Abstract
CD30 is a member of the tumor necrosis factor (TNF) receptor superfamily that is expressed on activated lymphocytes, as well as on neoplastic cells of Hodgkin disease (HD) and anaplastic large cell lymphoma (ALCL). A number of reports have shown that, depending on cellular context, CD30 signaling can exert a variety of effects, ranging from cell death to cellular proliferation. In the present study this disparity was examined, using a number of ALCL- and HD-derived cell lines. Activation of CD30 led to the induction of apoptotic death of ALCL cells, along with the selective reduction of TNF receptor-associated factor 2 and impairment in the ability of these cells to activate the pro-survival transcription factor nuclear factor κB (NF-κB). In contrast, HD cells, which constitutively express NF-κB, were not susceptible to CD30-induced apoptosis but could be sensitized following ectopic overexpression of a superdominant IκB. These studies suggest that NF-κB plays a determining role in the sensitivity or resistance of lymphoma cells to CD30-induced apoptosis, which may have important consequences in the clinical treatment of CD30-positive neoplasia.
Introduction
The tumor necrosis factor (TNF) receptor superfamily is a group of related cell-surface receptors that include the TNF and lymphotoxin receptors, CD95/Fas/Apo-1, CD40, and CD30.1-4 Signal transduction events mediated by members of this family have been shown to elicit a broad spectrum of cellular responses, including the induction of gene expression through the activation of transcription factors, the stimulation of proliferation, and the initiation of the apoptotic cell death pathway.5-8Several TNF receptor family members, including TNFR1 and Fas, contain an approximately 80-residue motif, designated the death domain.9-14 Signaling through these receptors has been shown to induce apoptosis in a manner that depends on oligomerization of the death domain of the receptors with similar death domains of cytoplasmic factors, such as FADD and TRADD, which, in turn, recruit caspases, the central effector proteases of the cell death program.15,16 In many experimental systems, inhibitors of protein synthesis are used in conjunction with TNF to induce apoptosis, because TNF alone is often insufficient to activate the apoptotic cascade. It is widely thought that this insensitivity to TNF is due to the activation of nuclear factor κB (NF-κB), a transcription factor that activates the expression of anti-apoptotic genes.17-20 The signaling cascade that leads to the activation of NF-κB is thought to be mediated by TNFR1 through TNF receptor-associated factor 2 (TRAF2), a signaling intermediate that has been shown to be recruited to the cytoplasmic tail of TNFR1 through a TNFR1-TRADD-TRAF2 interaction.16,21,22 It has been proposed that TNFR1 can either induce apoptosis through FADD and caspase recruitment or promote survival through TRAF recruitment and NF-κB induction.17-19,22-24 It is, therefore, likely that the sensitivity of cells to TNF might be modulated by additional signaling pathways. One possible stage at which additional signaling cascades could regulate this sensitivity would be to affect the ability of TNFR1 to activate NF-κB. Consistent with this hypothesis, impairment of NF-κB has been shown to greatly sensitize cells to TNF-induced death.17,18 25
Other members of the TNF receptor family, including TNFR2 and CD30, do not contain death domains within their cytoplasmic tails but in certain circumstances have also been shown to induce apoptosis.26-29 In the case of TNFR2 and CD30, several members of the TRAF family of signaling intermediates have been found to associate directly with short, acidic elements within their cytoplasmic domains.30-34 We previously suggested a model by which TNFR2 or CD30 indirectly induces apoptosis by sensitizing cells to TNFR1-induced cell death through a signal-mediated degradation of the TRAF proteins.25 TRAF degradation would compromise the ability of death domain-containing receptors (such as TNFR1) to activate NF-κB and, thus, would potentiate signaling through their death domains.
CD30 is normally found on the surface of activated T cells,35,36 but it has also been detected on a variety of cell types of hematopoietic origin, including neoplastic cells of Hodgkin disease (HD) and anaplastic large cell lymphoma (ALCL).37,38 Although its function is largely unknown, CD30 has been implicated both in cell death and proliferation.27,39 CD30-deficient mice have a mild impairment in thymic negative selection,40 and adoptive transfer studies suggest that the receptor is involved in limiting the expansion of autoreactive T lymphocytes.41
The aim of the present study was to determine whether sensitivity to CD30 activation in lymphoma cells correlates with TRAF2 stability and with the competence to activate NF-κB. The hypothesis was tested that CD30 activation would fail to induce NF-κB in CD30-sensitive cells. Activation of CD30 in ALCL cells was found to lead to the induction of apoptotic death, the selective reduction of TRAF2, and an impairment in the ability of these cells to activate NF-κB. In contrast, HD-derived cells that constitutively express NF-κB were found to be resistant to CD30-induced death. These cells became susceptible to CD30-induced death on inhibition of NF-κB. These studies suggest that NF-κB, but not TRAF2 stability, plays a determining role in the sensitivity or resistance of lymphoma cells to CD30-induced apoptosis.
Materials and methods
Cells and transfections
The ALCL cell lines Karpas 299 and Michel (a gift from Dr W. Murphy, National Cancer Institute, Frederick, MD) were maintained in RPMI with 10% fetal bovine serum, 2 mmol/L glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin and grown at 37°C in 5% CO2. Michel cells were transfected by electroporation. Cells were resuspended at a concentration of 2 × 107 cells/mL in complete media. The indicated plasmids were added to 400 μL of cells, followed by incubation at room temperature for 5 minutes. Cells and DNA were transferred to electroporation cuvettes (BTX, San Diego, CA) with a 4-mm gap and electroporated at 260 V, 1050 μF, and 720 Ω, using a BTX Electro Cell Manipulator 600. After electroporation, cells were placed immediately in 8 mL fresh media and incubated at 37°C for 24 hours before experiments were performed as indicated. The HD cell lines KM-H2, L428, and L591 were maintained in RPMI with 20% fetal bovine serum, 2 mmol/L glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin and grown at 37°C in 5% CO2. L428 cells were also transfected by electroporation, with the following modifications. Cells were washed once in sterile phosphate-buffered saline (PBS) and resuspended at a concentration of 2 × 107 cells/mL in PBS. A total of 15 μg plasmid DNA and 10 μg carrier DNA (Research Genetics, Huntsville, AL) were added to 400 μL of cells followed by incubation on ice for 10 minutes. Cells and DNA were transferred to electroporation cuvettes (4-mm gap) and electroporated at 600 V and 360 Ω. Following electroporation, cells were incubated on ice for 10 minutes and then diluted with 2.5 mL media. After incubation at room temperature for 10 minutes, cells were incubated at 37°C.
Plasmids
Immobilization of CD30 agonistic antibodies and viability assays
CD30 agonistic antibody M67 (a kind gift of Immunex Corporation) or immunoglobulin G1 (IgG1) isotype control antibody (Pharmingen) was resuspended in PBS at a concentration of 20 μg/mL, and 100 μL was added to wells of flat-bottom 96-well plates (Costar). After antibody immobilization at 4°C for 36 hours, wells were washed 3 times with PBS containing 1% bovine serum albumin (PBS-BSA) and blocked with 200 μL of PBS-BSA at 4°C overnight. Cells were resuspended at a concentration of 1 × 106 cells/mL, and 1 × 105 cells were added to each well. After incubation of cells with immobilized antibodies at 37°C, viability was measured by flow cytometry, using propidium iodide (PI) exclusion. Cells were washed once with PBS containing 1% BSA and then incubated with staining solution (10 mmol/L HEPES pH 7.9, 140 mmol/L NaCl, 2.5 mmol/L CaCl2) containing 2 μg/mL PI. Flow cytometry was performed as described previously.44 Experiments were performed in duplicate.
Reporter gene analysis
Michel cells were transfected with 15 μg of a κB-responsive luciferase reporter plasmid containing 2 canonical κB sites by electroporation. Transfectants were maintained for 24 hours and stimulated where indicated with either CD30 agonistic antibodies or with phorbol 12-myristate 13-acetate (PMA; 20 ng/mL). Cells were harvested 12 hours later, and luciferase reporter gene activity was assayed as described previously.33
Viability assays for transient transfections
L428 cells were transfected with 5 μg of a plasmid encoding green fluorescence protein (GFP, EGFP-C1; Clontech) along with either 10 μg of a plasmid encoding superdominant IκBα or a control plasmid as indicated in the text. Cells were maintained for 24 hours before incubation with either CD30 agonistic antibodies or isotype control antibodies. Flow cytometry was performed 18 hours later to determine viability of transfected cells. Viable, transfected cells were quantitated by gating on GFP-positive cells incubated with either isotype control antibodies or CD30 agonistic antibodies. Percentage of cell death due to CD30 activation was calculated as follows: [1 − (number of viable cells from wells with M67 antibodies / number of viable cells from wells with isotype control antibodies) × 100].
Immunoblot analysis
Cells were incubated with immobilized CD30 agonistic antibodies or isotype control antibodies for 12 hours, and lysates were prepared as follows: Cells were washed once in PBS and lysed in lysis buffer (25 mmol/L HEPES at pH 7.9, 100 mmol/L NaCl, 1 mmol/L EDTA, 1% Triton X-100, 10% glycerol, 10 mmol/L dithiothreitol, 1 mmol/L phenylmethylsulfonyl fluoride, and a cocktail of protease inhibitors [Complete tablets; Boehringer Mannheim, Indianapolis, IN]). Protein concentration was determined by the method of Bradford.45Protein lysates were resolved by 4% to 12% gradient sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Novex, Carlsbad, CA), transferred to nitrocellulose membranes (Novex), incubated with antibodies to human TRAF2 (C-20; Santa Cruz Biotechnologies, Santa Cruz, CA) or to human β-tubulin (Pharmingen, San Diego, CA), and visualized by enhanced chemiluminescence (ECL; Amersham. Piscataway, NJ). Lysates of unstimulated cells were prepared similarly to examine IκB and NF-κB subunits, using antibodies to human IκBα (Pharmingen), to IκBβ (Santa Cruz Biotechnologies), and to the NF-κB subunits p50, p52, p65 (RelA), RelB, and c-Rel (kindly provided by Dr N. Rice, NCI, Frederick, MD).
Flow cytometry analysis
Expression of CD30 on ALCL and Hodgkin cell lines was confirmed by flow cytometry, using a fluorescein isothiocyanate (FITC)-conjugated anti-CD30 antibody (Pharmingen).
Results
CD30 mediates apoptosis in ALCL cells but not HD cells
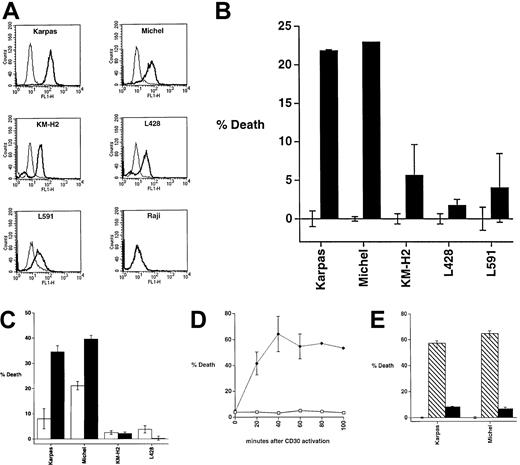
CD30 agonistic antibodies have been shown to inhibit the proliferation of ALCL cell lines.46 To determine whether CD30 activation induces death of ALCL cells, the ALCL cell lines Karpas 299 and Michel were incubated with the CD30 agonistic antibody M67 or isotype control antibodies, and cell death was evaluated by PI exclusion. For comparison, the HD cell lines KM-H2, L428, and L591 were also tested for susceptibility to CD30-induced death. The ALCL and HD cell lines express similar levels of the CD30 receptor (Figure1A). CD30 activation induced death in ALCL cells but not in HD cells (Figure 1B). Similar effects were observed with M44, another CD30-specific monoclonal antibody (data not shown).
CD30 activation induces apoptotic death of ALCL cells but not of HD cells.
(A) The ALCL cell lines Karpas 299 and Michel and the HD cell lines KM-H2, L428, and L591 were stained for CD30 and examined by flow cytometry for CD30 expression. The Raji cell line was used as a negative control. The thinner lines indicate the FITC-isotype control antibody and the bold lines indicate a FITC-conjugated anti-CD30 antibody. (B) The ALCL cell lines Karpas and Michel and the HD cell lines KM-H2, L428, and L591 were incubated for 24 hours with the immobilized CD30 agonistic antibody M67 (▪) or isotype control antibodies (■) and measured for viability by PI exclusion after 24 hours. (C) The ALCL cell lines Karpas and Michel and the HD cell lines KM-H2 and L428 were preincubated with either a media control (□) or with the protein synthesis inhibitor cycloheximide (10 μg/mL) (■) and then incubated for 24 hours with the immobilized CD30 agonistic antibody before viability was measured. For each cell line, the viability of cells incubated with control IgG antibody was defined as zero. The viabilities of cells treated with cycloheximide plus IgG was indistinguishable from IgG alone. (D) Michel cells were incubated with immobilized CD30 agonistic antibody (♦) or isotype control antibody (■) for the indicated periods of time prior to viability analysis. (E) Karpas and Michel cells were preincubated with cycloheximide (2 μg/mL) in addition to the general caspase inhibitor ZVAD-fmk (▪, 50 μmol/L in dimethyl sulfoxide; Enzyme Systems Products, Livermore, CA) or a mock control followed by incubation with the immobilized CD30 agonistic antibodies M67 (▧) or isotype control antibodies (■). Viability was measured by PI exclusion 24 hours later.
CD30 activation induces apoptotic death of ALCL cells but not of HD cells.
(A) The ALCL cell lines Karpas 299 and Michel and the HD cell lines KM-H2, L428, and L591 were stained for CD30 and examined by flow cytometry for CD30 expression. The Raji cell line was used as a negative control. The thinner lines indicate the FITC-isotype control antibody and the bold lines indicate a FITC-conjugated anti-CD30 antibody. (B) The ALCL cell lines Karpas and Michel and the HD cell lines KM-H2, L428, and L591 were incubated for 24 hours with the immobilized CD30 agonistic antibody M67 (▪) or isotype control antibodies (■) and measured for viability by PI exclusion after 24 hours. (C) The ALCL cell lines Karpas and Michel and the HD cell lines KM-H2 and L428 were preincubated with either a media control (□) or with the protein synthesis inhibitor cycloheximide (10 μg/mL) (■) and then incubated for 24 hours with the immobilized CD30 agonistic antibody before viability was measured. For each cell line, the viability of cells incubated with control IgG antibody was defined as zero. The viabilities of cells treated with cycloheximide plus IgG was indistinguishable from IgG alone. (D) Michel cells were incubated with immobilized CD30 agonistic antibody (♦) or isotype control antibody (■) for the indicated periods of time prior to viability analysis. (E) Karpas and Michel cells were preincubated with cycloheximide (2 μg/mL) in addition to the general caspase inhibitor ZVAD-fmk (▪, 50 μmol/L in dimethyl sulfoxide; Enzyme Systems Products, Livermore, CA) or a mock control followed by incubation with the immobilized CD30 agonistic antibodies M67 (▧) or isotype control antibodies (■). Viability was measured by PI exclusion 24 hours later.
One possible explanation for the different sensitivities of the ALCL and HD cell lines is that on CD30 activation, ALCL cells can induce the expression of pro-apoptotic factors, but that HD cells cannot. The alternative possibility is that ALCL cells cannot induce the expression of pro-survival factors, but that HD cells can. To distinguish between these 2 possibilities, the effect of protein synthesis inhibitors on CD30-mediated death was examined. ALCL and HD cell lines were preincubated with the protein synthesis inhibitor cycloheximide and then tested for susceptibility to CD30-induced death. Inhibition of protein synthesis potentiated CD30-induced death of ALCL cells but not of HD cells (Figure 1C). Cycloheximide alone did not induce cell death. These findings suggest that, in ALCL cells, the apoptotic machinery exists prior to CD30 activation and that the cell death is potentiated through the inhibition of de novo expression of pro-survival factors.
To determine the length of time required for CD30 activation to induce cell death, Michel cells were cross-linked with the CD30 agonistic antibody or an isotype control for specific periods of time, as indicated in Figure 1D. Interestingly, cell death was found to occur rapidly following CD30 activation, with the majority of death occurring within 40 minutes after CD30 cross-linking (Figure 1D).
To test whether CD30-induced death of ALCL cells occurs by an apoptotic pathway, Michel and Karpas 299 cells were incubated with the caspase inhibitor ZVAD-fmk and subsequently tested for susceptibility to CD30 activation. Inhibition of caspases markedly inhibited CD30-mediated death of these cells (Figure 1E), suggesting that CD30 induces death of ALCL cells through an apoptotic pathway.
Our findings suggested that ALCL and HD cells differ in their responsiveness to CD30. In the following set of experiments, comparable results were seen with Karpas 299, KM-H2, and L591 cells, but for clarity only the results of Michel and L428 cells are shown.
ALCL cells are unable to activate NF-κB in response to CD30 cross-linking
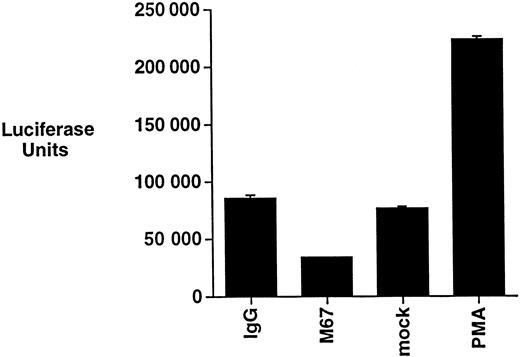
The regulation of NF-κB has been shown to modulate the apoptotic threshold of cells.17-19 CD30 has been reported to induce NF-κB activation in a manner that depends on the ability of the receptor to recruit TRAF2.32,33,47 The ability of CD30 to induce NF-κB activation was, therefore, examined in Michel cells by incubating cells with CD30 agonistic antibodies and then evaluating NF-κB activation by luciferase reporter gene assays. As a control, Michel cells were stimulated with PMA, a phorbol ester previously shown to activate NF-κB in many cell lines.48 CD30 was found not to induce NF-κB activation, whereas under the same conditions PMA resulted in a significant activation (Figure2). These data suggest that, although ALCL cells have retained the general ability to activate NF-κB, CD30 activation is unable to activate this pro-survival transcription factor in these cells.
CD30 activation does not induce NF-κB activation in ALCL cells.
Michel cells were transfected with a luciferase reporter containing NF-κB binding sites. Cells were incubated with the immobilized CD30 agonistic antibodies M67 or isotype control antibodies for 12 hours and assayed for luciferase activity. Transfected cells were also incubated with either PMA (20 ng/mL) or a media control under the same conditions and assayed for luciferase activity.
CD30 activation does not induce NF-κB activation in ALCL cells.
Michel cells were transfected with a luciferase reporter containing NF-κB binding sites. Cells were incubated with the immobilized CD30 agonistic antibodies M67 or isotype control antibodies for 12 hours and assayed for luciferase activity. Transfected cells were also incubated with either PMA (20 ng/mL) or a media control under the same conditions and assayed for luciferase activity.
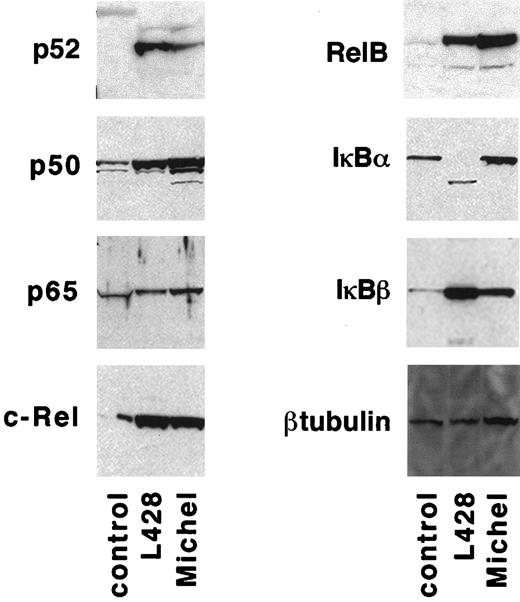
One possible explanation for the inability of CD30 activation to induce NF-κB in ALCL cells is that one or more of the NF-κB subunits might be defective or absent. Therefore, immunoblot analysis was performed to compare the levels of NF-κB subunits in protein lysates from Michel cells, the HD-derived L428 cells, and control cells (the 293 embryonic kidney cell line). The NF-κB subunits p50, p52, p65, RelB, and c-Rel were expressed similarly in lysates of HD and ALCL cells and co-migrated with the corresponding species in control lysates (Figure3). The inability of CD30 to activate NF-κB in ALCL cells might, therefore, be explained by a defect upstream of the NF-κB subunits themselves, most likely in the CD30 signaling pathway.
HD cells have a specific defect in IκBα.
Protein lysates were prepared from 1 × 107 control cells (293 cells), L428 cells, and Michel cells and examined for IκB and NF-κB species by immunoblot analysis as described in “Materials and methods.”
HD cells have a specific defect in IκBα.
Protein lysates were prepared from 1 × 107 control cells (293 cells), L428 cells, and Michel cells and examined for IκB and NF-κB species by immunoblot analysis as described in “Materials and methods.”
Inhibition of NF-κB in L428 cells sensitizes cells to CD30-induced death
HD cells have been reported to have constitutive activation of NF-κB49-53 due to a defect of its inhibitor IκBα. To determine whether ALCL cells contain a similar defect, immunoblot analysis was performed to compare levels of IκB subunits in lysates of L428 cells, Michel cells, and control cells (293 embryonic kidney cells). Although the species representing IκBβ in HD and ALCL cell lysates co-migrated with the species in the control lysate, L428 cells were found to contain an IκBα species that migrated faster than that of the ALCL and control lysate (Figure 3), supporting previous reports that HD cells have a specific defect in IκBα. Unstimulated L428 cells, but not Michel cells, were found to contain NF-κB constitutively present in the nucleus as measured by gel retardation analysis (data not shown). These data suggest that the ability to regulate NF-κB activation through IκB has been maintained in ALCL cells but not in HD cells.
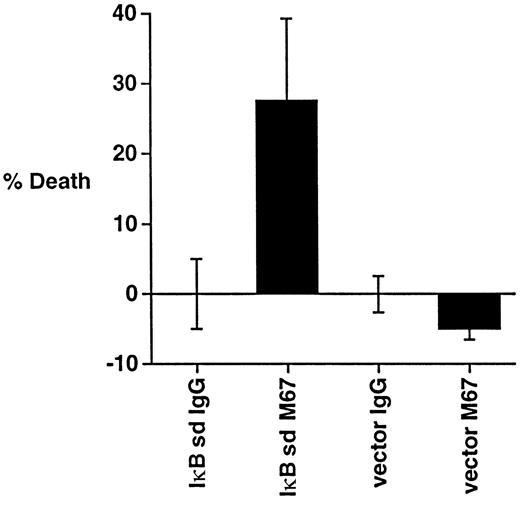
Previous studies have reported that constitutive NF-κB activation protects HD cells against apoptosis induced by growth factor withdrawal.54 To test whether inhibition of NF-κB might sensitize HD cells to CD30-induced death, L428 cells were transfected with a construct encoding an IκBα protein that is resistant to signal-induced degradation (superdominant IκBα), followed by incubation with CD30 agonistic antibodies. Expression of superdominant IκBα sensitized L428 cells to CD30-induced death (Figure4), suggesting that inhibition of NF-κB in HD cells renders these cells susceptible to CD30-induced death by other stimuli.
Inhibition of NF-κB sensitizes HD cells to CD30-induced death.
L428 cells were transfected with 5 μg of a GFP plasmid, along with 10 μg of either a control plasmid (pEBB) or a superdominant (sd) IκBα plasmid as indicated. Cells were incubated with the immobilized CD30 agonistic antibodies M67 or isotype control antibodies for 18 hours before measuring for viability as described in “Materials and methods.”
Inhibition of NF-κB sensitizes HD cells to CD30-induced death.
L428 cells were transfected with 5 μg of a GFP plasmid, along with 10 μg of either a control plasmid (pEBB) or a superdominant (sd) IκBα plasmid as indicated. Cells were incubated with the immobilized CD30 agonistic antibodies M67 or isotype control antibodies for 18 hours before measuring for viability as described in “Materials and methods.”
CD30 mediates the reduction of TRAF2 in ALCL and HD cells
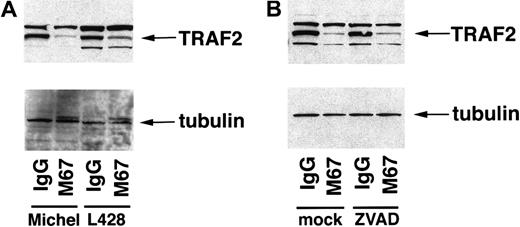
Overexpression of activated CD30 in 293 cells has been shown to sensitize cells to TNFR1-mediated apoptosis through a CD30-mediated degradation of the TRAF2 protein.25 These studies suggested that blocking TRAF2 signaling led to an inhibition of NF-κB, allowing TNF-induced apoptosis to proceed. TRAF2 protein levels were examined in ALCL cells as well as HD cells to determine whether reduction of endogenous TRAF2 occurs in these cells in response to CD30 activation. Michel and L428 cells were incubated with either CD30 agonistic antibodies or isotype control antibodies, followed by preparation of lysates and examination by immunoblot analysis for the presence of TRAF2 protein. The band designated as TRAF2 in lysates of Michel and L428 cells co-migrated with the TRAF2 species from lysates of 293 cells transfected with a plasmid encoding TRAF2 (data not shown). Endogenous TRAF2 in ALCL cells as well as HD cells was reduced in response to CD30 activation compared to the isotype control samples (Figure 5A). This reduction was specific to TRAF2, as a control protein (β-tubulin) was unaffected by CD30 activation. These data suggest that CD30 activation induces a signal-mediated degradation of endogenous TRAF2 protein in ALCL and HD cells.
CD30 mediates the reduction of TRAF2 in ALCL and HD cells.
(A) Michel and L428 cells were incubated with the immobilized CD30 agonistic antibodies M67 or isotype control antibodies. Cells were harvested 12 hours later and standardized for protein levels, and TRAF2 and β-tubulin were detected by immunoblot analysis as described in “Materials and methods.” Michel lysates (16 μg) and L428 lysates (26 μg) were loaded in the indicated lanes. (B) Michel cells were preincubated with either a media control or ZVAD-fmk (50 μmol/L) before incubation with the immobilized CD30 agonistic antibodies M67 or isotype control antibodies. Cells were harvested 12 hours later and standardized for protein levels, and TRAF2 and β-tubulin were detected by immunoblot analysis as described in “Materials and methods.” Michel lysates (12.3 μg) were loaded in the indicated lanes.
CD30 mediates the reduction of TRAF2 in ALCL and HD cells.
(A) Michel and L428 cells were incubated with the immobilized CD30 agonistic antibodies M67 or isotype control antibodies. Cells were harvested 12 hours later and standardized for protein levels, and TRAF2 and β-tubulin were detected by immunoblot analysis as described in “Materials and methods.” Michel lysates (16 μg) and L428 lysates (26 μg) were loaded in the indicated lanes. (B) Michel cells were preincubated with either a media control or ZVAD-fmk (50 μmol/L) before incubation with the immobilized CD30 agonistic antibodies M67 or isotype control antibodies. Cells were harvested 12 hours later and standardized for protein levels, and TRAF2 and β-tubulin were detected by immunoblot analysis as described in “Materials and methods.” Michel lysates (12.3 μg) were loaded in the indicated lanes.
To determine whether caspases are involved in CD30-mediated TRAF2 reduction, Michel cells were preincubated with the general caspase inhibitor ZVAD-fmk followed by incubation with immobilized CD30 agonistic antibodies or isotype control antibodies. Cell lysates were prepared and examined for the presence of TRAF2 or β-tubulin. Although ZVAD-fmk inhibited CD30-mediated apoptosis of ALCL cells (Figure 1D), the addition of ZVAD-fmk had no effect on the reduction of TRAF2 induced by CD30 activation (Figure 5B). These data suggest that CD30 mediates the reduction of TRAF2 independently of caspase activation in ALCL cells.
Discussion
The TNF receptor superfamily can be divided into 2 groups based on the presence or absence of death domain motifs within their cytoplasmic tails.55 Those receptors with death domains, such as TNFR1 and Fas, induce apoptosis of cells through dimerization of the death domains of these receptors with similar death domains of signaling intermediates such as FADD, which, in turn, recruit and activate caspases.55 It has been observed, however, that receptors with death domains can also promote the survival of cells through the recruitment of the TRAFs, signaling intermediates that promote the activation of NF-κB and thus the up-regulation of pro-survival genes.23
The question of how TNF receptors with death domains choose between initiating the pro-survival pathway or the pro-death pathway remains unanswered. One possibility is that death domain-containing receptors engage the pro-death pathway or pro-survival pathway in a manner that depends on crosstalk with receptors lacking death domains, such as TNFR2 or CD30. One outcome of CD30 activation is the degradation of the signaling intermediate TRAF2. We and others26-29 have previously predicted that this would serve to disrupt the NF-κB survival pathway, restricting receptors with death domains to transduce signals through the pro-death pathway.
This study demonstrates that activation of CD30 leads to the induction of apoptotic cell death in ALCL cells. Our observation that activation of CD30 on ALCL cells led to the reduction of TRAF2 and did not activate NF-κB may explain the sensitivity of these cells to CD30-induced apoptosis. Previous studies have demonstrated that CD30 activates NF-κB in a manner that depends on recruitment of the TRAF proteins.32 33 The ability of CD30 either to recruit TRAFs to activate NF-κB or to degrade TRAFs to compromise NF-κB activation may depend on the specific cell type examined and could explain previous contradictory observations that CD30 mediates pro-apoptotic as well as anti-apoptotic signals. The model we have proposed predicts that CD30 does not mediate apoptosis directly but that it potentiates death induced by a death domain-containing receptor, such as TNFR1, through the degradation of the TRAFs. We observed slightly increased levels of TNFR1 on CD30 activation (S.S.M. and C.S.D, unpublished data, 2000), raising the possibility that CD30 potentiates TNF receptor-induced cell death. However, neutralizing anti-TNF antibodies or TNF receptor-Fc fusion proteins failed to block CD30-mediated death of ALCL cells in our system (data not shown). The majority of CD30-induced death of ALCL cells occurred within 40 minutes of receptor activation (Figure 1D), which raises the possibility that TNF receptor potentiation occurs too rapidly for TNF-quenching reagents to take effect. In addition, these reagents might not target membrane-bound TNF as efficiently as soluble TNF. Although it is possible that CD30-mediated death occurs in a death domain-independent manner, CD30 might induce apoptosis through death domain-containing receptors other than TNFR1 yet to be identified.
HD cells have been shown to have constitutive activation of NF-κB,49-54 and constitutive NF-κB activation has been suggested to play a role in the pathogenesis of the disease.56 HD cells were found to be resistant to CD30-induced death, although CD30-mediated TRAF2 degradation was observed in these cells. When NF-κB was inhibited in HD cells, these cells became susceptible to CD30-induced death (Figure 4), suggesting that constitutive NF-κB activation confers resistance of HD cells to CD30-mediated death. The specific nature of this death is still unclear, because results with ZVAD-fmk on HD cells transfected with the superdominant IκB vector have been ambiguous (S.S.M. and C.S.D, unpublished data, 2000), possibly reflecting other intrinsic variables inherent to the experimental system that relies on electroporation. TRAF2 degradation induced by CD30 would be predicted not to affect the ability of HD cells to activate NF-κB because the ability of these cells to regulate NF-κB downstream of TRAF2 is compromised. However, because no specific inhibitor of TRAF degradation has been identified, the possibility remains that the degradation of TRAF2 is an epiphenomenon that is not centrally related to the induction of apoptosis by CD30.
The precise mechanisms by which NF-κB confers its protective effect in HD cells remain to be determined. Bargou et al54 have shown that the constitutive overexpression of NF-κB in HD cells was also required to prevent apoptotic cell death induced by growth factor withdrawal. Recent studies have implicated the cytokine interleukin 13 (IL-13) as an autocrine factor required for survival in HD cells,57 and neutralizing antibodies to IL-13 blocked the proliferation of HD cells. One possibility, therefore, is that, in addition to the presumed presence of IL-13 in growth serum, the constitutive activation of NF-κB in HD cells drives the autocrine expression of IL-13. Paradoxically, IL-13 has been shown to inhibit NF-κB activation, but this probably does not occur in HD cells in which IκB is nonfunctional.
CD30 is an attractive target for therapeutic intervention because of its restricted pattern of expression.35,36 Our finding that CD30 activation induces apoptotic death of ALCL cells in vitro supports the use of CD30 agonistic antibodies in the clinical treatment of the disease. Inhibition of constitutive NF-κB expression rendered HD cells sensitive to CD30-mediated death, suggesting that the combination of CD30 activation and NF-κB inhibition might be clinically useful. Interestingly, CD30 is also expressed on several subtypes of other lymphoid-derived tumors and cell lines, including cutaneous T-cell lymphoma, adult T-cell leukemia/lymphoma, T-acute lymphoblastic leukemia, and Epstein-Barr virus-transformed B cells.4 The ability to modulate the apoptotic threshold through CD30 signaling could ultimately lead to new therapeutic approaches in patients with CD30-positive neoplasia.
Acknowledgments
We thank Dr W. Murphy for providing cell lines; Dr N. Rice for antibodies to the NF-κB subunits; Dr B. Mayer for providing the pEBB vector; and the Immunex Corporation for providing the M67 CD30 agonistic antibody. We thank Dr N. Perkins and Dr L. Staudt for valuable discussions and the Duckett lab for critical reading of the manuscript.
S.S.M. is a Howard Hughes Medical Institute–National Institutes of Health Research Scholar.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Colin S. Duckett, Metabolism Branch, Division of Clinical Sciences, National Cancer Institute, National Institutes of Health, 10 Center Drive, Room 6B-05, Bethesda, MD 20892-1578; e-mail:duckettc@helix.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal