Abstract
We have developed a new method that allows detection and isolation of viable, antigen-specific, human T cells from a heterogeneous pool of T cells. We have engineered a self-inactivating retroviral vector containing multiple (3 or 6) nuclear factor of activated T-cell (NFAT)-binding sites, followed by the minimal IL2 promoter and the reporter gene GFP. Jurkat cells, primary T-cell blasts, and T-cell clones were transduced with high efficiency (20%-40%). Stimulation of the transduced cells with phorbol myristate acetate (PMA) and ionomycin resulted in a high level expression of GFP that was maximal after 12 to 14 hours and remained stable for another 12 hours. Activation of T cells carrying the construct containing 6 NFAT-binding sites resulted in the highest mean fluorescence intensity. Cyclosporin-A and FK506 were able to block the activation-dependent GFP expression. Activation of transduced T-cell blasts with the superantigen staphylococcal enterotoxin B or of transduced antigen-specific T-cell clones with cognate antigen resulted in GFP expression. After an overnight stimulation of a heterogeneous T-cell bulk culture with an HLA mismatched stimulator cell (JY), the GFP expressing cells were cloned. As expected, the cloning frequency of the antigen-specific GFP+ cells was considerably higher than that of the total T-cell population. Most of the T-cell clones were either cytolytic, or proliferative toward JY stimulator cells. Interestingly, we also isolated T-cell clones that were noncytolytic and nonproliferative toward JY cells, but specifically up-regulated GFP after an overnight stimulation with JY.
Triggering of the T-cell receptor (TCR) leads to signal transduction cascades and subsequent activation of gene transcription. Members of the nuclear factor of activated T cells (NFAT) family of transcription factors play a key role in activation of gene transcription in T cells. NFAT family members and the phosphatase calcineurin, which is found upstream of NFAT transcription factors in the signal transduction pathway, have been implicated in the regulation of expression of a number of cytokine genes, among which are IL-2, IFN-γ, and TNF-α (reviewed in Rau et al1).
Expression vector constructs have been used in the past containing multiple NFAT-binding sites and the bacterial reporter gene β-galactosidase (LacZ).2-4 It has been shown that T-cell lines transfected with such constructs express LacZ on activation through the TCR. In principle, one should be able to isolate antigen-specific T cells from a primary polyclonal T-cell population by sorting the cells that express the reporter gene product after stimulation with antigen. This approach would obviate the time-consuming in vitro stimulation and restimulations that are required to sufficiently enrich antigen-specific T cells before cloning these cells.5 Also, this approach would allow direct isolation of antigen-specific T cells from a polyclonal pool of T cells without prior knowledge of the antigen or the restriction element that is required for isolation of T cells with HLA/peptide tetramers. The methods that have been used to express LacZ in Jurkat cells are, however, of limited use for primary T cells. The transfection efficiencies of primary T cells using classical methods, like electroporation, are very low. Moreover, the staining procedures used, by using X-gal or FDG as substrates for the enzyme β-galactosidase, are suboptimal because the T cells have to be fixed or undergo an osmotic shock.
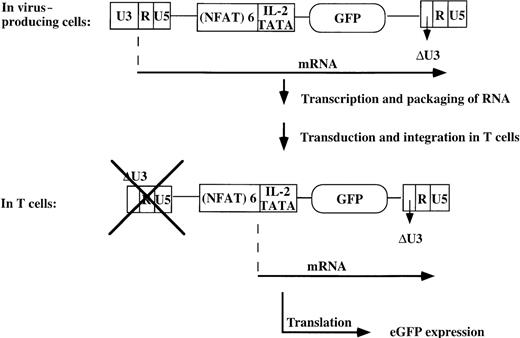
Here we describe the construction of a number of self-inactivating (SIN) retroviral constructs carrying multiple (3 or 6) NFAT-binding sites, followed by the minimal IL2 promoter and the reporter gene, green fluorescent protein (GFP). Because the 3′ LTR of the retroviral vector carries a deletion in the U3 region, the promoter activity of this LTR is abrogated on integration into the genome of the transduced cell and the expression of GFP has then become dependent on binding of NFAT and AP-1 transcription factors to the multiple NFAT-binding sites. Here we show the potentiality of this system to visualize viable activated T cells. Furthermore, we document the application of this system to isolate antigen-specific T-cell clones from a heterogeneous T-cell population.
Materials and methods
Cell lines
Phoenix cells, melanoma cells, and EBV-B cell lines, including JY cells, were grown in complete medium consisting of Iscove's (Life Technologies BV, Breda, The Netherlands) supplemented with 5% to 10% fetal calf serum (BioWhittaker, Verviers, Belgium), penicillin, and streptomycin (Boehringer Mannheim, Mannheim, Germany).
T-cell blasts and T-cell clones
T-cell blasts were prepared by incubation of 5 × 105 peripheral blood mononuclear cells (PBMCs) per milliliter in Yssels medium6 supplemented with 1% normal human serum (NHS) and 2 μg/mL phytohaemagglutinin (PHA). The resulting blasts were phenotyped after 1 week and were shown to contain T cells only, as all cells expressed CD3 in combination with CD4 or CD8 (data not shown). To maintain T-cell blasts and antigen-specific T-cell clones, the T cells (3 × 105 cells per well) were stimulated weekly with a feeder cell mixture of 10 × 105 irradiated (50 Gy) allogeneic PBMCs per milliliter and 1 × 105irradiated EBV-B cells (JY), supplemented with 100 ng/mL PHA and 10 IU/mL IL-2 (Chiron, Amsterdam, The Netherlands) in Yssels medium. Cell cultures were kept in incubators at 37°C in humidified air containing 5% CO2.
Isolation and cloning of antigen-specific T cells
T cells transduced with the reporter construct SIN-(NFAT)6-GFPs were isolated on the basis of GFP expression. After an overnight incubation with stimulator cells, GFP positive cells were sorted using a FACStar plus (Becton Dickinson, San Jose, CA) and cloned by single cell deposition in 96-wells plates containing 100 μL feeder cell mixture per well. The isolated T cells were grown for 2 to 3 weeks in these plates, weekly adding IL-2–containing medium, after which the clones were transferred to 24-well plates containing 1 mL of feeder cell mixture, as described above. Isolated clones were subsequently phenotyped and analyzed for antigen specificity and function.
Construction of retroviral vectors
We have made 2 SIN retroviral constructs carrying multiple (3 or 6) NFAT-binding sites, followed by the minimal IL2 promoter (IL-2Pmin) and the reporter gene, encoding the enhanced green fluorescent protein (GFP from Clontech Laboratories, Palo Alto, CA). We have constructed the SIN-(NFAT)x-GFP constructs as follows: the two 5′ phosphorylated primers NFAT I (AATTAGGAGGAAAAACTGTTTCATACAGAAGGCGTCAA- TTGTC) and NFAT II (CCGGGACCAATTGACGCCTTCTGTATGAAA- CAGTTTTTCCTCCT) (Perkin Elmer, Nieuwerkerk a/d Yssel, The Netherlands) were mixed in equimolar amounts, melted, reannealed, and subsequently ligated into pBluescript KS+ (Stratagene, La Jolla, CA) cut with EcoRI andXmaI to yield pBS-NFAT#1 (all restriction enzymes were obtained from Roche Diagnostics Nederland BV, Almere, The Netherlands). This plasmid was cut with MunI and XmaI to ligate the next NFAT-binding site generating pBS-NFAT#2. This last step was repeated once more to obtain pBS-NFAT#3. To double the number of NFAT-binding sites, pBS-NFAT#3 was cut with XhoI and EcoRV and in here the XhoI-SmaI fragment from pBS-NFAT#3 was ligated, resulting in pBS-NFAT#6.
The IL2 minimal promoter (pos.−60 to −6) was amplified by means of polymerase chain reaction (PCR) with primers IL2P-5′ (GAGCCCGGGACATTTTGACACCCCCATAAT) and IL2P-3′ (CGCGGATCCAACCCATGGAATTCAGGAGTTGAGGTTACTGTGAGTAG) from plasmid NFATZ,2 hereby also directly optimizing the translation initiation site for Kozak consensus.7 This amplicon was cut with XmaI and BamHI, ligated into pBS-NFAT#3, and pBS-NFAT#6 cut with the same enzymes obtaining pBS-NFAT#3-IL2Pmin and pBS-NFAT#6-IL2Pmin. Between the NcoI and BamHI sites of these vectors, the GFP was ligated generating pBS-NFAT#3(#6)-IL2Pmin-GFP.
The sequences of all subsequent steps were confirmed by fluorescent sequencing using Dye-terminator chemistry and 373A sequencer (Perkin Elmer). These NFAT#3/#6-GFP cassettes were shuttled to the SIN retroviral construct RetroTet8 using the uniqueXhoI and BamHI sites. These constructs were further modified by introducing the EBNA/OriP and Puromycin selection cassette from the LZRSpBMN vector,9 making use of the RcaI sites in both retroviral vectors. These final episomal retroviral self-inactivating vectors are designated SIN-(NFAT)3-GFP and SIN-(NFAT)6-GFP, respectively. As a positive control for transduction efficiencies, we have also used the LZRSpBMN retroviral vector9 (a kind gift from Dr G. Nolan, Stanford University, Stanford, CA), which we modified to contain the GFPgene.10
Production of retroviral supernatants
The constructs were transfected into a helper-virus free amphotropic producer cell line Phoenix-A, a derivative of the human embryonic kidney cell line 293 (a kind gift of Dr G. Nolan) using either calcium phosphate (Life Technologies BV, Breda, The Netherlands) or Fugene-6 (Roche Diagnostics), according to manufacturers protocols. Two days later, selection of transfected cells started by the addition of 2 μg/mL puromycin (Clontech Laboratories). Ten to 14 days after transfection, 6 × 106 cells were plated per 10-cm petri dishes (Becton Dickinson) in 10 mL complete medium without puromycin. The next day the medium was refreshed and, on the following day, retroviral supernatant was harvested, centrifuged, and frozen in cell-free aliquots at −70°C. This approach affords a reproducible rapid, large scale, and high titer retroviral production of over 3 × 106 infectious virus particles per milliliter.
Transduction method
The recombinant human fibronectin fragments CH-296 transduction procedure (RetroNectin; Takara, Otsu, Japan) was based on a method developed by Hanenberg et al.11 Nontissue culture-treated Falcon petri dishes (3 cm diameter) (Becton Dickinson) were coated with 2 mL of 30 μg/mL recombinant human fibronectin fragment CH-296 at room temperature for 2 hours or overnight at 4°C. The CH-296 solution was removed, followed by incubation of the petri dishes with 2% human serum albumin in phosphate-buffered saline (PBS) for 30 minutes at room termperature. Before use, the petri dishes were washed twice with PBS. T cells were prestimulated with PHA12 or with a feeder cell mixture containing PHA and IL-2 for 32 to 48 hours before transduction to get them into cycle. Subsequently, the target cells were plated on RetroNectin-coated dishes (maximum 5 × 106 cells per petri dish) in 0.5 mL of complete medium mixed with 1 mL of thawed retroviral supernatant. Cells were cultured at 37°C for 6 hours or overnight, washed, and transferred to 24-well culture plates (Falcon plastics, Becton Dickinson).
T-cell stimulations with PMA and ionomycin, staphylococcal enterotoxin B, EBV-B cells, or melanoma cells
The percentage GFP positive cells, as determined by flow cytometric analysis 3 to 4 days after transduction, was used as a measure of transduction efficiency with the standard LZRS-GFP construct. The efficiency of transduction of T cells with SIN-(NFAT)x-GFP constructs was determined by measuring the GFP expression after overnight stimulation of the cells with PMA (final concentration 10 ng/mL) in combination with ionomycin (final concentration 2.2 μmol/L), assuming that this stimulation protocol stimulates all transduced cells. To block induction of GFP, we used CsA (Sigma Chemical, St Louis, MO) or FK506 (Tacrolimus, Fujisawa Ireland Ltd, Kerry, Ireland) in the final concentrations mentioned in the figure legend.
T-cell stimulations with the superantigen SEB (Sigma) were performed as previously described.13 In brief, T cells (10 × 104) were incubated overnight (14-18 hours) in the presence or absence of autologous EBV-B cells (1 × 104) in round-bottom wells in a total volume of 200 μL Yssel's medium, in the presence or absence of the indicated concentrations of SEB.
T-cell stimulations with JY cells, autologous EBV-B cells, or melanoma cells were performed overnight (14-18 hours) in round-bottom wells in a total volume of 200 μL Yssel's medium. T cells (10 × 104) were incubated with target cells (10 × 104) in the presence or absence of saturating concentrations of blocking antibodies. The antibodies used were specific for HLA class I (hybridoma supernatant from clone W6/32, ATCC, Rockville, MD), HLA-DR (diluted ascitesfluid from clone R3E2; a kind gift from Dr RAW van Lier, CLB, Amsterdam, The Netherlands), or HLA-DQ (hybridoma supernatant from clone SPV-L3, isolated in our laboratory).
Flow cytometric analysis
Antibodies against the human molecules CD3, CD4, CD8, CD20, and HLA-DR (all from Becton Dickinson), directly labeled with phycoerythrin, were used for flow cytometric analysis. Stained cells were analyzed using a FACScan (Becton Dickinson) and FACS data were processed with Cell Quest computer software (Becton Dickinson).
Chromium release assays
Cytotoxicity of T-cell clones was determined using a standard chromium release assay described previously.14 All assays were performed in the presence of a 50-fold excess of unlabeled K562 cells to block nonspecific lysis of the target cells. The spontaneous release varied between 10% to 40% of the maximum. Standard deviation of triplicate determinations never exceeded 10% of the means. A clone was considered to be cytotoxic when the percentage specific chromium release on JY target cells was 30% or higher at an effector-to-target ratio of 30:1. A clone was considered to be noncytotoxic when the lysis was less than 15% at an effector-to-target ratio of 90:1.
Proliferation assays
T cells (105) were incubated in the absence or presence of target cells (104) in round-bottom plates in a total volume of 200 μL. As a control, T cells and target cells were incubated in separate wells. Cells were allowed to proliferate for 3 to 4 days, after which 3H-thymidine was added for an additional incubation period of 8 to 16 hours. The assay was harvested using the Packard Filtermate system and the radioactivities were measured on a TopCount (Packard Instruments, Meriden, CT). Clones were considered to be proliferative toward stimulator cells when the incorporated cells per minute in the presence of stimulator cells was at least 2 times as high as the sum of cells per minute of T cells alone plus stimulator cells alone. Clones were considered to be nonproliferative toward the stimulator cells when the counts per minute in the presence of stimulators did not exceed the counts per minute of either T cells alone or stimulators alone.
Cytokine production assays
The production of cytokines was measured after a 48-hour incubation of 1 million T cells in a total volume of 1 mL of complete medium in the absence or presence of PMA and ionomycin or equal numbers of EBV-B cells. Cell-free supernatants were collected and used in appropriate dilutions in sandwich enzyme-linked immunosorbent assays (ELISAs). Pelikine Compact ELISA kits were used for IL-4, IL-10, and IFNγ, according to manufacturer's protocols (CLB, Amsterdam, The Netherlands). The cytokine production experiments were performed 3 times, all determinations were performed in duplicate. Data from representative experiments are shown in the figures as production in picogram per million cells per 24 hours.
Results
T cells transduced with SIN-(NFAT)x-GFP are triggered, by PMA and ionomycin, to express the reporter gene GFP
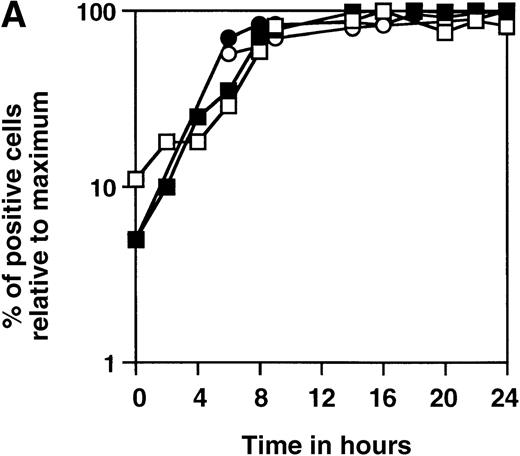
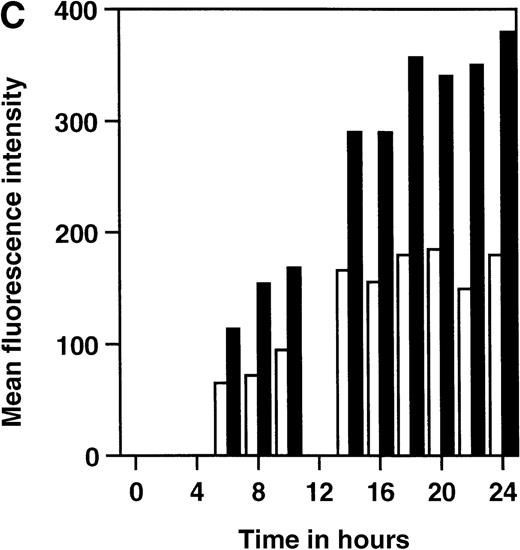
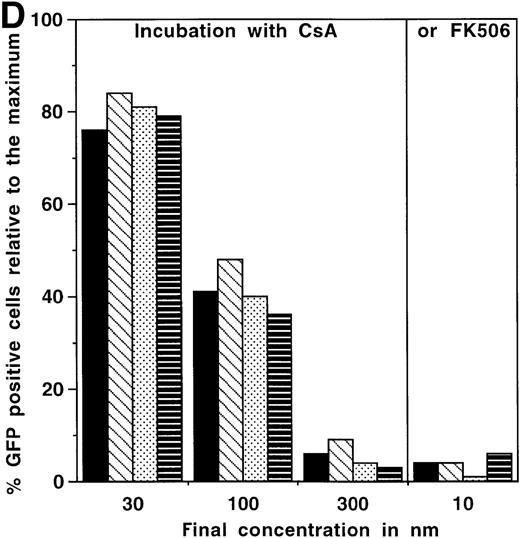
We have constructed a number of self-inhibiting retroviral vectors containing 3 or 6 NFAT-binding sites, followed by the minimal IL-2 promoter and the reporter gene GFP (Figure1). To test the functionality of the constructs, we transduced Jurkat cells and T-cell blasts derived from PBMC of healthy donors. As a control for the transduction efficiency, we also transduced part of the cells with a control virus containing GFP under transcriptional control of the intact viral LTR.10 The efficiency of transduction with SIN-(NFAT)x-GFP containing retroviruses was estimated from the GFP expression after overnight stimulation with PMA and ionomycin and varied between 20% and 40%, which is comparable to the results obtained with the control virus LZRS-GFP (data not shown). In Figure2A, we show the kinetics of induction of GFP expression in transduced Jurkat cells and bulk T-cell blasts from a healthy donor after stimulation with PMA and ionomycin. The results are expressed relative to the maximal level of GFP expression that was reached after 12 to 14 hours and remained stable for another 12 hours in all cases. The percentages of GFP positive cells that were induced from cells carrying either the 3 or the 6 NFAT-binding site constructs were similar. However, large differences between the 2 different NFAT constructs were observed in the mean fluorescence intensity (MFI). In Jurkat cells (Figure 2B), the MFI ratio of SIN-(NFAT)6-GFP over SIN-(NFAT)3-GFP is around 4 and in T-cell blasts (HSP) (Figure 2C), the ratio is 2 at any time point after stimulation. Similar results were obtained with T cells from 2 other donors (data not shown). Importantly, not only Jurkat cells and total T-cell blasts can be triggered to express GFP after stimulation with PMA plus ionomycin, but also sorted CD4+ and CD8+ T cells (Figure 2D and data not shown). Activation and subsequent GFP expression can be blocked by CsA and by FK506 (Figure 2D). Note that FK506 is a much more potent inhibitor of GFP expression than CsA. Only 10 nmol/L of FK506 is sufficient to block GFP expression completely, whereas 300 nmol/L of CsA is needed for the same effect. GFP expression is blocked completely in transduced Jurkat cells at 100 nmol/L CsA and 10 nmol/L FK506 (data not shown).
The SIN-(NFAT)x-GFP retroviral construct.
The SIN-(NFAT)x-GFP–retroviral vector contains a puromycin resistance gene for selection purposes, and EBNA sequences to maintain high copy numbers of the transfected construct in the amphotropic producer line Phoenix, enabling the production of high titer viral supernatants. Transduction of target T cells with the retrovirus, containing the 5′LTR-(NFAT)x-GFP-3′LTR only, ensures that expression of the reporter gene is dependent on binding of transcription factors to the multiple NFAT-binding sites, because an introduced deletion in the U3 region of the 3′LTR (which, on integration, will function as the upstream LTR) prevents promoter activity of this LTR. Thus only activation of the T cell will lead to expression of GFP. eGFP, enhanced EFP.
The SIN-(NFAT)x-GFP retroviral construct.
The SIN-(NFAT)x-GFP–retroviral vector contains a puromycin resistance gene for selection purposes, and EBNA sequences to maintain high copy numbers of the transfected construct in the amphotropic producer line Phoenix, enabling the production of high titer viral supernatants. Transduction of target T cells with the retrovirus, containing the 5′LTR-(NFAT)x-GFP-3′LTR only, ensures that expression of the reporter gene is dependent on binding of transcription factors to the multiple NFAT-binding sites, because an introduced deletion in the U3 region of the 3′LTR (which, on integration, will function as the upstream LTR) prevents promoter activity of this LTR. Thus only activation of the T cell will lead to expression of GFP. eGFP, enhanced EFP.
Time course of induction of GFP in SIN-(NFAT)x-GFP transduced Jurkat cells and primary T-cell bulk cultures.
(A-C) Unsorted, SIN-(NFAT)3-GFP– (open symbols and bars) or SIN-(NFAT)6-GFP– (filled symbols and bars) transduced, Jurkat T cells (panel B; panel A, squares) and bulk T cells from a healthy donor (H.S.P.) (panel C; panel A, circles) were stimulated with PMA in combination with ionomycin. The percentage of GFP expressing cells, relative to the maximal level, which varied between 20% to 40%, and the MFI of GFP positive cells are plotted against the time in hours after initial stimulation. Shown are representative data from 3 independent experiments. (D) GFP expression in SIN-(NFAT)6-GFP–transduced cells is blocked by CsA and by FK506. T cells transduced with the SIN-(NFAT)6-GFP construct were incubated overnight in medium containing PMA and ionomycin. The percentage of GFP positive cells reached was set to 100%. Next, CsA or FK506 (final concentrations are shown), which are inhibitors of calcineurin and thus of expression of GFP, were added 30 minutes before the addition of PMA and ionomycin. Indicated is the percentage of GFP positive cells. Bars represent the following: bulk T-cell blasts (black), sorted CD4+ T cells (hatched), and sorted CD8+ T cells (dotted) all derived from donor K.W.E., and bulk T-cell blasts from donor H.S.P. (striped). Shown are representative data from 3 independent experiments.
Time course of induction of GFP in SIN-(NFAT)x-GFP transduced Jurkat cells and primary T-cell bulk cultures.
(A-C) Unsorted, SIN-(NFAT)3-GFP– (open symbols and bars) or SIN-(NFAT)6-GFP– (filled symbols and bars) transduced, Jurkat T cells (panel B; panel A, squares) and bulk T cells from a healthy donor (H.S.P.) (panel C; panel A, circles) were stimulated with PMA in combination with ionomycin. The percentage of GFP expressing cells, relative to the maximal level, which varied between 20% to 40%, and the MFI of GFP positive cells are plotted against the time in hours after initial stimulation. Shown are representative data from 3 independent experiments. (D) GFP expression in SIN-(NFAT)6-GFP–transduced cells is blocked by CsA and by FK506. T cells transduced with the SIN-(NFAT)6-GFP construct were incubated overnight in medium containing PMA and ionomycin. The percentage of GFP positive cells reached was set to 100%. Next, CsA or FK506 (final concentrations are shown), which are inhibitors of calcineurin and thus of expression of GFP, were added 30 minutes before the addition of PMA and ionomycin. Indicated is the percentage of GFP positive cells. Bars represent the following: bulk T-cell blasts (black), sorted CD4+ T cells (hatched), and sorted CD8+ T cells (dotted) all derived from donor K.W.E., and bulk T-cell blasts from donor H.S.P. (striped). Shown are representative data from 3 independent experiments.
Stimulation with the combination of PMA and ionomycin to estimate the transduction efficiencies results in a slight underestimation thereof. Around 10% of cells properly transduced with either of the retroviral constructs remain unresponsive to such a stimulus (data not shown). The observed bimodal expression pattern is consistent with the data of others, using either a different NFAT-based reporter system2 or the mouse IL2 promoter region linked to the LacZ reporter gene.15
Expression of GFP after triggering of the TCR with SEB in bulk T-cell blasts transduced with SIN-(NFAT)6-GFP
Having demonstrated that the constructs are functional with the combination of PMA and ionomycin as stimulus for the transduced cells, we examined the consequences of triggering of the TCR by superantigens. We have used the superantigen SEB, which binds to MHC class II on antigen-presenting cells (eg, EBV-B cells) and to relevant Vβ regions of the TCR.16 17
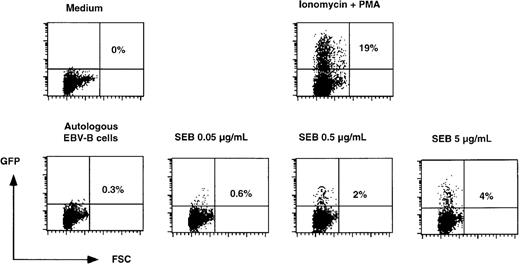
T-cell blasts derived from a healthy donor were transduced with SIN-(NFAT)6-GFP. In preliminary experiments, we observed that a small proportion (0.5%-2%) of the transduced cells expressed GFP constitutively. This is presumably due to insertion of the GFP gene in a transcriptionally active region. Therefore, the transduced T cells were first sorted to remove cells expressing GFP constitutively. Subsequently, these cells were incubated overnight with autologous EBV-B cells in the presence of increasing concentrations of SEB. Incubation of the T cells in medium alone or in the presence of autologous EBV-B cells did not lead to the expression of GFP. Stimulation of T cells with EBV-B cells and increasing concentrations of SEB gave rise to increasing numbers of T cells expressing GFP (Figure3). At the highest concentrations of SEB used, 4% of the cells expressed GFP, which equals around 20% of the cells carrying the SIN-(NFAT)6-GFP construct (as the maximum percentage of GFP positive cells after PMA and ionomycin stimulation was 19%). Although the percentage of SEB reactive T cells varies among individual donors, our findings are in good agreement with the expected percentage of SEB reactive T cells from an average donor, which ranges from 10% to 30% of total T cells.17 More important than the quantitative aspect of these findings is the notion that physiologic triggering of the TCR of SEB reactive T cells induces the expression of GFP.
SEB concentration-dependent induction of GFP in SIN-(NFAT)6-GFP–transduced unfractionated T-cell cultures.
SIN-(NFAT)6-GFP–transduced T cells were stimulated overnight in the presence of different concentrations of SEB presented by autologous EBV-B cells. As negative controls, T cells were incubated in medium alone or with the autologous EBV-B cells alone. As a positive control, we incubated cells with PMA in combination with ionomycin. Indicated in the figure is the percentage of GFP positive cells. Shown are representative data from 2 independent experiments.
SEB concentration-dependent induction of GFP in SIN-(NFAT)6-GFP–transduced unfractionated T-cell cultures.
SIN-(NFAT)6-GFP–transduced T cells were stimulated overnight in the presence of different concentrations of SEB presented by autologous EBV-B cells. As negative controls, T cells were incubated in medium alone or with the autologous EBV-B cells alone. As a positive control, we incubated cells with PMA in combination with ionomycin. Indicated in the figure is the percentage of GFP positive cells. Shown are representative data from 2 independent experiments.
Antigen-specific triggering of the TCR leads to expression of GFP in an alloreactive CD4+ T-cell clone and in a melanoma specific CD8+ T-cell clone.
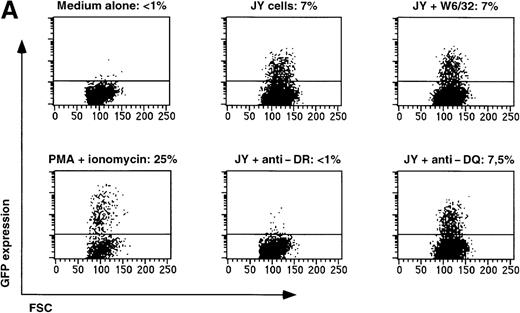
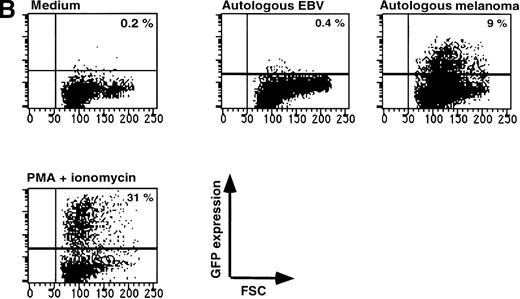
Triggering of the TCR by its cognate antigenic peptide bound to the appropriate MHC molecule should lead to GFP expression in T-cell clones transduced with the reporter construct SIN-(NFAT)6-GFP. To test this, we used a well-characterized T-cell clone, JS136, which is an HLA-DR6 restricted, CD4+T-cell clone able to lyse the B-cell line JY in vitro.18 19Incubation of SIN-(NFAT)6-GFP–transduced JS136 T cells with JY cells induced expression of GFP in the T cells (Figure4A). The expression of GFP could be blocked by antibodies directed against HLA-DR, but not by HLA-class I or by HLA-DQ antibodies. The same results were obtained in a chromium release assay using JS136 as effectors and JY cells as targets (results not shown). Not only SIN-(NFAT)6-GFP–transduced CD4+ T-cell clones can be triggered to express GFP after stimulation with the appropriate target cells, but also CD8+ T-cell clones. To demonstrate this point, we have used a melanoma-specific T-cell clone that is HLA-A2 restricted and specific for the Mart-1/MelanA27-35 epitope (Figure 4B and data not shown). These experiments show that T cells can be induced to express GFP after specific triggering of the TCR. As is the case in classical cytotoxicity assays, this specific activation of the T-cell clone via the TCR can be blocked with appropriate antibodies directed against the presenting MHC molecule.
Expression of GFP in JS136 and melanoma-specific T cell clone.
(A) Induction of GFP expression in T-cell clone JS136 transduced with SIN-(NFAT)6-GFP by its specific target JY is inhibited by anti–HLA-DR antibodies. JS136 T cells transduced with the reporter construct SIN-(NFAT)6-GFP were incubated with JY target cells in the presence or absence of anticlass I (W6/32) or class II (anti–HLA-DQ or -DR) antibodies. Indicated is the percentage of GFP positive T cells after an overnight incubation with JY target cells. Non-JY EBV-B targets were used as negative controls and did not induce GFP expression (data not shown). Stimulation of cells with PMA and ionomycin served as a positive control. Shown are representative data from 3 independent experiments. (B) Melanoma cells induce expression of GFP in a Mart1/MelanA specific CD8+ T-cell clone. Melanoma specific T cells transduced with the reporter construct SIN-(NFAT)6-GFP were incubated with autologous melanoma cells or EBV-B cells. Indicated is the percentage of GFP positive cells. Stimulation of cells with PMA and ionomycin served as a positive control. Shown are representative data from 2 independent experiments.
Expression of GFP in JS136 and melanoma-specific T cell clone.
(A) Induction of GFP expression in T-cell clone JS136 transduced with SIN-(NFAT)6-GFP by its specific target JY is inhibited by anti–HLA-DR antibodies. JS136 T cells transduced with the reporter construct SIN-(NFAT)6-GFP were incubated with JY target cells in the presence or absence of anticlass I (W6/32) or class II (anti–HLA-DQ or -DR) antibodies. Indicated is the percentage of GFP positive T cells after an overnight incubation with JY target cells. Non-JY EBV-B targets were used as negative controls and did not induce GFP expression (data not shown). Stimulation of cells with PMA and ionomycin served as a positive control. Shown are representative data from 3 independent experiments. (B) Melanoma cells induce expression of GFP in a Mart1/MelanA specific CD8+ T-cell clone. Melanoma specific T cells transduced with the reporter construct SIN-(NFAT)6-GFP were incubated with autologous melanoma cells or EBV-B cells. Indicated is the percentage of GFP positive cells. Stimulation of cells with PMA and ionomycin served as a positive control. Shown are representative data from 2 independent experiments.
Isolation of viable allo-specific T-cell clones from a heterogeneous T-cell pool
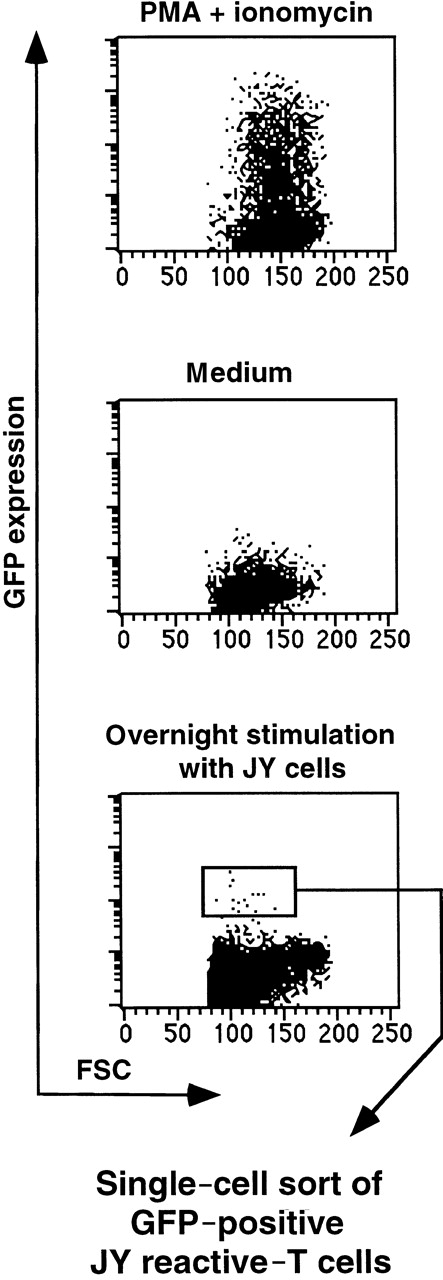
Next we wanted to test whether alloreactive T cells can be isolated from a heterogeneous T-cell pool using the NFAT-GFP reporter system. For this purpose, we used donor (W.B.O.)-derived T cells (HLA-A24,9 A29,19B7, B58,17 Cw7, DR115, DR13(w6), DR52, DQ1, DQ3) and HLA-mismatched (partly) stimulator cells: JY (HLA-A2, B7, DR4, DRw6). Donor T cells transduced with SIN-(NFAT)6-GFP were incubated overnight with JY cells. T cells expressing GFP were sorted by FACS and subsequently cloned by single cell deposition (Figure5). In the first experiment, we compared the plating efficiencies and the proportion of antigen-specific clones isolated from T cells that were cloned from unsorted or from GFP-sorted T-cell blasts. The plating efficiencies were comparable; 29% and 31%, respectively. Six of the 23 CD4+ clones obtained from the unsorted population proliferated to JY but not to the autologous EBV-transformed B-cell line. The proportion of clones proliferating to JY is quite high, but whether all of these clones were specific for class II MHC antigens was not further investigated. By using the reporter system, we found a 2-fold higher proportion of CD4+ T-cell clones (23 of 45) that specifically proliferated on JY targets and not on the autologous EBV-B cell line. Only 1 of 98 CD8+ clones obtained from unsorted T cells was able to lyse JY cells and not the autologous EBV-B cells, whereas 9 of 55 clones from the GFP-sorted cells were JY specific. Taken together, cloning from GFP-sorted cells yielded a greater than 5-fold higher proportion of JY-specific T-cell clones (32 of 100) than cloning from unsorted cells (7 of 121). The data from the random cloning indicate an alloreactive T-cell precursor frequency of about 6%. The transduction efficiency of the T-cell blasts used was 30%. On the basis of the data obtained with the JS136 T-cell clone and the melanoma specific T-cell clone, we estimate that one fourth to one third of the cells in a given clone can be triggered to express GFP after an overnight stimulation. Simple multiplication of these numbers (0.06 × 0.30 × 0.25 = 0.0045) would indicate that maximally 1 of 222 ( = 0.45%) of the T-cell blasts should up-regulate GFP after optimal stimulation with JY target cells. This is in good agreement with the percentage of GFP+ cells sorted (Figure 5).
Isolation of viable alloantigen specific T cells from a heterogeneous T-cell pool.
Donor (W.B.O.)-derived unfractionated T cells transduced with SIN-(NFAT)6-GFP were incubated overnight with JY cells as stimulators at a ratio of 1:1. T cells expressing GFP were sorted by means of flow cytometry and subsequently cloned at a single cell per well. As controls, we incubated T cells in medium in the presence or absence of PMA and ionomycin. All cells within the life gate are shown. Transduction efficiency was 30%. The percentage of GFP positive cells in the gate used to sort was approximately 0.2% to 0.3%.
Isolation of viable alloantigen specific T cells from a heterogeneous T-cell pool.
Donor (W.B.O.)-derived unfractionated T cells transduced with SIN-(NFAT)6-GFP were incubated overnight with JY cells as stimulators at a ratio of 1:1. T cells expressing GFP were sorted by means of flow cytometry and subsequently cloned at a single cell per well. As controls, we incubated T cells in medium in the presence or absence of PMA and ionomycin. All cells within the life gate are shown. Transduction efficiency was 30%. The percentage of GFP positive cells in the gate used to sort was approximately 0.2% to 0.3%.
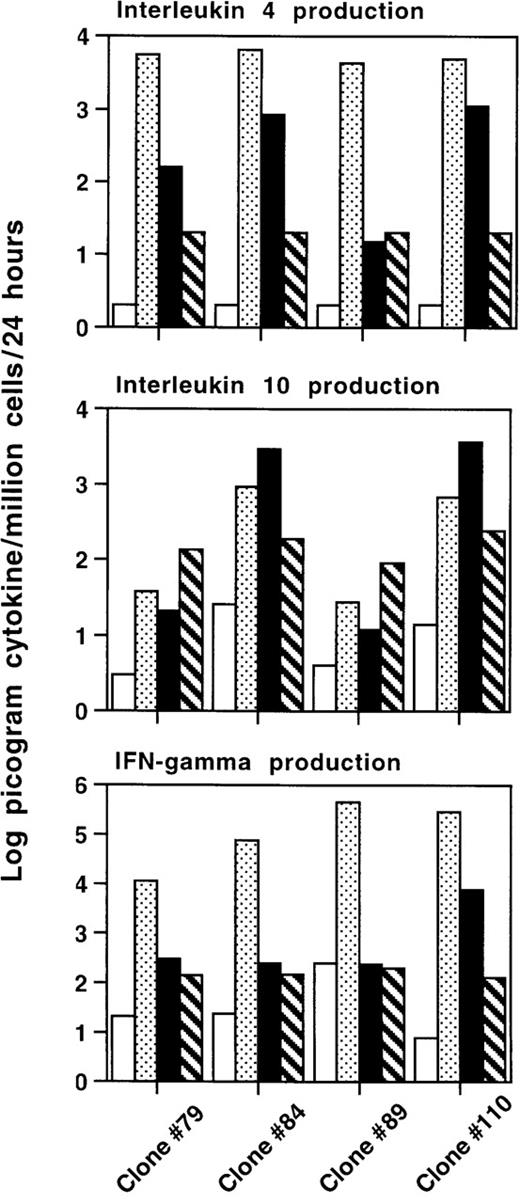
In the second experiment, we repeated the cloning of transduced T-cell blasts of the same donor W.B.O. to characterize in more detail the clones obtained from SIN-(NFAT)6-GFP–transduced T-cell blasts stimulated with JY cells. Twenty-three of the 41 isolated T-cell clones were specific for JY, consistent with the results of the first experiment. Both cytotoxic CD8+ T-cell clones (9 of 23 isolated clones) and proliferative CD4+ T-cell clones (10 of 23 isolated clones) were isolated. Apart from cytolytic and proliferative T-cell clones, we also isolated 4 clones that are not cytolytic, or proliferative (as defined in “Materials and methods”), toward JY cells, but specifically up-regulated GFP after stimulation with JY cells (4 of 23 isolated clones). A representative example of such a clone is shown in Figure6. The expression of GFP is up-regulated in these T cells after incubation with JY target cells but not after incubation in medium alone or in the presence of autologous EBV-B cells. Three of these clones could be evaluated in cytokine production assays. In parallel to the modest but significant up-regulation of GFP, clone 84 produced IL-4 (840 pg) and IL-10 (2960 pg), but no IFNγ after incubation with JY stimulator cells for 48 hours (Figure7). This clone 84 has the typical cytokine production profile of an antigen specific Th2 cell. Two other clones (79 and 89) also specifically up-regulated GFP after stimulation with JY cells (data not shown). Clone 79 produced 8 times more IL-4 in the presence of JY (160 pg) compared with the background levels in the presence of autologous EBV-B cells (20 pg). Clone 89 did not respond by producing any of the cytokines tested, but may produce yet other cytokines after specific stimulation with JY. Incubation with PMA and ionomycin showed the intrinsic capacity of these clones to produce large amounts of IL-4 and IFNγ. It is important to note that none of these clones produced IFNγ on stimulation with JY cells. As expected, stimulation of T cells with PMA and ionomycin did not stimulate IL-10 production. Cytolytic (CTL) clone 110 is lytic toward JY and was included in these experiments as a positive control as it specifically produces IL-4, IL-10, and IFNγ on stimulation with JY cells.
Up-regulation of GFP in T-cell clone 84 after stimulation with JY target cells.
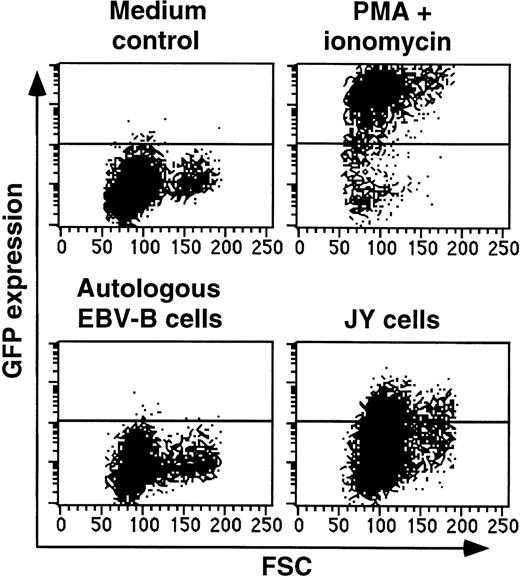
T cells of W.B.O. clone 84, which is noncytolytic and nonproliferative toward JY cells, were incubated in medium alone, or together with autologous EBV-B cells or with JY cells. Shown is the up-regulation of GFP after an overnight incubation. Gates were set to exclude CD20+ B cells.
Up-regulation of GFP in T-cell clone 84 after stimulation with JY target cells.
T cells of W.B.O. clone 84, which is noncytolytic and nonproliferative toward JY cells, were incubated in medium alone, or together with autologous EBV-B cells or with JY cells. Shown is the up-regulation of GFP after an overnight incubation. Gates were set to exclude CD20+ B cells.
Cytokine production by isolated T-cell clones that are not cytolytic, or proliferative, toward JY cells.
Cytokine production assays were performed with 3 W.B.O. T-cell clones (79, 84, and 89), which are noncytolytic and nonproliferative toward JY cells. CTL clone 110 was included as a positive control. Clone 84 is CD4+, the others are CD8+. Indicated is the production in log picogram cytokine per million cells per 24 hours. Bars indicate the following: T cells in medium (□), in medium plus PMA and ionomycin (), T cells incubated with JY (▪), or autologous EBV-B (▧) stimulator cells. Note that the scale for IFNγ is 2 logs higher that the other 2. Neither the autologous EBV-B cells nor the JY cells produce any of the 3 cytokines tested here.
Cytokine production by isolated T-cell clones that are not cytolytic, or proliferative, toward JY cells.
Cytokine production assays were performed with 3 W.B.O. T-cell clones (79, 84, and 89), which are noncytolytic and nonproliferative toward JY cells. CTL clone 110 was included as a positive control. Clone 84 is CD4+, the others are CD8+. Indicated is the production in log picogram cytokine per million cells per 24 hours. Bars indicate the following: T cells in medium (□), in medium plus PMA and ionomycin (), T cells incubated with JY (▪), or autologous EBV-B (▧) stimulator cells. Note that the scale for IFNγ is 2 logs higher that the other 2. Neither the autologous EBV-B cells nor the JY cells produce any of the 3 cytokines tested here.
Discussion
Here we describe a retroviral reporter system that can be used to isolate viable, antigen-specific, cytotoxic, or helper T cells. T-cell blasts transduced with SIN-(NFAT)n-GFP up-regulate GFP after activation with either polyclonal stimuli or with cognate antigen. As expected, the NFAT-controlled GFP expression was blocked by the calcineurin inhibitors CsA and FK506. We have tested 2 retroviral constructs differing in the number of NFAT-binding sites. No differences were observed in the percentage of GFP positive cells between the SIN-(NFAT)3-GFP and the SIN-(NFAT)6-GFP after triggering of the T cells with PMA and ionomycin. However, 6 NFAT-binding sites allow for a considerably higher expression of GFP after activation than 3 binding sites, indicating that the level of GFP expression is dependent on the promoter activity of complexes of transcription factors bound to the multiple NFAT-binding sites. It is possible that the level of GFP expression after activation of transduced T cells can be enhanced by a further increase of the number of binding sites incorporated in the reporter construct. We observed that a small percentage of cells expressed GFP constitutively. Single-cell sorting of these cells yielded clones that also expressed GFP constitutively whether they were activated or not. The proportion of cells expressing GFP constitutively is not significantly influenced by the number of triplets used in the reporter construct. We conclude therefore that constitutive GFP expression is a reflection of random integration near an active promoter region in the genome of the targeted cells.
The SIN-(NFAT)6-GFP construct was used to test some aspects of this reporter system in an antigen-specific CD4+ T-cell clone (JS136), which is both cytotoxic and proliferative toward JY cells. Incubation of this clone with its target cell JY resulted in expression of GFP. This interaction is specific because the reactivity can be blocked with antibodies directed against HLA-DR. Interestingly, not all the T cells within the population of cloned cells responded to stimulation with its target cell in an overnight incubation. We have observed that 7% of the JS136 T cells up-regulated GFP after stimulation with JY, whereas 25% of the cells responded to stimulation with the combination of PMA and ionomycin. We have also seen this discrepancy in other clones with different specificities (Figure 4B and results not shown). These observations indicate that approximately one fourth to one third of the cells within a given population of cloned T cells is capable of responding to antigen stimulation by up-regulating GFP. This finding is well in agreement with observations that use intracellular staining for the production of cytokines in T-cell clones,20 studies on cloned Jurkat cells stably transfected with an NFAT-LacZ expression vector,2 and studies on cloned T-cell hybridomas carrying a vector with the mouse IL-2 promoter region coupled to the LacZ reporter gene.15
After having obtained proof of concept, we tested the possibility of isolating alloreactive T cells from a heterogeneous T-cell pool in 2 separate experiments. A very high proportion of clones obtained from T cells that expressed GFP after overnight stimulation with JY cells were antigen specific. As expected, this proportion was considerably higher than that of clones obtained from random cloning (without selecting the GFP+ cells) of the same T-cell blasts. Both cytotoxic CD8+ and proliferative CD4+ T-cell clones were obtained, indicating that our reporter system allows for isolation not only of IL-2–producing CD4+ T cells but also of CD8+ CTL, that mostly do not produce IL-2. This finding is consistent with the fact that NFAT-binding sites are implicated in the control of expression of multiple cytokines, among which IL-2 (reviewed in Rau et al1). Interestingly, apart from these “classical” clones, we also isolated T-cells clones that specifically up-regulated GFP after an overnight stimulation with JY target cells but were not cytolytic or proliferative toward JY cells. Some of these clones also specifically produced IL-4 and/or IL-10, but no IFNγ after stimulation with JY cells but not with the autologous EBV-B cells. Such T-cell clones would not have been isolated using cytotoxicity or proliferation as the only read out of functional activity. Nor would these clones have been isolated using cytoplasmic staining or ELISPOT-assays, as these assays are based on the production of IFNγ. It can therefore be envisioned that the present reporter system allows for isolation of T-cell clones with functions other than classical helper (Th) or CTL activities, for example, T cells with regulatory activities (Tregcells).21
It is clear that the current NFAT-GFP reporter system allows for an easier and more rapid isolation of antigen-specific T-cell clones than the frequently used method of repeated in vitro stimulations of polyclonal T cells, followed by random cloning of the responding T cells by limiting dilution5 or single-cell deposition. The recently developed tetramer technology also allows the visualization and subsequent isolation of antigen-specific T-cell clones from a polyclonal population of T cells.22 The use of tetramers has the limitation that prior knowledge of the MHC restriction element and the antigenic peptide is required for their application. This is not necessary for the reporter system presented here.
However, some drawbacks of the current system are that the T cells need to be brought in cycle before being transduced and that the transduction efficiencies are well below 100%. Moreover, not all cells within a clonal population of T cells up-regulate GFP on activation. Future improvements of this system include insertion of a marker under control of a constitutive promoter element in the same retroviral construct, which would permit isolation of the transduced cells before activation. A further improvement of our system would be the use of vectors that allow transduction of quiescent T cells.
Acknowledgments
We thank Dr H. M. Blau for the original self-inactivating retroviral construct, Dr G. Nolan for the original LZRSpBMN retroviral vector and the virus producer cell line Phoenix-A, Dr L. A. Herzenberg for plasmid NFATZ, Dr J. Borst for the T cell line JS136, Dr R. A. W. van Lier for HLA-DR (R3E2) antibodies, and Mr E. Notenboom and Mrs A. Pfauth for expert technical assistance in FACS-sorting.
Supported by grant 96-1275 from the Dutch Cancer Society, Amsterdam, The Netherlands.
Reprints:Hergen Spits, Department of Immunology, The Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Plesmanlaan 121, NL-1066CX, Amsterdam, The Netherlands; e-mail:hergen@nki.nl.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal