Abstract
To define the basis for faulty granulopoiesis in patients with severe congenital neutropenia (SCN), the expression of granulocyte colony-stimulating factor receptor (G-CSFR) in primitive myeloid progenitor cells and their responsiveness to hematopoietic factors were studied. Flow cytometric analysis of bone marrow cells based on the expression of CD34, Kit receptor, and G-CSFR demonstrated a reduced frequency of CD34+/Kit+/ G-CSFR+cells in patients with SCN. The granulocyte-macrophage colony formation of CD34+/Kit+/G-CSFR+ cells in patients was markedly decreased in response to G-CSF alone and to the combination of stem cell factor, the ligand for flk2/flt3, and IL-3 with or without G-CSF in serum-deprived semisolid culture. In contrast, no difference in the responsiveness of CD34+/Kit+/G-CSFR− cells was noted between patients with SCN and subjects without SCN. These results demonstrate that the presence of qualitative and quantitative abnormalities of primitive myeloid progenitor cells expressing G-CSFR may play an important role in the impairment of granulopoiesis in patients with SCN.
Introduction
Severe congenital neutropenia (SCN) is characterized by onset in early childhood, recurrent life-threatening infections, and profound neutropenia of less than 200 absolute neutrophil count (ANC)/μL peripheral blood.1-3 The bone marrow usually shows a paucity of mature myeloid cells with a maturation arrest of neutrophil precursors at the promyelocyte-myelocyte stage of differentiation. To date, the underlying pathophysiology of SCN remains unclear, though the administration of recombinant human granulocyte colony-stimulating factor (G-CSF) has induced an increase in circulating neutrophil counts in most patients.2-8 Bone marrow cells from patients with SCN frequently show a markedly reduced or a complete lack of responsiveness to G-CSF in in vitro culture.5,9-12 The role of G-CSF and G-CSF receptor (G-CSFR) in the stimulation of granulopoiesis has been documented through the analysis of G-CSF–deficient and G-CSFR–deficient mice.13-16 However, the deficiency of G-CSF, the lack of G-CSFR, or the G-CSFR mutation itself may not be a sufficient contributor to severe neutropenia and the SCN phenotype.15-18
We recently reported a defective proliferation of primitive myeloid progenitor cells from patients with SCN in response to hematopoietic factors including G-CSF.12 To define the role of G-CSFR in the growth of primitive myeloid progenitor cells in patients with SCN, we have analyzed myeloid progenitor cells expressing G-CSFR and studied their responsiveness to hematopoietic factors involved in myelopoiesis.
Study design
Patients
Five patients with SCN were enrolled in this study. The diagnosis of SCN or Kostmann syndrome was made according to accepted criteria, such as less than 200 ANC/μL peripheral blood, maturation arrest at the promyelocyte or myelocyte level in the bone marrow, absence of circulating antineutrophil antibodies as determined by granulocyte indirect immunofluorescence test, and onset of severe infections in early childhood.1-3 All patients had histories of recurrent life-threatening infection. Patient 1 continued to have recurrent skin abscesses and chronic gingivitis, and he has been maintained on daily subcutaneous administration of G-CSF for the last 7 years. The other 4 patients have received intermittent administration of G-CSF when infections were observed. None of the patients acquired myelodysplastic syndrome or acute myelogenous leukemia during the administration of G-CSF.
Separation and purification of bone marrow cells
In accordance with the institutional guidelines of the committee on human experimentation, bone marrow samples were obtained after informed consent was given for all participants (patients, their guardians, and healthy adult volunteers). Bone marrow cells used in this study were taken when G-CSF was administered to patient 1 but not to the other 4 patients with SCN. Separation and purification of bone marrow cells were performed according to methods reported previously.12 19 Flow cytometric analysis and cell sorting were carried out by a FACS Vantage (Becton Dickinson Immunocytometry Systems, San Jose, CA) equipped with a 4-W argon laser and a 35-mW helium neon laser. The following reagents were used for flow cytometry: fluorescein isothiocyanate (FITC)-labeled monoclonal anti-CD34 antibody (clone 581; Beckman Coulter, Fullerton, CA), phycoerythrin (PE)-conjugated anti-c-Kit (clone 95C3; Beckman Coulter), biotin-conjugated anti-G-CSFR (clone LMM741; PharMingen, San Diego, CA), streptavidin labeled with allophycocyanin (Caltag Laboratories, San Francisco, CA), and propidium iodide (PI; Sigma Chemical, St Louis, MO). The appropriate isotype controls were used to identify background staining. Data acquisition and analysis were performed using CellQuest software (Becton Dickinson Immunocytometry Systems).
Clonal cultures
Clonal cell culture in serum-deprived conditions was performed according to methods reported previously.12 19 The following hematopoietic factors were used: recombinant human G-CSF, recombinant human IL-3 with a specific activity of 1.0 × 108 U/mg, and recombinant human stem cell factor (SCF), supplied by Kirin Brewery (Tokyo, Japan). Recombinant human ligand for flk2/flt3 (FL) was purchased from PeproTech (Rocky Hill, NJ).
Results and discussion
Flow cytometric analysis of bone marrow cells
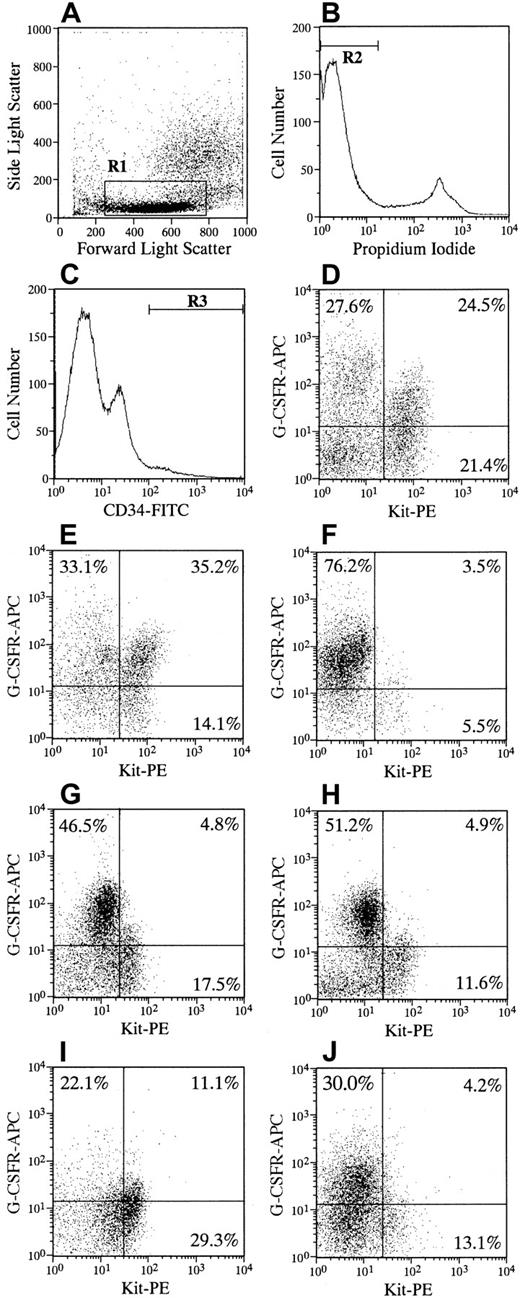
Figure 1 shows the results of the flow cytometric analysis of Kit and G-CSFR expression on CD34+ cells in 5 patients with SCN and in 2 representative subjects without SCN. The frequency of Kit+/G-CSFR+ expression on CD34+cells in patients was significantly decreased compared with that of subjects (P < .001). Some variations were seen in the frequencies of quadrant cells and in the staining pattern among patients with SCN. The total percentage of G-CSFR+ cells on CD34+ cells noted no significant difference between patients and subjects, resulting in a relative increase in the frequency of Kit−/G-CSFR+ cells in patients. The Kit−/G-CSFR+ on CD34+ cells yielded few granulocyte-macrophage (GM) colonies in response to hematopoietic factors, including G-CSF, in subjects and patients (data not shown). Thus, the significance of the inappropriate proportion of G-CSFR expression and the role of Kit−/G-CSFR+cells on CD34+ cells in patients with SCN in developing the myeloid progenitor cells remains to be elucidated. To exclude the possibility that endogenous or exogenous G-CSF in vivo affects the binding of G-CSFR antibody to G-CSFR, CD34+ cells from subjects were incubated with G-CSF before staining for biotin-conjugated G-CSFR antibody. However, the preincubation of G-CSF did not affect the expression of G-CSFR on CD34+/Kit+ cells (data not shown). These results indicate that the decrease in the frequency of CD34+/Kit+/G-CSFR+ expression in patients with SCN is not due to either high serum concentrations of G-CSF or the administration of G-CSF.
Flow cytometric analysis of CD34, Kit (CD117), and G-CSFR (CD114) expression of bone marrow cells.
One million cells were simultaneously incubated with FITC-labeled monoclonal anti-CD34, PE-conjugated anti–c-Kit, and biotin-conjugated anti–G-CSFR for 30 to 40 minutes at 4°C. Cells were then washed twice and stained with streptavidin labeled with allophycocyanin for 15 minutes at 4°C. After the addition of PI at a concentration of 1 μg/mL, cells were applied to FACS Vantage. The appropriate isotype controls, FITC-, PE-, and biotin-conjugated mouse IgG1a were used to identify background staining. More than 3 × 105 events were collected and then analyzed. Low to medium forward scatter and low side scatter (A, R1) negative for PI fluorescence (B, R2), and positive for CD34 (C, R3) gates were used. The expressions of Kit and G-CSFR within gated cells are shown for representative subjects without SCN (D, E) and 5 patients with SCN (F, patient 1; G, patient 2; H, patient 3; I, patient 4; J, patient 5). Quadrant percentages are indicated in each cytogram. The quadrant percentages (mean ± SD) of Kit+/G-CSFR+, Kit+/G-CSFR−, Kit−/G-CSFR+, and Kit−/G-CSFR− cells in CD34+ cells in 9 subjects were 33.4 ± 8.8, 16.1 ± 6.2, 26.4 ± 9.5, and 24.1 ± 13.6, respectively. Those from 5 patients were 5.9 ± 3.5, 15.4 ± 8.9, 45.2 ± 21.0, and 33.5 ± 13.6, respectively. The difference in the frequency of Kit+/G-CSFR+cells between subjects without SCN and patients with SCN was statistically significant (P < .001).
Flow cytometric analysis of CD34, Kit (CD117), and G-CSFR (CD114) expression of bone marrow cells.
One million cells were simultaneously incubated with FITC-labeled monoclonal anti-CD34, PE-conjugated anti–c-Kit, and biotin-conjugated anti–G-CSFR for 30 to 40 minutes at 4°C. Cells were then washed twice and stained with streptavidin labeled with allophycocyanin for 15 minutes at 4°C. After the addition of PI at a concentration of 1 μg/mL, cells were applied to FACS Vantage. The appropriate isotype controls, FITC-, PE-, and biotin-conjugated mouse IgG1a were used to identify background staining. More than 3 × 105 events were collected and then analyzed. Low to medium forward scatter and low side scatter (A, R1) negative for PI fluorescence (B, R2), and positive for CD34 (C, R3) gates were used. The expressions of Kit and G-CSFR within gated cells are shown for representative subjects without SCN (D, E) and 5 patients with SCN (F, patient 1; G, patient 2; H, patient 3; I, patient 4; J, patient 5). Quadrant percentages are indicated in each cytogram. The quadrant percentages (mean ± SD) of Kit+/G-CSFR+, Kit+/G-CSFR−, Kit−/G-CSFR+, and Kit−/G-CSFR− cells in CD34+ cells in 9 subjects were 33.4 ± 8.8, 16.1 ± 6.2, 26.4 ± 9.5, and 24.1 ± 13.6, respectively. Those from 5 patients were 5.9 ± 3.5, 15.4 ± 8.9, 45.2 ± 21.0, and 33.5 ± 13.6, respectively. The difference in the frequency of Kit+/G-CSFR+cells between subjects without SCN and patients with SCN was statistically significant (P < .001).
The proportion of CD34+ cells in the bone marrow of patients with SCN was comparable to that in subjects, resulting in a reduction in the absolute number of CD34+/Kit+cells expressing G-CSFR in patients. The CD34 antigen and Kit receptor identify cell populations that are enriched for pluripotent and lineage-restricted hematopoietic progenitor cells in vitro, and some of them are capable of bone marrow reconstitution in vivo.20 21 The decreased number of CD34+/Kit+/G-CSFR+ cells may be indicative of a defective origin of the myeloid progenitor for neutropenia in patients with SCN.
Granulocyte-macrophage colony formation of CD34+/Kit+ cells
According to the expression of CD34, Kit, and G-CSFR, the CD34+/Kit+/G-CSFR+ and CD34+/Kit+/G-CSFR− cells were purified. Results of GM colony formation of purified cells are presented in Table 1. The number of GM colonies of CD34+/Kit+/G-CSFR+cells in patients with SCN was significantly reduced in response to G-CSF alone and to the combination of SCF, FL, and IL-3 with or without G-CSF, which are primarily involved in myelopoiesis,22-24compared with the number of colonies in subjects. The CD34+/Kit+/G-CSFR− cells failed to respond to G-CSF in both subjects and patients. There was no difference in the number of GM colonies of CD34+/Kit+/G-CSFR− cells supported with SCF, FL, and IL-3 with or without G-CSF between subjects and patients. Similarly, the CD34+/Kit+/G-CSFR+, but not CD34+/Kit+/G-CSFR−, cells showed a decreased proliferation in response to SCF, FL, and IL-3 with or without G-CSF in liquid suspension culture (data not shown). We recently reported direct evidence, using a single-cell proliferation assay, of the defective proliferation of CD34+/Kit+ cells in patients with SCN.12 Taken together, the faulty granulopoiesis of CD34+/Kit+/G-CSFR+ cells in patients suggests that abnormal responsiveness to hematopoietic factors in patients with SCN lies in primitive myeloid progenitor cells expressing G-CSFR.
Formation of granulocyte-macrophage colonies by purified cells supported with various factors
| Factors . | No. of GM colonies . | |||
|---|---|---|---|---|
| Patients (n = 5) . | Subjects (n = 9) . | |||
| Mean ± SD . | Range . | Mean ± SD . | Range . | |
| CD34+/Kit+/G-CSFR+cells | ||||
| G-CSF (1 ng/mL) | 0 ± 0 | 0-1 | 3 ± 1 | 2-6 |
| G-CSF (10 ng/mL) | 2 ± 1* | 1-5 | 16 ± 3 | 11-20 |
| G-CSF (100 ng/mL) | 7 ± 1* | 6-8 | 29 ± 6 | 21-35 |
| G-CSF (1000 ng/mL) | 8 ± 2* | 6-11 | 30 ± 7 | 21-40 |
| SCF, FL, IL-3 | 14 ± 3† | 11-16 | 30 ± 8 | 25-46 |
| SCF, FL, IL-3, G-CSF (100 ng/mL) | 28 ± 3* | 23-30 | 67 ± 8 | 56-76 |
| CD34+/Kit+/G-CSFR−cells | ||||
| G-CSF (10 ng/mL) | 1 ± 1 | 0-2 | 1 ± 1 | 0-2 |
| G-CSF (100 ng/mL) | 3 ± 2 | 1-5 | 2 ± 1 | 1-4 |
| G-CSF (1000 ng/mL) | 3 ± 1 | 1-4 | 4 ± 2 | 1-5 |
| SCF, FL, IL-3 | 23 ± 6 | 17-30 | 18 ± 6 | 14-29 |
| SCF, FL, IL-3, G-CSF (100 ng/mL) | 41 ± 8 | 28-48 | 36 ± 5 | 31-48 |
| Factors . | No. of GM colonies . | |||
|---|---|---|---|---|
| Patients (n = 5) . | Subjects (n = 9) . | |||
| Mean ± SD . | Range . | Mean ± SD . | Range . | |
| CD34+/Kit+/G-CSFR+cells | ||||
| G-CSF (1 ng/mL) | 0 ± 0 | 0-1 | 3 ± 1 | 2-6 |
| G-CSF (10 ng/mL) | 2 ± 1* | 1-5 | 16 ± 3 | 11-20 |
| G-CSF (100 ng/mL) | 7 ± 1* | 6-8 | 29 ± 6 | 21-35 |
| G-CSF (1000 ng/mL) | 8 ± 2* | 6-11 | 30 ± 7 | 21-40 |
| SCF, FL, IL-3 | 14 ± 3† | 11-16 | 30 ± 8 | 25-46 |
| SCF, FL, IL-3, G-CSF (100 ng/mL) | 28 ± 3* | 23-30 | 67 ± 8 | 56-76 |
| CD34+/Kit+/G-CSFR−cells | ||||
| G-CSF (10 ng/mL) | 1 ± 1 | 0-2 | 1 ± 1 | 0-2 |
| G-CSF (100 ng/mL) | 3 ± 2 | 1-5 | 2 ± 1 | 1-4 |
| G-CSF (1000 ng/mL) | 3 ± 1 | 1-4 | 4 ± 2 | 1-5 |
| SCF, FL, IL-3 | 23 ± 6 | 17-30 | 18 ± 6 | 14-29 |
| SCF, FL, IL-3, G-CSF (100 ng/mL) | 41 ± 8 | 28-48 | 36 ± 5 | 31-48 |
GM indicates granulocyte-macrophage, G-CSFR, granulocyte colony-stimulating factor receptor; SCF, stem cell factor; FL, flk2/flt3 ligand; IL-3, interleukin-3; SCN, severe congenital neutropenia.
Cultures were grown under serum-deprived conditions. One milliliter of the culture mixture contained 250 purified cells, 1% deionized crystallized bovine serum albumin, 300 μg/mL fully iron-saturated human transferrin, 10 μg/mL soybean lecithin, 6 μg/mL cholesterol, 1 × 10−7 mol/L sodium selenite, 10 μg/mL insulin, 4.5 mmol/L L-glutamine, 1.5 mmol/L glycine, 5 × 10−5mol/L 2-mercaptoethanol, 1.2% 1500 cP methylcellulose, and designated factors. Cultures were incubated for 14 days at 37°C in a humidified atmosphere with 5% CO2/95% air. The concentrations of SCF, FL, and IL-3 were 100 ng/mL, 50 ng/mL, and 100 U/mL, respectively. GM colonies included pure granulocyte colonies consisting primarily of neutrophils and their precursors and of mixed GM colonies consisting mainly of neutrophils, macrophages-monocytes, and their precursors. All assays were carried out in triplicate cultures. The CD34+/Kit+/G-CSFR+ and CD34+/Kit+/G-CSFR− cells were purified according to flow cytometric analysis based on the expression of Kit and G-CSFR on CD34 cells, as shown in Figure 1.
P < .001, compared with subjects without SCN.
P < .01, compared with subjects without SCN.
G-CSFR–deficient mice show a modest but significant reduction in the total number of hematopoietic colonies formed in response to pokeweed mitogen-stimulated conditioned media, IL-3, GM-CSF, or SCF.16 These data demonstrate that G-CSFR is required for the maintenance of a normal number of hematopoietic progenitor cells. In the current study, CD34+/Kit+/G-CSFR+ cells of patients with SCN showed a reduced number of GM colony formations in response to a combination of SCF, FL, and IL-3, irrespective of the presence or absence of G-CSF. This evidence also suggests that functional G-CSFR is necessary for the full stimulation of hematopoietic cells in response to hematopoietic factors. Alternatively, the decrease in the responsiveness of CD34+/Kit+/G-CSFR+ cells might reflect the functional abnormality of the G-CSFR or G-CSFR–mediated signal pathway in patients with SCN.
On the basis of abnormalities in CD34+/Kit+/G-CSFR+ cells in patients with SCN, the key to detecting the underlying pathophysiology of SCN is to clarify the significance of G-CSFR expression on primitive myeloid progenitor cells in the development of hematopoietic progenitor cells associated with cell survival.25 It is likely that the quantitative abnormality of CD34+/Kit+/G-CSFR+ cells might be a consequence of an underlying primary cellular defect of patients with SCN. Further studies are required to search for the origin of the quantitative and qualitative abnormalities of primitive myeloid progenitor cells expressing G-CSFR in patients with SCN.
Acknowledgment
We thank Kirin Brewery (Tokyo, Japan) for providing the cytokines.
Supported in part by Grants-in-Aid for Scientific Research (C) from the Ministry of Education, Science, Sports, and Culture of Japan (M.K., K.U.).
K.N. and M.K. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Masao Kobayashi, Department of Child Health, Faculty of Education, Hiroshima University, 1-1-1 Kagamiyama Higashi-Hiroshima, Hiroshima, 739-8524 Japan; e-mail:masa@mcai.med.hiroshima-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal