry. (A) K562 cells + NK lysates, (B) ML-2 cells + NK lysates, (C) K562 cells + 10 U/mL complement, and (D) K562 cells + 0.02% NP-40.
Correlation of perforin binding and subsequent cell death of K562 and ML-2 cells.
The cells were incubated for 4 hours with 100 μg total protein of the NK lysates, 0.02% NP-40, or 10 U/mL complement. After staining dead cells with PI and perforin on the cell surface with a FITC-labeled perforin antibody, cells were analyzed in FL1 (FITC) versus FL2 (PI) dot plots by flow cytometry. (A) K562 cells + NK lysates, (B) ML-2 cells + NK lysates, (C) K562 cells + 10 U/mL complement, and (D) K562 cells + 0.02% NP-40.
Perforin binding and killing of native tumor cells
Leukemia cells from 6 patients with AML were used as targets to obtain information on the clinical relevance of deficient perforin binding as a cause of tumor cell resistance. As for ML-2 and K562, the number of perforin-binding tumor cells was correlated with the susceptibility of leukemia cells to NK-granule–mediated killing. Figure 8 demonstrates the high correlation observed between perforin binding and killing by NK lysates. Tumor cells resistant to perforin-mediated cell death demonstrate only a weak binding of perforin, whereas a high proportion of susceptible tumor cells bind perforin.
Killing and perforin binding on the surface of tumor cells from 6 AML patients by NK lysates.
Target cells and 100 μg total protein of the NK lysates were coincubated for 4 hours at 37°C. After staining dead cells with PI and perforin on the cell surface with an FITC-labeled perforin antibody, cells were analyzed in FL1 versus FL2 dot plots by flow cytometry. K562 cells served as a positive control.
Killing and perforin binding on the surface of tumor cells from 6 AML patients by NK lysates.
Target cells and 100 μg total protein of the NK lysates were coincubated for 4 hours at 37°C. After staining dead cells with PI and perforin on the cell surface with an FITC-labeled perforin antibody, cells were analyzed in FL1 versus FL2 dot plots by flow cytometry. K562 cells served as a positive control.
Discussion
The data presented here reveal that resistance to perforin might be frequent in hematologic malignancies. Moreover, they suggest that impaired binding of perforin to the surface of tumor cells is one mechanism responsible for target cell resistance to NK-cell–mediated cytotoxicity. Perforin, which mediates its biologic effects by formation of pores in target cell membranes,10-12,19 is cytolytic in its own right, but it is not able to activate the apoptotic machinery.31,32 The formation of pores allows the delivery of various granule-associated proteins into subcellular compartments, either by direct access through the perforin pores or by repair endocytosis of the target cell, resulting in uptake of the extracellular fluid by pinocytosis.33 The best known of these proteins, which finally induce target cell apoptosis, is the serine esterase granzyme B.33,34 Granzyme A seems to play an important role as a “back-up system” in case granzyme B is inhibited in the target cell.35
Because complete granula (ie, perforin and apoptosis-inducing proteins) are essential for the induction of programmed target cell death, we adopted the method described by Lee et al29 to isolate granules from purified human NK cells to analyze the susceptibility of leukemia cells to molecules of the secretory pathway. It turned out that resistance to granule-mediated cytotoxicity was responsible for the failure of NK cells to kill ML-2 leukemia cells. The lack of activatory signals (eg, an impaired binding of NK cells to ML-2 target cells) as a cause for reduced killing of ML-2 cells was ruled out by a cold target inhibition assay, which showed no differences in effector-target conjugate formation for K562 and ML-2 cells.
To separate the role of perforin from that of other granule-associated proteins, we utilized a perforin-specific antibody that blocked the lysis of erythrocytes and K562 cells, thereby indicating that perforin is fundamental for the process of granule-mediated cytotoxicity assessed by our method.
To evaluate whether the resistance to perforin is associated with resistance to other pore-forming molecules, target cells were incubated with the complement membrane attack complex or the bacterial exotoxin streptolysin O, which are both able to cause lysis of nucleated target cells.36,37 No target cell resistance could be observed when ML-2 cells were exposed to lytic doses of these agents, indicating that resistance of ML-2 cells is not a general defense mechanism against pore-forming proteins but is specific for perforin. Similar observations were made regarding perforin-resistant CTLs that can be killed easily using streptolysin O.38 One might speculate that tumor cells and CTLs utilize similar mechanisms to protect themselves from perforin-mediated cytotoxicity.
Because impaired binding of perforin to the target cell surface was suspected as one potential mechanism through which tumor cells may prevent their destruction by cytotoxic effector cells, we examined the amount of perforin bound on the surface of susceptible K562 and resistant ML-2 cells by flow cytometry. The finding that most ML-2 cells, in contrast to K562 cells, were not able to bind perforin and the observation that perforin binding was highly correlated with cell death lead us to the conclusion that deficient binding of perforin is responsible for target cell resistance.
Perforin resistance as a tumor escape mechanism against cytotoxic effector cells of the immune system is a novel finding that has not been described before. To evaluate its biologic relevance in a more clinical context, we tested tumor cells of patients with acute leukemias for their susceptibility toward cytotoxic NK lysates. Interestingly, the binding of perforin on the surface of myeloid leukemia cells was always predictive for cell killing by NK lysates (which, in turn, is a prerequisite for successful NK-cell–mediated cytotoxicity). Thus, perforin binding and granule-induced cell death can provide relevant information regarding the susceptibility of human tumor cells as targets of immunotherapeutic approaches.
Although perforin binding has not been systematically investigated with regard to tumor cell resistance, there are some data concerning its role in the context of CTL effector functions: With the help of a simple competition assay, Jiang et al28 were first to describe an association between the resistance of CTLs and their ability to bind perforin and proposed that weak binding of perforin to the surface of the effector cell is a mandatory mechanism to protect the killer cell from its own weapons.27Muller and Tschopp,38 however, were not able to detect any correlation between perforin binding and resistance to lysis with the use of radioactively labeled perforin.
Because, until recently, perforin-specific antibodies were not available, the detection of perforin binding was restricted to competition assays28 or the use of prelabeled perforin.38 The major disadvantage of prelabeling is possible effects of the labeling process itself on the binding abilities of perforin. Because the method described here allows the detection of perforin on a single-cell level after binding has occurred, this problem does not exist. Moreover, the method has the advantage of being technically simple and sensitive. It is possible, however, that the perforin antibody detects a conformationally altered perforin molecule.
Tumor cells as well as virally infected cells are able to develop numerous strategies to prevent destruction through cytotoxic cells, antibodies, or cytostatic drugs. Because most of these mechanisms rely on the active suppression or induction of proteins that are physiologically involved in the regulation of cellular functions, the next logical step would be to ask which proteins are involved in perforin resistance. The precise process of perforin binding and pore formation is still unknown; no binding or receptor structures have been generally accepted to function as perforin ligands. It has been suggested that phosphorylcholine, the headgroup of the ubiquitous phospholipids phosphatidylcholine and sphingomyelin, is the specific receptor of perforin.39 However, there are contradictory data, indicating that the spacing of outer leaflet lipids is more critical.40 Because it has been demonstrated that lipid spacing is closer in CTLs than in target cells, changes in the lipid composition of the cell membrane may render tumor cells resistant to perforin. Another candidate for the inhibition of perforin-mediated cell death is the histone H2B that prevents perforin-induced hemolysis and is found in relatively high density on the surface of CTLs.41 Because it is also present on the surface of perforin-sensitive tumor cells, it may impair perforin binding in a concentration-dependent manner. In a first attempt to test whether perforin resistance relies on the synthesis of a protein that actively inhibits perforin binding, we treated target cells with an inhibitor of RNA-transcription (actinomycin D) and of protein synthesis (cycloheximide). Preliminary data showed no reversal of perforin resistance after blocking protein de novo synthesis. Similar negative results have been reported with regard to perforin resistance of cytotoxic T lymphocytes.42
Taken together, our results show that impaired binding of perforin to the tumor cell membrane (caused by a yet unknown mechanism) is responsible for the resistance of many tumors against cell death induced by granule exocytosis. We have formally demonstrated that this phenomenon accounts for resistance against NK-cell–mediated cytotoxicity but, because the effector mechanisms are identical, resistance will also occur with T-cell–mediated cytotoxicity. The description of perforin resistance as an important tumor cell escape mechanism could help to optimize cellular immunotherapy. First, the simple technique used to detect target cell resistance might allow to restrict cellular immunotherapy to those patients who have a chance to benefit from this approach. Attempts to optimize tumor cell recognition (eg, through vaccination or gene therapy) may fail if the target demonstrates resistance to perforin-mediated cell death. Second, the finding that tumor cells differ in their capacity to bind perforin should stimulate the search for perforin-binding structures to develop techniques to overcome this mechanism of tumor escape.
Acknowledgments
We thank Maria Niggemeier for expert technical assistance and Marianne Helweg for reviewing the manuscript.
Supported by a grant from the Deutsche Krebshilfe.
Reprints:Christof Lehmann, Department of Hematology, University of Leipzig, Johannisallee 32, 04103 Leipzig, Germany; e-mail: christof_lehmann@yahoo.com.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal