Abstract
CD33 is a myeloid specific member of the sialic acid-binding receptor family and is expressed highly on myeloid progenitor cells but at much lower levels in differentiated cells. Human CD33 has two tyrosine residues in its cytoplasmic domain (Y340 and Y358). When phosphorylated, these tyrosines could function as docking sites for the phosphatases, SHP-1 and/or SHP-2, enabling CD33 to function as an inhibitory receptor. Here we demonstrate that CD33 is tyrosine phosphorylated in the presence of the phosphatase inhibitor, pervanadate, and recruits SHP-1 and SHP-2. Co-expression studies suggest that the Src-family kinase Lck is effective at phosphorylating Y340, but not Y358, suggesting that these residues may function in the selective recruitment of adapter molecules and have distinct functions. Further support for overlapping, but nonredundant, roles for Y340 and Y358 comes from peptide-binding studies that revealed the recruitment of both SHP-1 and SHP-2 to Y340 but only SHP-2 to Y358. Analysis using mutants of SHP-1 demonstrated that binding Y340 of CD33 was primarily to the amino Src homology-2 domain of SHP-1. The potential of CD33 to function as an inhibitory receptor was demonstrated by its ability to down-regulate CD64-induced calcium mobilization in U937. The dependence of this inhibition on SHP-1 was demonstrated by blocking CD33-mediated effects with dominant negative SHP-1. This result implies that CD33 is an inhibitory receptor and also that SHP-1 phosphatase has a significant role in mediating CD33 function. Further studies are essential to identify the receptor(s) that CD33 inhibits in vivo and its function in myeloid lineage development.
CD33 is a 67 kd transmembrane cell surface glycoprotein receptor that is specific for the myeloid lineage. Its expression is not seen in the earliest pluripotent progenitor cells, but it is expressed highly on cells committed to the myeloid lineage.1 CD33 expression is down-regulated with development of the myeloid lineage, resulting in low-level expression on peripheral granulocytes and tissue macrophages.2
Consistent with its expression in the myeloid lineage, CD33 is highly expressed in, and considered a marker of, acute myeloid leukemias.3 In fact, several recent studies have utilized toxin-conjugated CD33 monoclonal antibodies to mediate lysis of myeloid leukemia cells in vivo.4 In addition, CD33 × CD64 bispecific antibodies have been used to enhance the lysis of leukemic cells by cytokine-activated monocytes.5 Despite more than 15 reports of anti-CD33-based therapies, the biological significance of the high expression of CD33 in acute myeloid leukemias and normal myeloid progenitors remains unclear.
Biochemically, CD33 belongs to a growing family of sialic acid-binding, immunoglobulin-like lectins (siglecs), eight of which have been identified thus far.6 Sialoadhesin,7CD22,8 and CD339 are classified as siglecs 1-3, siglecs 4a and 4b are myelin-associated glycoprotein (MAG)10 and Schwann cell myelin protein (SMP),11 and Siglecs 5, 7, and 8 have been recently described.12-15 The siglecs recognize sialylated glycoproteins as ligands. They are sialic acid-dependent adhesion receptors, each recognizing differently linked terminal sialic acids on glycoproteins. CD22 requires a 2,6-linked sialic acid on ligands and CD33, MAG, SMP, and sialoadhesin all require a 2,3-linked sialic acid for recognition and binding to the ligands.16 Each of the members of the siglec family has an amino-terminal V-set immunoglobulin domain followed by various numbers of C3-set immunoglobulin domains.6 In addition to the various numbers of immunoglobulin-like domains and diverse patterns of expression, these proteins also have different numbers of tyrosine-containing motifs within their cytoplasmic domains, suggesting differences in cellular function. For example, siglec 1 (Sialoadhesin) has a total of 17 immunoglobulin-like domains but no cytoplasmic tyrosine residues in the immunoreceptor tyrosine-based inhibitory motif (ITIM) configuration, whereas CD33 has two immunoglobulin-like domains and two cytoplasmic tyrosines. The most studied siglec, (CD22), has six tyrosine residues in its cytoplasmic domain.17 Detailed analysis of CD22 has demonstrated that it functions largely as an inhibitor of B-cell activation and that its inhibitory activity is mediated by the four cytoplasmic tyrosine residues found within ITIMs. These motifs, defined by the sequence I/VxYxxL/I, become tyrosine phosphorylated on CD22 ligation. Tyrosine-phosphorylated CD22 is then capable of recruiting cytoplasmic phosphatases that, in turn, act in suppressing B-cell activation.18
Interestingly, subtle differences in ITIM sequences can result in the differential recruitment of phosphatases by various receptor systems, presumably because of differences in the binding specificity of the phosphatase SH2 domains. Therefore, some ITIMs recruit the Src homology (SH) 2 domain-containing protein tyrosine phosphatases SHP-1 and SHP-2, whereas others bind the inositol polyphosphate 5′-phosphatase, SHIP, and some bind all three.19
In addition to CD22, a large number of receptors have now been identified as “inhibitory receptors” because of the presence of ITIMs in their cytoplasmic domains and/or their demonstrated ability to inhibit cellular activation or to recruit phosphatases. Some of the best known of these are the killer cell immunoglobulin-like receptors (KIRs)20 that act to inhibit the natural killer cell and T-cell activation, FcγRIIB,21 an inhibitory receptor in B cells, and the paired immunoglobulin-like receptors type B (PIR-B) of B cells and myeloid cells.22 Current models suggest that these receptors are co-ligated to activation receptors, mainly those that utilize ITAMs. The protein tyrosine kinases activated by the ITAM-containing receptor system lead to the phosphorylation of ITIM tyrosines of the inhibitory receptor.23 Therefore, the presence of ITIM-like sequences in the cytoplasmic tail of CD33 makes it an excellent candidate as an inhibitory receptor. However, it is unknown whether CD33 functions as an inhibitory receptor and, if so, what are its target receptor systems within myeloid precursors.
Here we have tested the ability of CD33 to become phosphorylated on tyrosine residues, recruit phosphatases, and negatively regulate cell activation. We show that after phosphorylation, CD33 is capable of recruiting the protein tyrosine phosphatases SHP-1 and SHP-2. Interestingly, phosphopeptide-binding data suggest that the tyrosine motifs of CD33 may serve distinct functions. In addition, for the first time, we demonstrate the ability of CD33 to function as an inhibitory receptor by co-ligating it with CD64. These studies demonstrate the first example of CD33 mediation of intracellular inhibitory signaling and suggest a biochemical basis for the differential conservation of tyrosine motifs within the siglec family.
Materials and methods
Cells and antibodies
U937, THP-1, and Mo7e cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mmol/Ll-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. A biotinylated antiphosphotyrosine monoclonal antibody, 4G10, and rabbit anti-SHP-1 were purchased from Upstate Biotechnology, Inc (Lake Placid, NY). Anti-SHP-2, anti-CD33, and anti-CD64 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) and rabbit anti-goat IgG were purchased from Boehringer Mannheim (Indianapolis, IN). Mouse IgG1, used as an isotype control for anti-hCD33, was obtained from Pharmingen (San Diego, CA). Additional, anti-CD33 antibodies (Clones 1C7/1 and 3D6/1) were provided by the Imperial Cancer Research Fund, London, U.K.
Peptides
Biotinylated and nonbiotinylated as well as phosphorylated and nonphosphorylated peptides that spanned the tyrosines (Y340 and Y358) of CD33 were synthesized by Chiron Biotechnologies (Raleigh, NC). Each peptide was constructed with an additional four nonspecific amino acids (SGSG) at the amino terminus for anchoring to the biotin moiety. Peptide sequences were as follows: (1) SGSGDTSTEYSEVERT, (2) SGSGDTSTEpYSEVERT, (3) SGSGDEELHYASLNF, (4) SGSGDEELHpYASLNF, and (5) SGSGGHDGLpYQGLST. Peptide 5 is a TcR zeta chain used as a negative control. Each peptide was dissolved in DMSO at a concentration of 10 mg/mL, and 10 μg of this stock was used per milliliter of the cell lysates for peptide capture.
Mutagenesis and cDNA constructs
The complementary DNA (cDNA) for CD33 was a gift from Dr Brian Seed (Department of Molecular Biology, Massachusetts General Hospital, Boston, MA).2 The cDNA was cloned into pcDNA3 vector and site-directed mutagenesis performed with the use of the Transformer Site-Directed Mutagenesis Kit as per the vendor's instructions (Clonetech Laboratories, Palo Alto, CA). The two mutagenic primers used for the mutation of the tyrosines in the ITIMs to phenylalanine are 5′GAG CTG CAT TTT GCT TCC CTC 3′ (Y340 containing ITIM) and 5′ CTC CAC CGA ATT CTC AGA GGT C 3′ (Y358 containing ITIM). Three different mutants were prepared. In the first mutant, Y340F, the tyrosine at position 340 was changed to phenylalanine. In the second mutant, Y358F, the tyrosine at position 358 was changed to phenylalanine; and, in the third mutant (Y340F/Y358F), both tyrosines were changed to phenylalanine. The mutants were verified by sequencing.
Expression plasmids for Lck and Kinase dead Lck (LckK273R), Syk and kinase dead Syk (SykK395R), and Zap70 and kinase dead Zap-70 (Zap70K369R) were provided by Dr Andre Veillette (McGill University, Montreal, Canada), Dr Robert Geahlen (Purdue University, Indianapolis IN), and Dr Ronald Wange (National Institute of Aging, Baltimore, MD), respectively. SHP-1 expression constructs were the gift of Dr Taolin Yi (Cleveland Clinic, Cleveland, OH).
Immunoprecipitations and Western blotting
Cells were cultured in RPMI-1640 supplemented with 10% fetal calf serum (FCS) until they reached the mid-log phase when they were harvested. The cells were washed once in RPMI-1640 medium supplemented with 10% FCS and suspended in 1 mL of the media at a concentration of 1 × 106/mL or 10 × 106/mL. The cells were maintained at 37°C with shaking. The cells were stimulated with 1 mmol/L pervanadate for 10 minutes. The cells were washed once with ice-cold phosphate-buffered saline (PBS) containing 1 mmol/L Na3VO4 and then lysed on ice for 10 minutes in Triton X-100 lysis buffer (25 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 1% Triton X-100, 10 mmol/L NaF, 1 mmol/L Na3VO4, 1 mmol/L phenylmethylsulfonyl fluoride, and 10 μg/mL each leupeptin and aprotinin). The cell lysate was clarified by centrifugation at 15 000 rpm in a refrigerated microcentrifuge. The clarified lysates were incubated for 4 hours at 4°C with protein G-Sepharose that had the specific antibody bound. The sepharose beads were collected and washed four times with Triton X-100 containing lysis buffer. For immunoblotting, whole-cell lysates or immunoprecipitates were separated by SDS-PAGE and transferred to a PVDF membrane. The membranes were blocked with 5% nonfat dry milk in PBS/0.1%Tween overnight at 4°C. The membranes were probed with the primary antibodies (anti-phosphotyrosine, anti-SHP-1, anti-SHP-2) for 1 hour at room temperature. The bound antibodies were detected with horseradish peroxidase-conjugated secondary antibodies and detected with the use of the ECL system (Amersham Pharmacia, Piscataway, NJ), according to the manufacturer's protocol.
Expression studies
For co-expression studies, 293T epithelial cells were transfected with 0.5 μg/well of CD33 and 0.5 μg/well of kinase cDNA. Control cells were transfected with CD33 alone. The cDNA content of each transfection was balanced, using an empty expression construct (pCDNA3). Twenty-four hours after transfection, the cells were harvested and tested for CD33 expression with the use of flow cytometry. Transfections typically resulted in 80%-90% expression of CD33 (data not shown). Cells were lysed in Triton X-100 lysis buffer, immunoprecipitated with anti-CD33, and immunoblotted with antiphosphotyrosine as above. Parallel blots were probed with anti-CD33.
For SHP-1 expression studies, 293T cells were transfected with expression constructs encoding either wild-type SHP-1 or SHP-1 carrying point mutations within either the amino SH2 domain (SHP-1R30K) or carboxyl SH2 domain (SHP-1R136K). Twenty-four hours later, the cells were harvested and lysed, and SHP-1 expression was tested by immunoblot. Equal amounts of each isoform of SHP-1 was then precipitated with phosphopeptide-loaded beads as above. After washing, bound SHP-1 was eluted with sample buffer and detected by immunoblot. An aliquot of each isoform was loaded to demonstrate equal availability of SHP-1.
Vaccinia infections
The vaccinia constructs pSC65 and SHP-1S235C were the gifts of Dr Deborah Burshtyn (NIAID, Rockville, MD). The Myc-Zap-70K369R vaccinia was the gift of Dr Paul Leibson (Mayo Clinic, Rochester, MN). Vaccinia virus stocks were maintained and propagated as described.24 For vaccinia infections, cells were suspended in serum-free DMEM at a concentration of 4 × 106 cells/mL. Infection was with an MOI of 20 for 1.5 hours at 37°C. After the initial infection, the cells were diluted in complete medium to a concentration of 0.4 × 106 cells/mL and incubated for an additional 2.5 hours. Cells were then washed and loaded with calcium dyes as described below. Expression of vaccinia encoded Zap70 was confirmed by Western blotting.
Calcium flux
Analysis of the changes in intracellular calcium concentration ([Ca++]i) was carried out with the use of a FACSort Flow Cytometer (Becton Dickinson, Mountain View, CA) and the calcium-sensitive fluorochromes Fluo-3 and Fura Red (Molecular Probes, Eugene, OR). Briefly, cells (5 × 106/mL) were incubated at 37°C in complete medium containing 5 μg/mL Fluo-3-am and 5 μg/mL Fura Red-am. After 30 minutes, cells were washed in 5% FBS-DMEM containing 50 mmol/L Tris (pH 7.5) and held at room temperature in the dark until analysis. The [Ca++]i was monitored with the loaded cells (40 μL) diluted to 500 μL with DPBS with Ca++ (130 μg/mL), Mg2+ (100 μg/mL), glucose (1 mg/ml), and sodium pyruvate (36 μg/mL) at 37°C. Cells were kept at 37°C during analysis. Baseline data were collected for 20-30 seconds, then cells were stimulated with primary monoclonal antibody followed 20-25 seconds later by goat anti-mouse antibody as described in the figure legends. Data were analyzed with the use of the MultiTime Kinetic Experiment Analysis Software (Phoenix Flow Systems, San Diego, CA) and are expressed as the percentage of responding cells relative to unstimulated baseline measurements.
Results and discussion
The high expression of CD33 on myeloid progenitors and its regulation during development suggests a critical role for this siglec in the control of myelopoiesis. However, to date little is known regarding the potential biochemical functions of CD33. Sequence analysis of the cytoplasmic domain of CD33 revealed the presence of two tyrosine residues (Y340 and Y358). In both cases, these tyrosine residues were followed three residues later by a hydrophobic residue, suggesting a resemblance to the ITIMs of many inhibitory receptors.2 The rapidly emerging superfamily of inhibitory receptors now includes CD22,17FcγRIIB,21,25,26 KIR,27 the more recently described immunoglobulin-like transcripts,28,29PIR-B,30,31 and the leukocyte-associated immunoglobulin-like receptor,32 and others. On co-ligation with activation receptors, these proteins become tyrosine phosphorylated, and recruit one or more of the protein tyrosine phosphatases SHP-1, SHP-2, and/or the inositol phosphatase, SHIP.33 The recruited phosphatases then dephosphorylate cytoplasmic substrates otherwise involved in cellular activation. With the recent explosion in the discovery of inhibitory receptors, nearly every ITAM-containing receptor system in immunity has been shown to be regulated by an inhibitory receptor system, including the B-cell antigen receptor, the TcR, and various Fc receptors.34
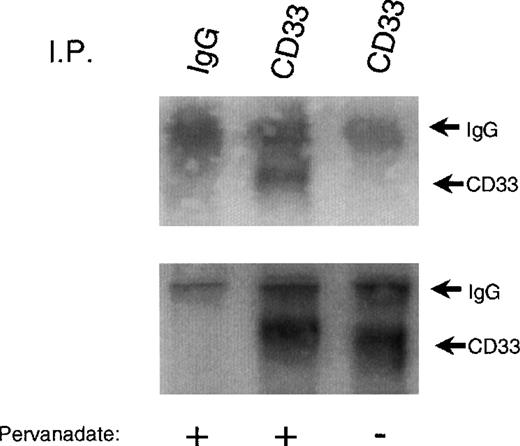
To begin to assess whether CD33 might function as an inhibitory receptor, we first studied the expression of CD33 in several different cell lines. Mo7e, U937, and THP-1 were ideal in that they all expressed significant amounts of the receptor when analyzed by flow cytometry after labeling with PE-conjugated CD33 (data not shown). However, to be effective as an inhibitory receptor, CD33 would have to be tyrosine phosphorylated. Therefore, we treated U937 with the tyrosine phosphatase inhibitor pervanadate, immunoprecipitated CD33, and immunoblotted with antiphosphotyrosine under nonreducing conditions. Figure 1 shows that pervanadate treatment resulted in tyrosine phosphorylation of CD33 (upper panel). Immunoprecipitation of CD33 was confirmed by immunoblotting parallel samples with anti-CD33 (bottom panel). CD33 exists primarily as a homodimer on cells as evidenced by the approximately 130 kd band seen in phosphotyrosine blots of nonreduced immunoprecipitates. In some experiments, other tyrosine-phosphorylated proteins appear to be co-immunoprecipitated with phosphorylated CD33 (data not shown). These proteins varied in mass from 50-130 kd. The protein tyrosine phosphatases SHP-1 and SHP-2 are known to be tyrosine phosphorylated35 36 and bind phosphotyrosine motifs in inhibitory receptors, suggesting that they could be one or more of the phosphoproteins co-precipitating with CD33.
Tyrosine phosphorylation of CD33.
U937 cells were treated with (lanes 1 and 2) or without (lane 3) the phosphatase inhibitor pervanadate, lysed in Triton X-100 lysis buffer, and immunoprecipitated with anti-CD33 (lanes 2 and 3) or control antibody (IgG, lane 1). Immunoprecipitates were blotted with anti-phosphotyrosine (upper panel). Parallel blots were immunoblotted with anti-CD33 (lower panel).
Tyrosine phosphorylation of CD33.
U937 cells were treated with (lanes 1 and 2) or without (lane 3) the phosphatase inhibitor pervanadate, lysed in Triton X-100 lysis buffer, and immunoprecipitated with anti-CD33 (lanes 2 and 3) or control antibody (IgG, lane 1). Immunoprecipitates were blotted with anti-phosphotyrosine (upper panel). Parallel blots were immunoblotted with anti-CD33 (lower panel).
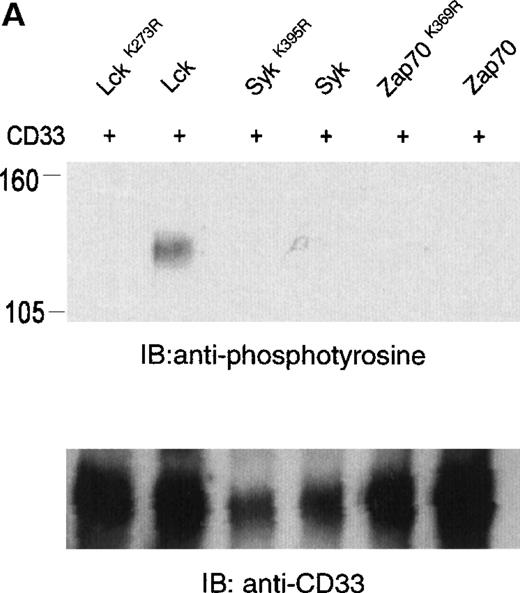
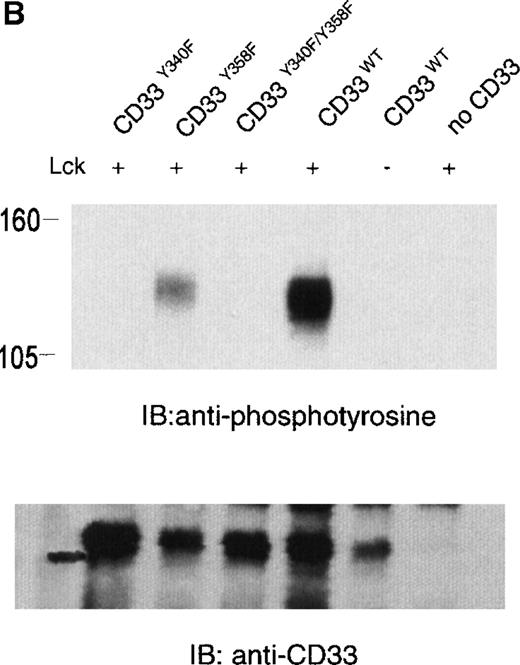
The phosphorylation of CD33 by pervanadate could be mediated by any of several protein tyrosine kinases expressed in U937 cells. However, studies of the KIRs and FcγRIIb have demonstrated most efficient phosphorylation of ITIMs when inhibitory receptors are cross-linked with ITAM-containing receptors, leading to speculation that the Src family kinases activated by these receptors mediate ITIM phosphorylation.37-39 Therefore, we wanted to assess the ability of various kinases to phosphorylate the cytoplasmic tyrosines of CD33. In these experiments, 293T human kidney epithelial cells were transiently transfected with CD33 and various tyrosine kinases. After 24 hours, the cells were harvested and CD33 phosphorylation was assayed with the use of immunoprecipitation, followed by immunoblotting with antiphosphotyrosine (Figure 2A). CD33 expression was verified by flow cytometry before lysis (data not shown) and by immunoblotting of parallel samples with anti-CD33 (lower panels). These experiments determined that Lck, but not Syk or Zap70, was capable of mediating phosphorylation of CD33 in vivo (Figure 2A). Although Zap70 is relatively inactive under these conditions and serves as a transfection control, transfected Syk is active as evidenced by its ability to phosphorylate co-transfected LAT (data not shown). These data are consistent with the ability of Src-family kinases to efficiently phosphorylate both biliary glycoprotein and platelet and endothelial cell adhesion molecule-1 (PECAM-1), because the amino acids flanking the cytoplasmic tyrosines of these two receptors are similar to those of CD33.2,40,41 We next expressed CD33 carrying tyrosine to phenylalanine mutations of Y340, Y358, or both (CD33Y340F, CD33Y358F, and CD33Y340/358F) together with Lck in 293T cells and analyzed CD33 phosphorylation with immunoprecipitation and Western blotting (Figure 2B). Even though each of the mutant receptors was expressed in 293T cells as detected by flow cytometry (data not shown) and immunoblotting of parallel samples with anti-CD33, we detected significant tyrosine phosphorylation only of wild-type CD33 and CD33Y358F (Figure 2B, lanes 2 and 4). These data suggest that Lck phosphorylates primarily Y340 of CD33. Interestingly, the study of PECAM-1 suggested that Lck primarily phosphorylated tyrosine 686, the distal tyrosine of PECAM-1, despite the fact that the sequence surrounding this tyrosine 686 of PECAM, TETVY686SEIR, is most homologous to the distal ITIM of CD33, TSTEY358SEVR.41 The basis for the apparent contradiction in Lck phosphorylation sites in PECAM-1 and CD33 is currently unknown, however, together these findings may suggest that residues well outside of the putative ITIM may be critical in directing the phosphorylation of these receptors. Alternatively, other differences in these experiments, for example the use of activated Lck in the PECAM studies and wild-type Lck here, may be the explanation for the differences in our findings.41 Regardless, the finding that different ITIM-like sequences in a given receptor are differentially phosphorylated by a given kinase lends support to a model in which the two tyrosine motifs may function independently as opposed to being redundant.
Lck-mediated phosphorylation of CD33.
(A) 293T cells were transfected with Lck, kinase dead Lck (LckK273R), Syk, kinase dead Syk (SykK395R), Zap70 or kinase dead Zap70 (Zap70K369R), and wild-type CD33 cDNA. After 24 hours, cells were lysed, immunoprecipitated with anti-CD33, and immunoblotted with anti-phosphotyrosine antibody (upper panel). (B) 293T cells were transfected with the indicated CD33 cDNA and Lck kinase then analyzed as in A. In both cases, expression of CD33 was verified using flow cytometry (data not shown) and immunoblotting of parallel samples with anti-CD33 (lower panels).
Lck-mediated phosphorylation of CD33.
(A) 293T cells were transfected with Lck, kinase dead Lck (LckK273R), Syk, kinase dead Syk (SykK395R), Zap70 or kinase dead Zap70 (Zap70K369R), and wild-type CD33 cDNA. After 24 hours, cells were lysed, immunoprecipitated with anti-CD33, and immunoblotted with anti-phosphotyrosine antibody (upper panel). (B) 293T cells were transfected with the indicated CD33 cDNA and Lck kinase then analyzed as in A. In both cases, expression of CD33 was verified using flow cytometry (data not shown) and immunoblotting of parallel samples with anti-CD33 (lower panels).
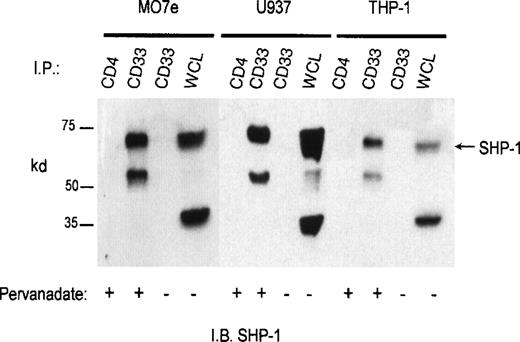
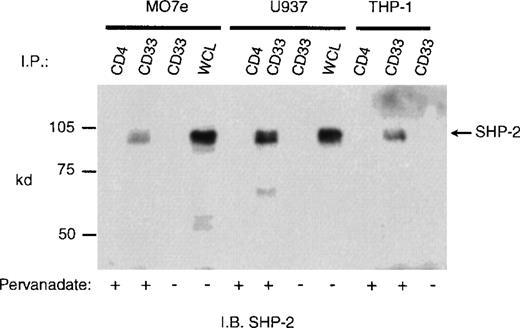
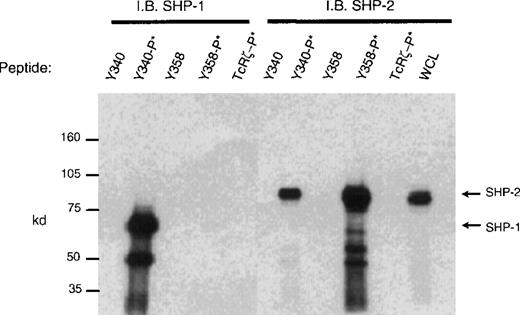
CD33 has two tyrosines in its cytoplasmic tail in which adjoining amino acid residues are close to the consensus for an ITIM. Phosphorylated tyrosines in these motifs could serve as docking sites for the tyrosine phosphatases SHP-1 and/or SHP-2. CD33 could then function as an inhibitory receptor, regulating the phosphorylation of an activating counterpart or other proteins associated with macrophage activation. To test this possibility, the cell lines (Mo7e, THP-1, and U937) were grown to mid-log phase and treated with pervanadate to induce tyrosine phosphorylation. The cells were then lysed and immunoprecipitated with anti-CD33. Western blot analysis of the immunoprecipitates showed that the phosphatases, SHP-1 (Figure 3) and SHP-2 (Figure 4) co-immunoprecipitated with phosphorylated CD33. Similar analysis using anti-SHIP demonstrated no interaction between CD33 and SHIP (data not shown). SHP-1, however, appears to be co-immunoprecipitated much more efficiently than SHP-2, suggesting that it is the primary effector molecule in the CD33 pathway. In Figures 3 and 4, whole-cell lysate lanes represent 105 cells (1% of the available SHP-1). Comparison of the levels of SHP-1 in lanes 2 and 4 suggests, therefore, that approximately 2%-3% of the available SHP-1 co-immunoprecipitated with CD33. As CD33 has two tyrosines in the cytoplasmic tail, either one of these could function in recruiting the phosphatases. To further assess whether the CD33 cytoplasmic tyrosines are functionally redundant or play specific roles with regards to phosphatase recruitment, we used phosphorylated and nonphosphorylated peptides spanning Y340 and Y358 in peptide-capture experiments. In these experiments, lysates from unstimulated U937 cells were incubated withbiotinylated peptides bound to streptavidin-agarose beads. After incubation and extensive washing, proteins bound to the peptides were eluted and analyzed with the use of Western blotting with antibodies specific for SHP-1 or SHP-2. Figure5 shows that the phosphorylated Y340 peptide bound both SHP-1 and SHP-2. In marked contrast, the phosphorylated Y358 peptide bound SHP-2 well but did not bind significant amounts of SHP-1. The specificity of these interactions was demonstrated by the fact that neither nonphosphorylated CD33-derived peptides nor a control phosphopeptide derived from the TcR zeta bound detectable amounts of phosphatase (Figure 5). Taken together, our findings that Y358 binds SHP-2 the best but is only weakly phosphorylated during co-expression studies may, in part, explain the low levels of SHP-2 co-immunoprecipitated with CD33 in monocyte cell lines (Figure 4). Studies by others42,43 have begun to address the specificity of phosphatase recruitment by defining the amino acid specificity of the SHP-1 SH2 domains. These studies include broad amino acid consensus motifs, V/IxYxxV/L for SHP-1 docking43 and VxYI/VxV/I/L for SHP-2 docking.44In a study by Burshtyn et al,42 the significance of the amino acids flanking the tyrosine residue in the KIR ITIM was studied by assaying the ability of peptides carrying various substitutions to activate SHP-1. These authors found that a change in the amino acid −2 relative to the phosphotyrosine from valine to either isoleucine or leucine did not show significant difference in the phosphatase activity of the peptides. Interestingly, the amino acid at position −2 relative to tyrosine Y340 of CD33 is leucine. In contrast, changing the amino acid at +3 from leucine to valine resulted in a significantly lower induction of SHP-1 phosphatase activity.42 The presence of valine at +3 of Y358 could, therefore, decrease the ability of this phosphopeptide to bind SHP-1. In addition, T356 of CD33 does not conform to the ITIM consensus and may, in part, be responsible for the selective recruitment of SHP-2 by this ITIM-like motif. Further study of the CD33 tyrosine residues will be required to fully address these questions.
Recruitment of SHP-1 phosphatase by CD33.
Mo7e, U937, and THP-1 cells (1 × 107/point) were treated with the phosphatase inhibitor pervanadate as indicated and lysed in Triton X-100 lysis buffer, and the cleared lysates immunoprecipitated with anti-CD33 or control antibody (CD4). The immunoprecipitates were blotted with SHP-1 antibody. WCL indicates the presence of SHP-1 in whole cell lysate (1 × 105cells).
Recruitment of SHP-1 phosphatase by CD33.
Mo7e, U937, and THP-1 cells (1 × 107/point) were treated with the phosphatase inhibitor pervanadate as indicated and lysed in Triton X-100 lysis buffer, and the cleared lysates immunoprecipitated with anti-CD33 or control antibody (CD4). The immunoprecipitates were blotted with SHP-1 antibody. WCL indicates the presence of SHP-1 in whole cell lysate (1 × 105cells).
Recruitment of SHP-2 phosphatase by CD33 in pervanadate treated cells.
Mo7e, U937, or THP-1 cells (1 × 107) were treated with the phosphatase inhibitor pervanadate, lysed, and immunoprecipitated as in Figure 3. The immunoprecipitates were blotted with anti-SHP-2.
Recruitment of SHP-2 phosphatase by CD33 in pervanadate treated cells.
Mo7e, U937, or THP-1 cells (1 × 107) were treated with the phosphatase inhibitor pervanadate, lysed, and immunoprecipitated as in Figure 3. The immunoprecipitates were blotted with anti-SHP-2.
Peptide capture of SHP-1 and SHP-2.
Biotinylated peptides spanning tyrosines Y340 or Y358 were linked to streptavidin sepharose beads and used to capture proteins from cell lysates of U937 (1 × 107). Precipitated proteins were blotted with antibodies to SHP-1 or SHP-2. Nonphosphorylated and phosphorylated peptides (denoted as −P*) spanning Y340, Y358, or control TcRζ phosphopeptide were used as indicated. WCL = whole cell lysate (1 × 105).
Peptide capture of SHP-1 and SHP-2.
Biotinylated peptides spanning tyrosines Y340 or Y358 were linked to streptavidin sepharose beads and used to capture proteins from cell lysates of U937 (1 × 107). Precipitated proteins were blotted with antibodies to SHP-1 or SHP-2. Nonphosphorylated and phosphorylated peptides (denoted as −P*) spanning Y340, Y358, or control TcRζ phosphopeptide were used as indicated. WCL = whole cell lysate (1 × 105).
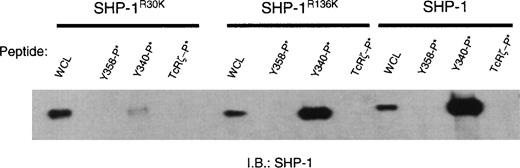
SH2 domain-containing tyrosine phosphatases can bind to phosphotyrosine-containing receptors or phosphopeptides with one or both of their SH2 domains. Detailed study of SHP-1 has demonstrated that phosphopeptide binding to the amino SH2 domain is required for significant activation of phosphatase activity. In contrast, binding of the carboxyl SH2 domain may serve primarily in the physical recruitment of the enzyme.45 Because of the high stoichiometry of the CD33-SHP-1 interaction, we sought to determine which SH2 domain of SHP-1 bound CD33. For these experiments, we expressed SHP-1 or SHP-1 carrying point mutations within either the amino SH2 domain (SHP-1R30K) or carboxyl SH2 domain (SHP-1R136K) in 293T cells. Twenty-four hours later, the cells were harvested and lysed, and equal amounts of each isoform of SHP-1 were then incubated with phosphopeptide-loaded beads as above. After washing, bound SHP-1 was eluted with sample buffer and detected by immunoblot (Figure 6). Like Figure 5, this analysis demonstrated the ability of Y340, but not Y358, to bind SHP-1. Interestingly, mutation of the amino-terminal SH2 domain (SHP-1R30K) ablated binding of SHP-1 to Y340, suggesting this SH2 domain as the primary site of interaction between the receptor and the phosphatase. The strong binding of SHP-1 via its amino terminal SH2 domain suggests the interaction between CD33 and SHP-1 would result in significant activation of SHP-1.45
The amino SH2 domain of SHP-1 binds Y340 of CD33.
SHP-1 or SHP-1 carrying point mutations within either the amino SH2 domain (SHP-1R30K) or carboxyl SH2 domain (SHP-1R136K) was expressed in 293T cells. Equal amounts of each isoform of SHP-1 was then incubated with phosphopeptide-loaded beads as defined in Figure 5. Bound SHP-1 was eluted with sample buffer and detected by immunoblot. WCL = whole cell lysate demonstrating equal availability of SHP-1.
The amino SH2 domain of SHP-1 binds Y340 of CD33.
SHP-1 or SHP-1 carrying point mutations within either the amino SH2 domain (SHP-1R30K) or carboxyl SH2 domain (SHP-1R136K) was expressed in 293T cells. Equal amounts of each isoform of SHP-1 was then incubated with phosphopeptide-loaded beads as defined in Figure 5. Bound SHP-1 was eluted with sample buffer and detected by immunoblot. WCL = whole cell lysate demonstrating equal availability of SHP-1.
The differential binding of SHP-1 and SHP-2 to the CD33 ITIMs prompted us to look more closely at the putative ITIMs of sialic acid-binding proteins of the siglec family. Aligning the different siglecs, sialoadhesin, CD22,8 CD33,2MAG,10 SMP,11 and the more recently identified siglec, siglec-5,12 showed that all of these proteins except sialoadhesin have at least one ITIM-like motif in their cytoplasmic domains (Table 1). CD22 has four tyrosine residues in ITIM configuration, CD33 and siglec-5 each have two such motifs, and MAG and SMP have only one tyrosine in apparent ITIM consensus. The membrane proximal tyrosine of CD33 and siglec-5 are similar in configuration and have a hydrophobic residue at position −2 relative to the phosphotyrosine. In contrast, the distal ITIM-like sequences of CD33, siglec-5, MAG, and SMP all possess threonine at position −2.2,10,11 On the basis of our peptide-binding studies, one could speculate that the proximal tyrosine functions in recruiting SHP-1, whereas the distal tyrosine (the only one present in MAG and SMP) functions primarily in the recruitment of SHP-2, if at all. This theory fits well with overlapping expression patterns of SHP-1 and SHP-2 and the various receptors. SHP-1 is expressed primarily in hematopoietic cells, whereas SHP-2 is expressed ubiquitously.46 Therefore, MAG and SMP would preferentially bind SHP-2 consistent with their expression in nonhematopoietic cells. In contrast, the hematopoietic receptors of the siglec family would have access to both SHP-1 and SHP-2, and the presence of the membrane proximal ITIM would allow interaction with both phosphatases. One could then imagine a lack of selective pressure for the maintenance of the proximal ITIM of the cytoplasmic tails of the nonhematopoietic siglecs.
Comparison of the ITIMs of some of the members of the siglec family, PECAM-1 and biliary glycoprotein
| . | Proximal tyrosine . | Distal tyrosine . |
|---|---|---|
| CD33 | ELHYASL | TEYSEV |
| PECAM-1 | DVEYTEV | TVYSEI |
| Biliary glycoprotein | DVAYTVL | TVYSEV |
| CD22 | TVSYAIL | IHYSEL |
| Siglec-5 | ELHYASL | TEYSEI |
| MAG | AEYAEI | |
| SMP | PEYAEI |
| . | Proximal tyrosine . | Distal tyrosine . |
|---|---|---|
| CD33 | ELHYASL | TEYSEV |
| PECAM-1 | DVEYTEV | TVYSEI |
| Biliary glycoprotein | DVAYTVL | TVYSEV |
| CD22 | TVSYAIL | IHYSEL |
| Siglec-5 | ELHYASL | TEYSEI |
| MAG | AEYAEI | |
| SMP | PEYAEI |
ITIM indicates immunoreceptor tyrosine-based inhibitory motif; PECAM-1, platelet and endothelial cell adhesion molecule-1; siglec, sialic acid-binding, immunoglobulin-like lectins; MAG, myelin-associated glycoprotein; SMP, Schwann cell myelin protein.
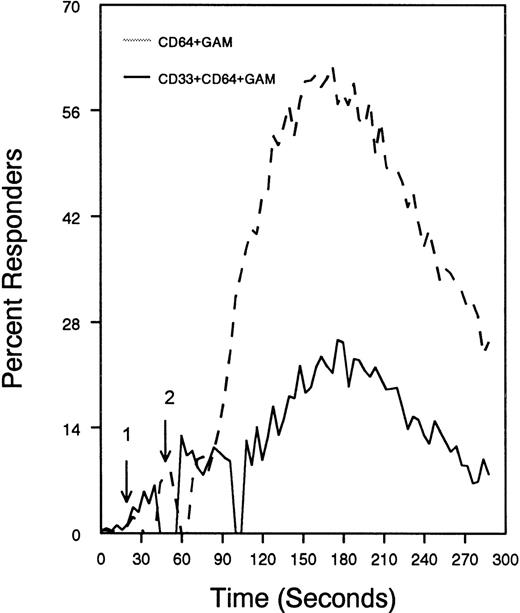
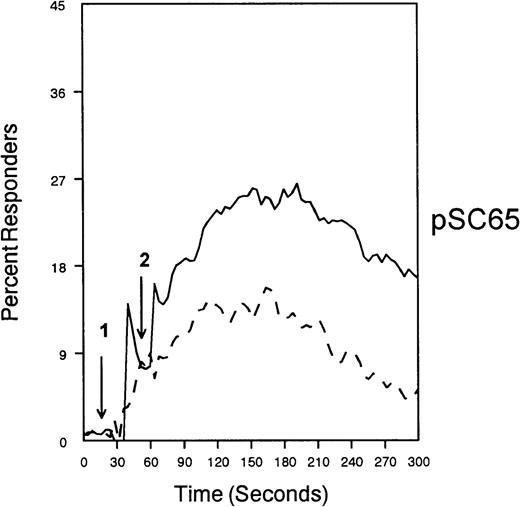
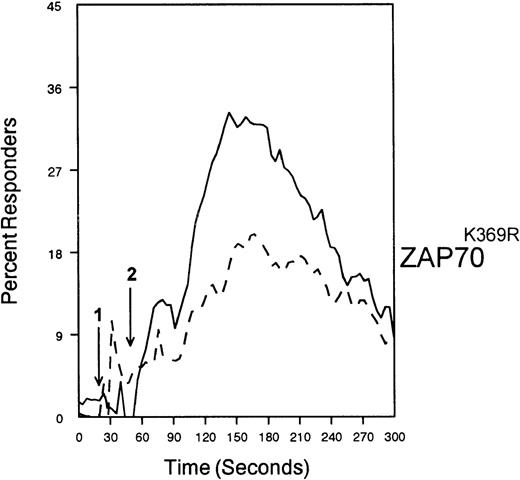
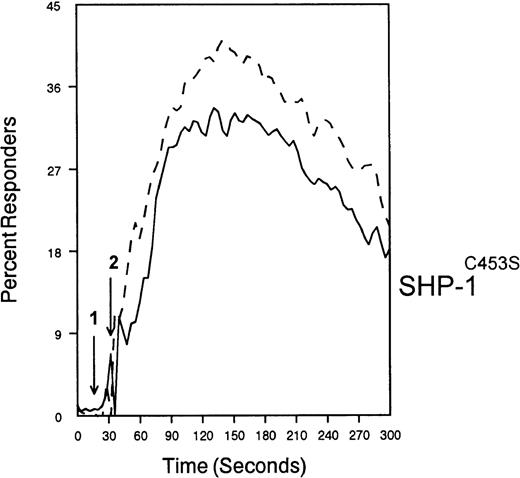
The ability of CD33 to become tyrosine phosphorylated and recruit protein tyrosine phosphatases strongly suggests that it would function as an inhibitory receptor in the myeloid compartment. Many of the cells that express CD33 also express the high-affinity receptor for IgG, CD64. Moreover, CD64 signal transduction involves FcεRI gamma chain, an ITAM-containing protein.47 Most of the inhibitory receptors characterized to date are efficient at suppressing signals generated by ITAM-containing receptor systems.34 Therefore, we speculated that CD33 might, when co-cross-linked, suppress CD64 signals. To test this possibility, we induced CD64 expression with interferonγ, and then cross-linked CD64 on U937 along with anti-CD33 as we monitored [Ca++]i (Figure7). Cross-linking of control antibody or CD33 alone resulted in no increase in [Ca++]i. In contrast, cross-linking of CD64 resulted in robust mobilization of intracellular calcium as demonstrated by the increase in [Ca++]i (Figure 7). Co-cross-linking of CD64 with CD33, however, resulted in diminished calcium mobilization as compared with cross-linking of CD64 alone. Co-cross-linking of CD64 with control IgG had no effect (data not shown). Notably, CD33 cross-linking did not completely abolish CD64-mediated calcium mobilization but only reduced it. To verify that CD33-mediated inhibition was due to recruitment of phosphatases to CD33, we used a vaccinia-based overexpression system to express dominant negative SHP-1.24 In these experiments, U937 were infected with recombinant vaccinia virus carrying empty expression vector (pSC65), catalytically inactive SHP-1 (SHP-1C453S), or catalytically inactive Zap70 as a control (Figure 8). Four hours after infection, the cells were washed and calcium mobilization was monitored as CD64 was co-cross-linked with control antibody (solid trace) or anti-CD33 (dotted trace; Figure 8). Anti-CD33 co-cross-linking inhibited CD64-mediated increases in [Ca++]i after infection with pSC65-containing virus and after expression of kinase dead Zap70 even though expression of dead Zap70 was evident by Western blotting (data not shown). In contrast, infection with the dominant negative SHP-1 abolished the inhibitory activity of CD33. In addition, to preventing CD33-mediated inhibition, the SHP-1C453S infection relieved the basal control of CD64-mediated calcium mobilization, resulting in increased [Ca++]i following CD64 cross-linking. These experiments, together with the preferential co-immunoprecipitation of SHP-1, and the relative lack of phosphorylation of CD33 on Y358, the tyrosine that binds SHP-2, suggest that the inhibition of CD64-mediated calcium mobilization by CD33 is largely mediated by SHP-1 phosphatase.
CD33-mediated inhibition of FcγRI-induced calcium mobilization in interferon γ-treated (250 U/mL for 48 hours) U937 cells.
Cells were loaded with calcium-sensitive dyes then stimulated with anti-CD64 or anti-CD64 + anti-CD33 as indicated (arrow 1) followed by GAM (arrow 2). Calcium was monitored with cells at 37°C.
CD33-mediated inhibition of FcγRI-induced calcium mobilization in interferon γ-treated (250 U/mL for 48 hours) U937 cells.
Cells were loaded with calcium-sensitive dyes then stimulated with anti-CD64 or anti-CD64 + anti-CD33 as indicated (arrow 1) followed by GAM (arrow 2). Calcium was monitored with cells at 37°C.
SHP-1 mediates CD33-induced inhibition of Ca++ mobilization.
U937 cells were primed with IFNγ as above, infected with the indicated vaccinia virus, then loaded with calcium-sensitive dyes and stimulated with anti-CD64 and IgG (solid trace) or anti-CD64 and anti-CD33 (broken trace).
SHP-1 mediates CD33-induced inhibition of Ca++ mobilization.
U937 cells were primed with IFNγ as above, infected with the indicated vaccinia virus, then loaded with calcium-sensitive dyes and stimulated with anti-CD64 and IgG (solid trace) or anti-CD64 and anti-CD33 (broken trace).
Taken together, our data extend a recent study of CD33 by Taylor et al48 by demonstrating the inhibitory activity of CD33 in the myeloid compartment. In addition, we have shown the ability of the CD33 tyrosine motifs to differentially interact with the endogenous phosphatases of monocytes. Moreover, our co-expression data suggest that the phosphorylating kinase may, in part, direct the recruitment of phosphatases to CD33. Our data suggest a model in which CD33 is tyrosine phosphorylated by a Src-family kinase activated in response to CD64 cross-linking. This Src family-mediated phosphorylation would be most prominent on Y340, a tyrosine capable of efficiently recruiting both SHP-1 and minimally recruiting SHP-2. Perhaps a second kinase phosphorylates Y358 exclusively to increase the recruitment of SHP-2, a model consistent with the conservation of Y358-like tyrosine motifs in the siglec family members expressed in tissues that lack SHP-1. Regardless, the result is significant inhibition of CD64-mediated activation. Such a mechanism would allow for CD33brightcells to ignore stimuli that would otherwise result in monocytic activation. As the myeloid compartment develops and CD33 expression falls, the cells would be expected to become more and more responsive to stimulation through CD64 and/or other activation receptors. Definitive examination of these possibilities, however, will require further characterization of CD33 signaling, definition of the CD33 ligand(s), and/or genetic studies of CD33−/− mice.
Reprints:Daniel W. McVicar, NCI-FCRDC Building 560, Rm 31-93, Frederick, MD 21702; e-mail: mcvicar@nih.gov.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal