Abstract
Bernard-Soulier syndrome is a rare bleeding disorder caused by a quantitative or qualitative defect in the platelet glycoprotein (GP) Ib-IX-V complex. The complex, which serves as a platelet receptor for von Willebrand factor, is composed of 4 subunits: GPIb, GPIbβ, GPIX, and GPV. We here describe the molecular basis of a novel form of Bernard-Soulier syndrome in a patient in whom the components of the GPIb-IX-V complex were undetectable on the platelet surface. Although confocal imaging confirmed that GPIb was not present on the platelet surface, GPIb was readily detectable in the patient's platelets. Moreover, immunoprecipitation of plasma with specific monoclonal antibodies identified circulating, soluble GPIb. DNA-sequence analysis revealed normal sequences for GPIb and GPIX. There was a G to A substitution at position 159 of the gene encoding GPIbβ, resulting in a premature termination of translation at amino acid 21. Studies of transient coexpression of this mutant, W21stop-GPIbβ, together with wild-type GPIb and GPIX, demonstrated a failure of GPIX expression on the surface of HEK 293T cells. Similar results were obtained with Chinese hamster ovary IX cells, a stable cell line expressing GPIb that retains the capacity to re-express GPIX. Thus, we found that GPIbβ affects the surface expression of the GPIb-IX complex by failing to support the insertion of GPIb and GPIX into the platelet membrane.
Platelet adhesion to damaged blood vessels is a critical hemostatic mechanism. This adhesion is initiated by the interaction between von Willebrand factor (vWF) exposed on the subendothelium and its platelet receptor, the glycoprotein (GP) Ib-IX-V complex. GPIb-IX-V is a hetero-oligomeric protein complex assembled from 4 distinct gene products and uniquely expressed on the membranes of platelets and megakaryocytes. GPIbα, the largest protein (145 kd), contains the binding site for vWF1 and α-thrombin2 in its extracellular domain and has binding sites for 14.3.33 and the actin cytoskeleton4in its short cytoplasmic region. GPIbα is linked by means of an extracellular disulfide bond to GPIbβ (25 kd) and exists in a 1:1 covalent complex with this protein. The other components of the oligomer, GPIX and GPV, are noncovalently linked to the GPIb complex in a ratio of 2:2:1 (GPIb:GPIX:GPV). The functional roles of GPIbβ and GPIX in the complex are not clear, although in vitro evidence suggests that both these proteins act as complex-specific chaperones that protect GPIbα from lysosomal degradation while it is transported to the membrane.5 GPV has a role in the high-affinity binding of thrombin to the platelet6 but does not seem to be essential for the surface expression of a functional complex.7
Abnormalities of the GPIb-IX-V protein complex are associated with abnormal platelet function and appearance, giving rise to a syndrome first described by Bernard and Soulier in 1948.8Bernard-Soulier syndrome (BSS) is an autosomal recessive disorder characterized by moderate to severe thrombocytopenia, enlarged (giant) platelets, and a tendency to have profuse and often spontaneous bleeding.9 Twenty-one causes of BSS have been characterized at a molecular level. Of these, 14 are due to mutations in GPIbα, 5 in GPIX, and 2 in GPIbβ.9-12 There are no reports of BSS affecting the GPV gene. Furthermore, mice without GPV expressed normal levels of GPIb-IX protein complex and had no BSS-like symptoms.7
In this report, we describe a novel, homozygous, single-nucleotide substitution (G159→A) in the coding region for GPIbβ at a tryptophan codon (TGG), which results in the premature termination of translation (TAG) at amino acid 21. No sequence abnormalities were observed in either the GPIbα or the GPIX subunit of the complex. Although GPIbα, GPIX, and GPV were undetectable on the platelet surface, GPIbα was readily demonstrated in platelets. Moreover, soluble GPIbα was present in plasma. Thus, we found that the defective GPIbβ is unable to support the expression or maintenance of a functional complex at the platelet surface.
Patients, materials, and methods
Case history
A 57-year-old Irish man with a severe bleeding diathesis was given a tentative diagnosis of BSS because of the presence of thrombocytopenia (platelet count, 20-120 × 109/L), large platelets on peripheral blood smear, and a profuse bleeding tendency requiring transfusion. Bleeding occurred both spontaneously and after minor surgical procedures. The spontaneous bleeding apparently stopped when the patient was about 20 years of age, but the thrombocytopenia persisted. The patient's bleeding time was longer than 15 minutes. During childhood, the patient was treated with steroids. The patient had 2 living siblings, neither of whom had any history of abnormal bleeding. Two other siblings died in infancy, 1 from pneumonia and 1 from unknown causes. The patient's parents were first cousins and had no history of abnormal bleeding. Investigations of the patient's thrombocytopenia showed that the bone marrow aspirate was normal except for the presence of megakaryocytes with an excessively granular appearance. Coagulation studies showed no deficiency of factor V, VII, or VIII.
Monoclonal antibodies (mAbs) and reagents
The anti-GPIbα antibody AP1 blocks vWF binding to GPIbα. MBC 142.2, 142.6, and 142.11 are mAbs raised against purified GPIbα that do not inhibit the binding of vWF to GPIbα. GC is a rabbit polyclonal antibody raised against purified GPIbα. Anti-GPIX mAbs FMC 25 and GRP were purchased from Harlan Bioproducts (Indianapolis, IN). Antibodies SZ2, SZ1, and SW16 (Beckman Coulter Immunotech, France) are mAbs that bind GPIbα, GPIX, and GPV, respectively, and are components of a kit for diagnostic estimation of these antigens on platelet surfaces (BioCytex, Marseilles, France). AP2 is a mAb against the GPIIb-IIIa complex, and LYP18 is a mAb that recognizes GPIIIa in complex with GPIIb. AK1, a mAb that recognizes an epitope that requires the intact GPIb-IX complex,13 was a generous gift of Dr Michael C. Berndt (Baker Medical Research Institute, Victoria, Australia).
Platelet isolation
Venous blood samples from the patient and healthy controls were collected into 0.15- vol ACD (38 mmol/L citric acid anhydrous, 75 mmol/L sodium citrate, and 124 mmol/L dextrose) and centrifuged to obtain platelet-rich plasma (PRP). The PRP was acidified to pH 6.5 with ACD, and prostaglandin E1 (final concentration, 1 μmol/L) was added. The platelets were then pelleted through the plasma by centrifugation at 900g for 12 minutes at room temperature. The supernatant was removed, and platelets were resuspended in a modified Tyrode buffer containing 130 mmol/L sodium chloride, 10 mmol/L trisodium citrate, 9 mmol/L sodium bicarbonate, 6 mmol/L dextrose, 0.9 mmol/L magnesium chloride, 0.81 mmol/L potassium phosphate (monobasic), and 10 mmol/L Tris (pH 7.4). Calcium chloride (final concentration, 1.8 mmol/L) was added to the washed platelets for aggregation and agglutination studies.
Flow cytometric analysis of whole blood
Platelets were analyzed by flow cytometry with a platelet GP quantification kit (BioCytex). Briefly, 50 μL of whole blood was diluted 1:4 in modified Tyrode buffer and incubated for 10 minutes at room temperature with 20 μL of the following antibodies: negative control mouse IgG, LYP18 (anti-GPIIIa), SZ2 (anti-GPIbα),14,15 SZ1 (anti-GPIX),13,16 and SW16 (anti-GPV).17 18 Secondary antibody (polyclonal antimouse IgG [fluorescein isothiocyanate, conjugated], 20 μL) was incubated with each sample for an additional 10 minutes. Samples were diluted by adding 2 mL of buffer and were then analyzed on a flow cytometer (FACS Star; Becton Dickinson, San Jose, CA). A calibration-bead suspension coated with increasing and known quantities of mouse IgG was incubated in parallel with secondary antibody and used to construct a standard curve for fluorescence intensity compared with known numbers of binding sites. This standard curve was used to convert results from test samples to number of sites per platelet, and histogram analysis was performed with CellQuest (version 3.1f; Becton Dickinson).
Polymerase chain reaction (PCR) amplification of genomic DNA
Genomic DNA was isolated from peripheral blood lymphocytes as described previously.19 DNA was amplified by PCR20 using primer pairs based on the published genomic sequence of GPIbα, GPIbβ, and GPIX.21-23 For DNA-sequence analysis, the full-length coding region for mature GPIbα was amplified with primers 162 to 181 and 2634 to 2653.22For GPIbβ, the primers 8 to 30 and 767 to 79121 were used for amplification; for GPIX, we used primers 792 to 816 and 1547 to 1560.23 The target sequences were amplified in a 50-μL reaction volume containing 500 to 1000 ng of genomic DNA, 30 pmol of each primer, and 0.2 mmol/L of each nucleoside triphosphate in a reaction buffer consisting of 60 mmol/L Tris-hydrochloric acid (pH 9.0), 15 mmol/L ammonium sulfate, 2 mmol/L magnesium chloride, 1 U Taq polymerase (Perkin Elmer, Foster City, CA), and 4% (vol/vol) dimethyl sulfoxide. PCR amplification was performed in a programmable thermal cycler (model 9600; Perkin Elmer) for 35 cycles of 45 seconds of denaturation at 96°C, annealing for 1 minute at 60°C, and extension for 1 minute at 72°C. PCR products containing the entire coding regions of GPIbα, GPIbβ, and GPIX were cloned into the pCRII cloning vector by using the TA cloning kit (Invitrogen, San Diego, CA).
DNA sequencing
Direct sequence analysis of the entire coding region of PCR-amplified GPIbα, GPIbβ, and GPIX from the patient was performed with the Prism Ready Reaction DyeDeoxy terminator cycle sequencing kit and a DNA sequencer (model 373A; Applied Biosystems, Foster City, CA). Sequencing primers were synthesized on a DNA synthesizer (model 394; Applied Biosystems).
Fluorescent in situ hybridization (FISH)
FISH analysis was performed on G-banded metaphase chromosomes by using the D22S75 probe that maps to the region 22q11.2.24 25 This chromosomal region is frequently deleted in DiGeorge syndrome, velocardiofacial syndrome, and in platelets from patients with familial or isolated congenital heart defects.
Confocal imaging of platelets
Glass slides were coated with 100 μL fibrinogen (20 μg/mL) for 2 hours at 37°C and blocked with 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS; pH 7.4) for 1 hour in a humidified staining tray. Washed platelets (50 μL), prepared as described above, were diluted to 3.0 × 108/mL and allowed to adhere for 60 minutes at room temperature to the fibrinogen-coated glass slides. Nonadhered platelets were removed by washing in PBS (pH 7.4), and adhered platelets were either stained immediately or fixed and permeabilized in ice-cold methanol (7 minutes) followed by ice-cold acetone (2 minutes). Primary antibodies (1:50 dilutions of monoclonal LYP18 for GP IIb-IIIa or polyclonal anti-GC for GPIbα) were incubated for 45 minutes at room temperature for fixed preparations or for 90 minutes at 4°C for nonpermeabilized platelets. Goat antimouse Alexa 488-conjugated IgG or goat antirabbit Alexa 546 IgG was then added (Molecular Probes, Leiden, Netherlands). The immunostained slides were washed 3 times in PBS and mounted in fluorescent mounting medium (Dako, Carpinteria, CA) before imaging on a Zeiss Axioplan 2 confocal microscope with a 63 × (1.4 n/a) lens.
Immunoprecipitation of plasma GPIb
GPIbα mAb AP1 was coupled to cyanogen bromide–activated Sepharose 4B beads (Sigma, St Louis, MO). Platelet-poor plasma was precleared by incubating it with uncoupled Sepharose CL-4B beads for 1 hour at room temperature. The beads were centrifuged at 1000g, and the plasma was added to the antibody-coupled beads and incubated overnight at 4°C. The beads were washed, and the immunoprecipitated complexes from plasma were eluted in a 1 × nonreducing lane marking sample buffer (Pierce, Rockford, IL). All samples were boiled at 100°C for 3 minutes. The samples were then analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis on a 4% to 20% exponential gradient in the presence of 5% β-mercaptoethanol. The separated proteins were electroblotted onto a polyvinylidene fluoride membrane (Novex, San Diego, CA) and detected as described previously.11
Transient expression
For transient-expression studies, HEK 293T cells were used. The parent 293T cell line is a human renal epithelial cell transformed with SV40 large T antigen.26 The 293T cells were maintained at 37°C in a 5% carbon dioxide humidified chamber in modified Eagle medium (Sigma) supplemented with 10% fetal calf serum.
An XhoI/MluI restriction fragment containing the entire coding region for GPIbα was inserted into the mammalian expression vector pCI-Neo (Promega, Madison, WI). EcoRI restriction fragments containing the entire coding region of both GPIbβ and GPIX amplified from genomic DNA were inserted into pCI-Neo. Constructs containing wild-type GPIbα, GPIbβ, and GPIX, and mutant GPIbβ were sequenced to ensure that no additional mutations had been introduced and that they were inserted in the expression vector in the correct orientation. Expression plasmids were introduced into 293T cells in the presence of lipofectamine (Gibco BRL, Gaithersburg, MD).
Transient-expression studies were also performed in Chinese hamster ovary (CHO) α IX cells (kindly provided by Dr José A. López, Baylor College of Medicine, Houston TX). CHOαIX cells are CHO cells that stably express human GPIbα on their surface.16 When these cells are additionally transfected with GPIbβ, the surface expression of GPIX becomes readily detectable.27 Cells from this stable cell line were also transiently transfected with either the plasmid pCI-Neo alone, wild-type GPIbβ, or the construct containing the mutation in GPIbβ. Expression plasmids were introduced into CHOαIX cells in the presence of lipofectamine and lipofectamine plus (Gibco BRL) by following the protocol of Felgner et al.28 Briefly, either 1.5 × 106 CHOαIX cells or 4 × 106 293T cells were plated in 100-mm dishes and grown overnight. Then, 8 mL of OPTI-MEM–reduced serum medium (Gibco BRL) containing 36 μg lipofectamine and 6 μg of the appropriate plasmid DNA was added to the CHOαIX cells and 120 μg lipofectamine and 8 μg DNA was added to the 293T cells. After transfection and 5 hours of incubation, the transfection medium was removed, 8 mL of culture medium was added, and incubation was reinitiated at 37°C and continued for 60 hours.
Flow cytometry studies in transfected cells
Transfected cells were detached from tissue culture plates with 3 mmol/L EDTA, centrifuged at 250g, and resuspended in Hanks balanced salt solution with 1% BSA and 1% normal donkey serum. Then, 3 × 105 cells were transferred to each well of a 96-well V-bottomed plate (Dynatech, Chantilly, VA) and incubated with either a rabbit antiglycocalicin polyclonal antibody (5 μg/mL), the anti-IX mAb FMC-25 or GRP (5 μg/mL), or the complex-specific mAb AK1 (ascites 1:1200). The cells were washed twice and incubated for an additional 30 minutes in a darkened room with a 1:100 dilution of phycoerythrin-conjugated affinity-purified F(ab′)2 donkey antimouse or antirabbit IgG (Jackson Immunoresearch Laboratories, West Grove, PA). The cells were washed twice, resuspended in 2% paraformaldehyde, and incubated for at least 1 hour at 4°C before analysis on a flow cytometer (FACScan; Becton Dickinson).
Results
Platelet GPs
Platelet function studies showed normal aggregation in the presence of ADP (10 μmol/L), collagen (10 μg/mL), and thrombin receptor actuating peptide (TRAP) (10 μmol/L), but platelets failed to agglutinate in the presence of 1.2 mg/mL ristocetin. The diagnosis of BSS was confirmed by the presence of clinically insignificant binding of antibodies to GPIbα, GPIX, and GPV on flow cytometric analysis (Figure 1). Binding of LYP18 to the unrelated platelet GP, GPIIb-IIIa, was increased. This finding is consistent with other reports of BSS9 and probably reflects the larger size of the platelets. When calibration beads were used to estimate the number of sites per platelet, platelets from healthy controls were found to express 54 000 ± 7000 (n = 3) GPIIb-IIIa molecules on their surface, whereas platelets from the patient with BSS expressed 98 000 ± 10 000 sites per platelet (n = 3; Table 1). All these findings are consistent with a diagnosis of BSS.
Flow cytometric analysis of platelets from a patient with Bernard-Soulier syndrome (BSS).
Analysis was performed on whole blood with monoclonal antibodies (mAbs) against glycoprotein (GP) IIb-IIIa (A), GPIbα (B), GPIX (C), and GPV (D). As expected BSS patients' platelets (BSP), which are larger than normal, this patient's platelets (clear area) showed an increase in surface fluorescence compared with platelets from healthy controls (CTL, shaded area) in reaction to the anti-GPIIb-IIIa mAb LYP18. There was no detectable GPIbα (B) or GPIX (C) on the platelets of the patient (clear area), whereas both were present on control platelets (shaded area).
Flow cytometric analysis of platelets from a patient with Bernard-Soulier syndrome (BSS).
Analysis was performed on whole blood with monoclonal antibodies (mAbs) against glycoprotein (GP) IIb-IIIa (A), GPIbα (B), GPIX (C), and GPV (D). As expected BSS patients' platelets (BSP), which are larger than normal, this patient's platelets (clear area) showed an increase in surface fluorescence compared with platelets from healthy controls (CTL, shaded area) in reaction to the anti-GPIIb-IIIa mAb LYP18. There was no detectable GPIbα (B) or GPIX (C) on the platelets of the patient (clear area), whereas both were present on control platelets (shaded area).
Characteristics of platelets from healthy (control) donors and from a patient with Bernard-Soulier syndrome (BSS)
| Characteristic . | Control platelets . | BSS platelets . |
|---|---|---|
| Glycoprotein (GP) IIb-IIIa (sites/platelet) | 54 613 ± 6993 | 98 551 ± 9841 |
| GPIb (sites/platelet) | 33 891 ± 6600 | 21 ± 16 |
| GPIX (sites/platelet) | 30 566 ± 5030 | 67 ± 13 |
| GPV (sites/platelet) | 9046 ± 4156 | 224 ± 142 |
| Mean platelet diameter (μm) | 3.3 ± 0.13 | 5.3 ± 0.24 |
| Mean platelet height (μm) | 4.1 ± 0.77 | 6.6 ± 1.8 |
| Height–diameter ratio | 1:24 | 1:25 |
| Characteristic . | Control platelets . | BSS platelets . |
|---|---|---|
| Glycoprotein (GP) IIb-IIIa (sites/platelet) | 54 613 ± 6993 | 98 551 ± 9841 |
| GPIb (sites/platelet) | 33 891 ± 6600 | 21 ± 16 |
| GPIX (sites/platelet) | 30 566 ± 5030 | 67 ± 13 |
| GPV (sites/platelet) | 9046 ± 4156 | 224 ± 142 |
| Mean platelet diameter (μm) | 3.3 ± 0.13 | 5.3 ± 0.24 |
| Mean platelet height (μm) | 4.1 ± 0.77 | 6.6 ± 1.8 |
| Height–diameter ratio | 1:24 | 1:25 |
Platelet immunophenotyping was performed on whole blood with monoclonal antibodies against GPIIb-IIIa (LYP18), GPIbα (SZ2), GPIX (SZ1), and GPV (SW16). Samples were analyzed for fluorescent intensity by flow cytometry. Fluorescent intensity was translated into sites per platelet by using a calibration curve generated from beads coated with known densities of IgG molecules. The data represent mean ± SEM values in blood samples from 3 healthy donors (controls) or samples obtained from the BSS patient on 3 different days. Values for GPIb and GPIX density on platelets from the BSS patient were at the threshold of detection but significantly higher than background levels. Platelet size was determined by measuring the maximal diameter of platelets stained with Alexa 488–LYP18, spread on fibrinogen-coated glass slides, and imaged on a confocal fluorescent microscope. Platelet height was the depth of the adhered platelets calculated from z-sections. All the platelets in a microscopical field were measured to avoid selection bias. Data represent mean ± SEM values for 40 individual platelets obtained from 3 healthy donors or from the BSS patient on 3 different days.
Sequence analysis of GPIb-IX complex
Sequence analysis of the entire coding regions of the genes for GPIbα and GPIX identified no abnormalities. However, a novel G to A mutation in codon 21 of the gene for GPIbβ was observed. This mutation results in premature termination of protein synthesis at W21 by replacing a TGG codon specifying a tryptophan residue with a TAG codon that specifies termination of translation (Figure 2). BSS due to a single-allele mutation in GPIbβ was reported previously to be accompanied by a deletion in the DiGeorge-velocardiofacial chromosomal region in 22q11.2. The gene for GPIbβ is in the center of this region.29 Our patient had no clinical signs or symptoms of this syndrome. However, because the syndrome may be subtle, FISH studies were performed. Using G-banded metaphase chromosomes, we detected no deletion on either of the chromosome 22 homologues by FISH analysis with a probe (D22S75) that mapped to the region 22q11.2 (data not shown).
Mutation in GPIbβ.
DNA-sequence analysis of GPIbβ from a healthy volunteer (A) and the patient with BSS (B). Sequence analysis of DNA amplified by polymerase chain reaction showed that nucleotide 159, part of a TGG codon, was replaced by an A, yielding a TAG stop signal.
Mutation in GPIbβ.
DNA-sequence analysis of GPIbβ from a healthy volunteer (A) and the patient with BSS (B). Sequence analysis of DNA amplified by polymerase chain reaction showed that nucleotide 159, part of a TGG codon, was replaced by an A, yielding a TAG stop signal.
Confocal microscopical analysis of BSS platelets
Initial studies to investigate the diagnosis of BSS included analysis of platelet characteristics. Platelet size was determined by assessing the maximal diameter of a random selection of 40 normal platelets and 40 platelets from the patient, imaged by confocal microscopy. All the platelets in a field were measured to avoid the possibility of selection bias. The mean (± SD) diameter of the BSS platelets was 5.3 ± 1.5 μm (range, 3.1-9.1 μm), whereas that of control platelets was 3.3 ± 0.8 μm (range, 1.3-5.7 μm) (Table1). Interestingly, the BSS platelets and the platelets from healthy volunteers adhered to and spread on the immobilized fibrinogen in an identical manner. As a measure of cell spreading, we estimated the ratio of height to diameter for a random selection of platelets (Table1), with selection bias ruled out by including all platelets in a field. The ratio in the 2 platelet populations was almost identical, indicating that spreading on fibrinogen, a function of the GPIIb-IIIa integrin adhesion protein, was normal in our patient.
Normal platelets plated on fibrinogen-coated glass slides were stained for GPIbα (anti-GC) and for the integrin GPIIb-IIIa (CD41). These platelets showed normal surface expression of both antigens on fluorescent microscopical imaging (Figure3A-B). BSS platelets also stained normally for GPIIb-IIIa on their surface and in their cytoplasm (Figure 3H,K). This staining showed a focal distribution, consistent with activation of the platelet by the immobilized fibrinogen substrate. However, staining for GPIbα was absent in these platelets (Figure 3G). In contrast, when platelets were permeabilized before staining, GPIbα staining was readily detectable in the BSS platelets, though not with a distribution equivalent to that of GPIIb-IIIa, suggesting a cytosolic distribution (Figure 3J). To investigate a role for GPIbβ in intracellular complex formation, we used fluorescent microscopy to determine whether GPIbα and GPIX formed a complex in the BSS platelets in the absence of functional GPIbβ. The antibody AKI recognizes the GPIbα-GPIX complex but not its individual components alone.13 AK1 failed to bind to either permeabilized or nonpermeabilized BSS platelets but did bind substantially to normal platelets (Figure 4). These data show that the expression of GPIbα-GPIX complex is dependent on functional GPIbβ. The fluorescent images of GPIbα staining in the BSS platelets demonstrated a circumferential pattern, suggesting that GPIbα is transported toward the platelet membrane even in the absence of an association with GPIbβ or GPIX. Flow cytometric analysis of the BSS platelets (Figure 1 and Table 1) found minimal expression of GPIbα on the surface of BSS platelets, indicating that there is a residual capacity for this protein to be expressed alone on the cell surface.
Fluorescent imaging of platelet GPs.
Washed platelets from healthy volunteers (A-F) and the patient with BSS (G-L) were plated onto glass slides precoated with fibrinogen (20 μg/mL) and allowed to adhere for 1 hour at room temperature. Platelets were then either stained directly (A-C and G-I) or permeabilized with ice-cold methanol and acetone (D-F and J-L) before staining. Slides were dual stained for GPIbα with a rabbit anti-GC polyclonal antibody and for GPIIb-IIIa with a mouse mAb, LYP18. Platelets were imaged on a Zeiss Axioplan II confocal microscope with a 63 × oil immersion lens (1.4 n/a). The platelets were permeabilized in the images shown in A to C and G to I to allow antibody probes to react with cytoplasmic epitopes. Data are presented as triplets of images separated to show GPIbα staining with an Alexa 546–conjugated anti-rabbit antibody (red: A, D, G, and J), GPIIb-IIIa staining with Alexa 488 antimouse antibody (green: B, E, H, and K), or the confocal image showing both colors (C, F, I, and L). One image is shown for each treatment; this is representative of 3 independent experiments in which up to 50 platelets were analyzed. GPIbα was present in permeabilized and nonpermeabilized normal platelets (A, D) and permeabilized BSS platelets (J) but absent from the surface of nonpermeabilized preparations of the BSS platelets (G). In contrast, the platelet integrin GPIIb-IIIa was present on the surface of both normal and BSS platelets, regardless of permeabilizing treatment of the cell membrane (B, E, H, and K).
Fluorescent imaging of platelet GPs.
Washed platelets from healthy volunteers (A-F) and the patient with BSS (G-L) were plated onto glass slides precoated with fibrinogen (20 μg/mL) and allowed to adhere for 1 hour at room temperature. Platelets were then either stained directly (A-C and G-I) or permeabilized with ice-cold methanol and acetone (D-F and J-L) before staining. Slides were dual stained for GPIbα with a rabbit anti-GC polyclonal antibody and for GPIIb-IIIa with a mouse mAb, LYP18. Platelets were imaged on a Zeiss Axioplan II confocal microscope with a 63 × oil immersion lens (1.4 n/a). The platelets were permeabilized in the images shown in A to C and G to I to allow antibody probes to react with cytoplasmic epitopes. Data are presented as triplets of images separated to show GPIbα staining with an Alexa 546–conjugated anti-rabbit antibody (red: A, D, G, and J), GPIIb-IIIa staining with Alexa 488 antimouse antibody (green: B, E, H, and K), or the confocal image showing both colors (C, F, I, and L). One image is shown for each treatment; this is representative of 3 independent experiments in which up to 50 platelets were analyzed. GPIbα was present in permeabilized and nonpermeabilized normal platelets (A, D) and permeabilized BSS platelets (J) but absent from the surface of nonpermeabilized preparations of the BSS platelets (G). In contrast, the platelet integrin GPIIb-IIIa was present on the surface of both normal and BSS platelets, regardless of permeabilizing treatment of the cell membrane (B, E, H, and K).
GPIbα-IX complex formation in BSS platelets.
Washed platelets from healthy volunteers (A-D) and the patient with BSS (E-H) were plated onto glass slides precoated with fibrinogen (20 μg/mL) and allowed to adhere for 1 hour at room temperature. Platelets were then either permeabilized with ice-cold methanol and acetone before staining (A, B and E, F) or stained directly without permeabilizing (C, D and G, H). Slides were stained for GPIbα-IX with the mAb AK1. Platelets were imaged in parallel in fluorescent (A, C, E, and G) and differential-interference contrast (DIC) mode (B, D, F, and H). The platelets were permeabilized to allow antibody probes to react with cytoplasmic epitopes. Data are presented as paired images separated to show AK1 staining with an Alexa 546–conjugated antimouse antibody or DIC images to indicate the platelet position in the nonstaining cells. One image is shown for each treatment; this is representative of 3 independent experiments in which more than 20 platelets were analyzed. GPIbα-IX complex was present in the cytoplasm and on the surface of normal platelets (A, C) but absent from BSS platelets (E, G). The DIC images show the larger size of the BSS platelets.
GPIbα-IX complex formation in BSS platelets.
Washed platelets from healthy volunteers (A-D) and the patient with BSS (E-H) were plated onto glass slides precoated with fibrinogen (20 μg/mL) and allowed to adhere for 1 hour at room temperature. Platelets were then either permeabilized with ice-cold methanol and acetone before staining (A, B and E, F) or stained directly without permeabilizing (C, D and G, H). Slides were stained for GPIbα-IX with the mAb AK1. Platelets were imaged in parallel in fluorescent (A, C, E, and G) and differential-interference contrast (DIC) mode (B, D, F, and H). The platelets were permeabilized to allow antibody probes to react with cytoplasmic epitopes. Data are presented as paired images separated to show AK1 staining with an Alexa 546–conjugated antimouse antibody or DIC images to indicate the platelet position in the nonstaining cells. One image is shown for each treatment; this is representative of 3 independent experiments in which more than 20 platelets were analyzed. GPIbα-IX complex was present in the cytoplasm and on the surface of normal platelets (A, C) but absent from BSS platelets (E, G). The DIC images show the larger size of the BSS platelets.
Soluble GPIb in the plasma
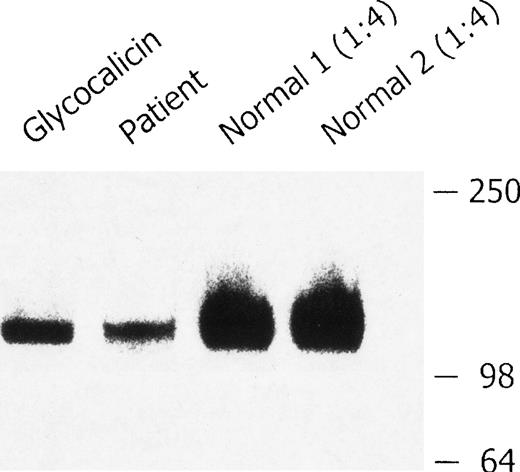
Because GPIbα was readily detectable in the patient's platelets but not on their surface, we hypothesized that circulating, soluble GPIbα might be detectable in the plasma. There was less soluble GPIbα in plasma from the patient than in that from healthy volunteers (Figure 5). This finding is consistent with the reduced number of platelets in the patient's blood and the reduced surface expression of the GP complex.
Western blot analysis of plasma GPIb.
Platelet-poor plasma from 2 healthy volunteers and the patient with BSS was immunoprecipitated with antibody AP1, analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on a 4% to 20% gradient gel in the presence of β-mercaptoethanol, and immunoblotted with anti-GPIbα monoclonal antibody MCB142.11. The glycocalicin-positive control (3.8 ng) is shown in lane 1; BSS plasma is shown in lane 2. The control plasma samples were diluted (1:4) before SDS-PAGE to avoid overloading of the gel (lanes 3 and 4). There was less soluble GPIbα in the plasma from the patient with BSS.
Western blot analysis of plasma GPIb.
Platelet-poor plasma from 2 healthy volunteers and the patient with BSS was immunoprecipitated with antibody AP1, analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on a 4% to 20% gradient gel in the presence of β-mercaptoethanol, and immunoblotted with anti-GPIbα monoclonal antibody MCB142.11. The glycocalicin-positive control (3.8 ng) is shown in lane 1; BSS plasma is shown in lane 2. The control plasma samples were diluted (1:4) before SDS-PAGE to avoid overloading of the gel (lanes 3 and 4). There was less soluble GPIbα in the plasma from the patient with BSS.
Expression of GPIb-IX complex
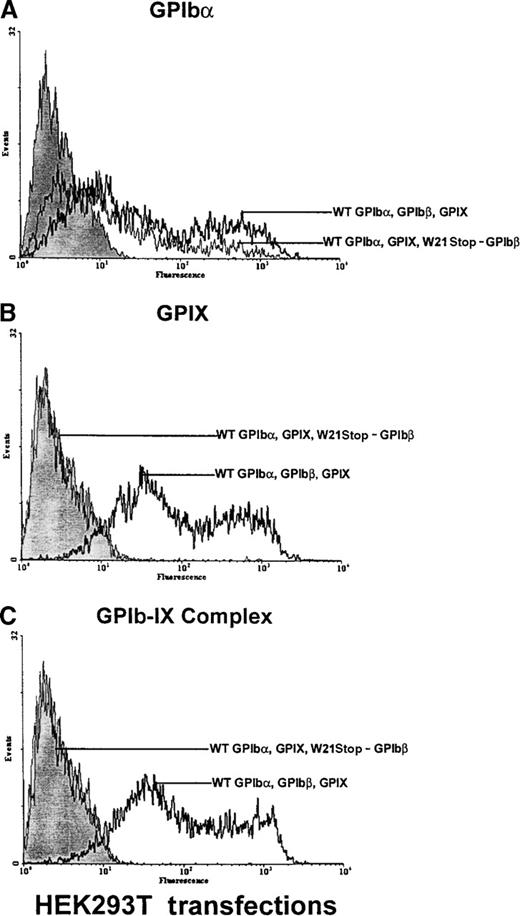
To test the hypothesis that the observed mutation in the GPIbβ gene was responsible for the defects in the expression of the GPIb-IX complex on the platelet surface, the effect of this mutation was evaluated in HEK 293T cells transiently transfected with either wild-type GPIbβ or W21stop-GPIbβ. GPIbα, GPIbβ, and GPIX are all required for efficient expression of the ligand-binding entity on the surface of transfected cells.30 Therefore, to investigate the effects of W21stop-GPIbβ on complex expression by the cells, plasmids encoding GPIbα, GPIbβ, and GPIX were transiently transfected into HEK 293T cells. Transient coexpression of normal GPIbβ in HEK 293T cells with wild-type GPIbα and GPIX resulted in enhanced surface expression of GPIbα and expression of GPIX (Figure6). In contrast, the mutant W21stop-GPIbβ gave rise to a lesser expression of GPIbα and failed to support GPIX expression. In these cells, in the presence of wild-type GPIbβ—but not W21stop-GPIbβ—GPIbα and GPIX were expressed as a complex on the platelet surface and recognized by a complex-specific antibody, AKI. Thus, it appears that wild-type GPIbβ facilitates the transport and expression of GPIbα and GPIX on the cell surface but W21stop-GPIbβ does not achieve this function.
Analysis of GPIb and GPIX in HEK 293T cells transfected with GPIb, GPIbβ, and GPIX.
HEK 293T cells were transiently transfected with wild-type GPIbα, GPIbβ, and GPIX or with wild-type GPIbα and GPIX and mutant W21stop-GPIbβ. The cells were analyzed with an anti-GPIbα polyclonal antibody, the anti-GPIX antibody FMC25, or the complex-specific antibody AK1 (each graph is representative of 4 different experiments). (A) In cells transfected with wild-type GPIbα, GPIbβ, and GPIX, there was a significant increase in fluorescence in cells in reaction to the anti-GPIbα polyclonal antibody (boldface lines) compared with mock-transfected cells (shaded area). In cells transfected with W21stop-GPIbβ and wild-type GPIbα and GPIX, GPIbα was detectable on the cell surface (thin line) but in significantly smaller amounts than in triple wild-type transfections. (B) In cells transfected with wild-type GPIbα, GPIbβ, and GPIX, GPIX was readily detectable on the cell surface (boldface line). However, GPIX was not detectable when W21stop-GPIbβ was transfected with wild-type GPIbα and GPIX (thin line). Mock-transfected cells are shown for comparison (shaded area). (C) There was a marked increase in surface fluorescence in cells transfected with wild-type GPIbα, GPIbβ, and GPIX in reaction to the complex-specific antibody, AK1 (boldface lines), compared with mock-transfected cells (shaded area) and cells transfected with wild-type GPIbα, GPIX, and mutant GPIbβ (thin line). Thus, GPIbα that was expressed on the cell surface (A) in the transfections involving the mutant GPIbβ was not recognized by AK1, thereby confirming the lack of surface expression of GPIX or complex formation.
Analysis of GPIb and GPIX in HEK 293T cells transfected with GPIb, GPIbβ, and GPIX.
HEK 293T cells were transiently transfected with wild-type GPIbα, GPIbβ, and GPIX or with wild-type GPIbα and GPIX and mutant W21stop-GPIbβ. The cells were analyzed with an anti-GPIbα polyclonal antibody, the anti-GPIX antibody FMC25, or the complex-specific antibody AK1 (each graph is representative of 4 different experiments). (A) In cells transfected with wild-type GPIbα, GPIbβ, and GPIX, there was a significant increase in fluorescence in cells in reaction to the anti-GPIbα polyclonal antibody (boldface lines) compared with mock-transfected cells (shaded area). In cells transfected with W21stop-GPIbβ and wild-type GPIbα and GPIX, GPIbα was detectable on the cell surface (thin line) but in significantly smaller amounts than in triple wild-type transfections. (B) In cells transfected with wild-type GPIbα, GPIbβ, and GPIX, GPIX was readily detectable on the cell surface (boldface line). However, GPIX was not detectable when W21stop-GPIbβ was transfected with wild-type GPIbα and GPIX (thin line). Mock-transfected cells are shown for comparison (shaded area). (C) There was a marked increase in surface fluorescence in cells transfected with wild-type GPIbα, GPIbβ, and GPIX in reaction to the complex-specific antibody, AK1 (boldface lines), compared with mock-transfected cells (shaded area) and cells transfected with wild-type GPIbα, GPIX, and mutant GPIbβ (thin line). Thus, GPIbα that was expressed on the cell surface (A) in the transfections involving the mutant GPIbβ was not recognized by AK1, thereby confirming the lack of surface expression of GPIX or complex formation.
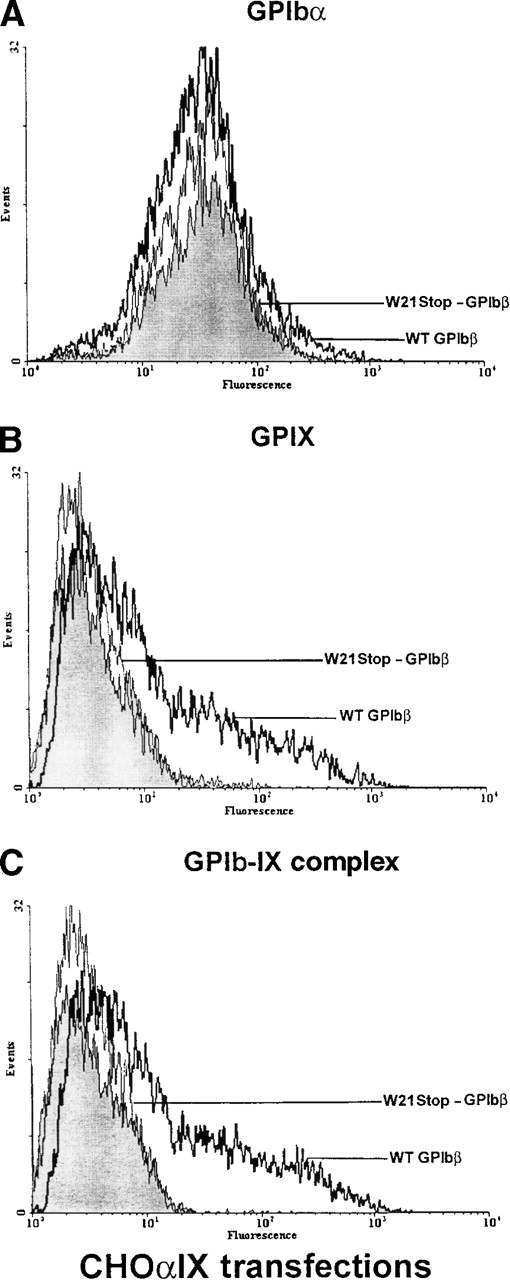
Similar results were obtained when plasmids encoding wild-type GPIbβ were transiently transfected into CHOαIX cells stably expressing GPIbα (ie, CHO cells that retain the capacity to re-express GPIX). There was a significant increase in the surface expression of GPIX and in the binding of the complex-specific antibody. In contrast, when W21stop-GPIbβ was transiently transfected into these cells, GPIX was not re-expressed on the plasma surface (Figure7), thereby confirming a role for wild-type GPIbβ in maintaining functional GPIb-IX complex on the cell surface.
Analysis of GPIb and GPIX in Chinese hamster ovary (CHO) IX cells transiently transfected with GPIbβ.
(A) CHOαIX cells expressing GPIbα and GPIX were additionally transfected with wild-type GPIbβ or the mutant W21stop-GPIbβ or were mock transfected with the expression plasmid alone. GPIbα was readily detectable in cells transfected with plasmid alone (shaded area), the mutant W21stop-GPIbβ (thin lines), or wild-type GPIbβ (boldface lines). (B) There was a significant increase in the surface expression of GPIX when wild-type GPIbβ (boldface lines) was transfected into CHOαIX cells compared with mock-transfected controls (shaded area) or cells transfected with the mutant W21stop-GPIbβ (thin line). (C) CHOαIX cells transfected with wild-type GPIbβ (boldface lines) were allowed to react with the complex-specific antibody AK1. Again, there was a marked increase in surface fluorescence compared with either the mock-transfected cells (shaded area) or the cells transfected with W21stop-GPIbβ (thin lines). Thus, the mutant GPIbβ failed to support the efficient expression of the GPIbα-IX complex on the surface of these cells.
Analysis of GPIb and GPIX in Chinese hamster ovary (CHO) IX cells transiently transfected with GPIbβ.
(A) CHOαIX cells expressing GPIbα and GPIX were additionally transfected with wild-type GPIbβ or the mutant W21stop-GPIbβ or were mock transfected with the expression plasmid alone. GPIbα was readily detectable in cells transfected with plasmid alone (shaded area), the mutant W21stop-GPIbβ (thin lines), or wild-type GPIbβ (boldface lines). (B) There was a significant increase in the surface expression of GPIX when wild-type GPIbβ (boldface lines) was transfected into CHOαIX cells compared with mock-transfected controls (shaded area) or cells transfected with the mutant W21stop-GPIbβ (thin line). (C) CHOαIX cells transfected with wild-type GPIbβ (boldface lines) were allowed to react with the complex-specific antibody AK1. Again, there was a marked increase in surface fluorescence compared with either the mock-transfected cells (shaded area) or the cells transfected with W21stop-GPIbβ (thin lines). Thus, the mutant GPIbβ failed to support the efficient expression of the GPIbα-IX complex on the surface of these cells.
These results suggest that in the absence of normal GPIbβ, the larger α subunit is not maintained on the platelet surface but is released into the plasma. Furthermore, GPIbα-IX complex formation and surface expression are impaired in the absence of functional GPIbβ.
Discussion
We observed a novel, homozygous mutation in the gene for GPIbβ that results in BSS. The single base substitution converts the TGG sequence at codon 21 of the GPIbβ gene to TAG, which causes a premature termination of translation. Because the patient's parents had a consanguineous marriage, the patient might be predicted to be homozygous. However, 2 other reports of BSS due to mutations in GPIbβ identified a single-allele mutation accompanied by a partial chromosomal deletion at 22q11.2.11,31 This deletion gives rise to a clinical condition known as DiGeorge-velocardiofacial syndrome and is often referred to as the DiGeorge chromosomal region. The GPIbβ gene has been localized to position q11.2 on chromosome 2232,33 and is in the middle of the DiGeorge region.34 We therefore assessed our patient for deletion of the DiGeorge region of chromosome 22 by FISH analysis using a D22S75 probe specific for this region. We found a normal male karyotype with dual labeling of both chromosomes 22.
There are 3 other reports of BSS resulting from defects in GPIbβ synthesis.11,29,35 In 2 of these, the mutations were associated with macrodeletions of chromosome 22q11.2, the locus for the GPIbβ gene.11,29 The third patient was a compound heterozygote with 2 independent mutations at amino acid positions 88 and 108 of the GPIbβ gene.35 This patient was described as having a variant form of BSS in which giant platelets were present but neither thrombocytopenia nor a definite tendency to have abnormal bleeding was observed. Notably, however, there was a reduced density of GPIb-IX complexes on the platelet surface and absence of disulfide linkage between the GPIbα and GPIbβ subunits.
All these reports confirm a critical role for GPIbβ in the functioning of the vWF receptor on the platelet surface. However, the precise role of GPIbβ in the GPIb-IX complex remains elusive. The ligand-binding features of the complex reside in the sequence of GPIbα. This large GP protein contains the binding sites for vWF and α-thrombin in its large extracellular domain, and a serine phosphorylation site36 and a binding site for 14-3-33,37 have been identified in its cytoplasmic domain. Thus, it has been suggested that the role of the other GPs is in the successful assembly and transport of the intact GPIb-IX-V complex to the platelet surface. Indeed, mutations in GPIX, which give rise to BSS, were shown to adversely affect the stability of the GPIb-IX-V complex on the platelet surface by inhibiting the association of GPIX with GPIbβ. Furthermore, in vitro studies examining the biosynthesis and assembly of the individual components of the GPIb-IX-V complex in stably transfected CHO cells confirmed an essential role for GPIX and GPIbβ in the assembly of the complex.27 38 In this study, we found that GPIbα was virtually absent from the surface of platelets from our patient with BSS but present in the platelet cytoplasm and detectable in plasma. These findings confirm a role for GPIbβ in the stability of the GPIb-IX complex on the platelet surface.
GPIbα and GPIbβ are normally held together by an intermolecular disulfide bond.27 The W21stop mutation in GPIbβ reported here cannot sustain this disulfide bridge and is therefore not disulfide linked to GPIbα in the membrane. Similar results occur when GPIbβ is mutated at Ala80.11 Furthermore, our studies in which W21stop-GPIbβ was cotransfected into HEK 293T cells with wild-type GPIbα and GPIX demonstrated a similar lack of surface expression of the intact complex. Identical results were obtained when the stable cell line CHOαIX was transiently transfected with the mutant GPIbβ. The fluorescent images of GPIbα staining in the BSS platelets demonstrated a circumferential pattern, suggesting that GPIbα is transported to the platelet membrane even in the absence of an association with GPIbβ or GPIX. Flow cytometric analysis of the BSS platelets (Figure 1 and Table 1) showed some residual expression of GPIbα on the surface of those platelets, indicating a capacity for this protein to be expressed alone on the cell surface. This may explain why the CHOαIX cells and the HEK cells transfected with GPIbα had some surface expression of GPIbα, even in the absence of a functional GPIbβ.
Our study demonstrates that in the absence of a functional GPIbβ, GPIbα can be synthesized by BSS platelets. The fluorescent images of GPIbα staining in the BSS platelets showed that GPIbα is protected from proteolytic degradation and is transported to the platelet membrane. There, it is either not inserted into the membrane (which would result in a complete absence of membrane expression) or it is inserted and then shed (resulting in transient surface expression). Our detection of soluble GPIbα in the patient's plasma lends support to the latter alternative. The data also suggest that GPIbβ acts in normal platelets to stabilize the GPIbα-IX complex in the platelet cytoplasm and to enhance its tenure in the platelet membrane.
To our knowledge, this is the first report of a novel, homozygous mutation in the gene for GPIbβ. This mutation affects the synthesis of GPIbβ and the expression and functions of the vWF receptor, the GPIb-IX complex. The mutation also results in the absence of the surface expression of the GPIb-IX complex on platelets and the presence of circulating soluble GPIbα, and it accounts for the BSS phenotype.
Supported in part by grants from the Higher Education Authority (Ireland), the Irish Heart Foundation, the RCSI Research Trust, and the Wellcome Trust, and by US Public Health Service grant HL56027.
Reprints:Dermot Kenny, Department of Clinical Pharmacology, Royal College of Surgeons in Ireland, 123 St Stephen's Green, Dublin 2, Ireland; e-mail: dkenny@rcsi.ie.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal