Abstract
Dissolution of the fibrin blood clot is regulated in large part by plasminogen activator inhibitor-1 (PAI-1). Elevated levels of plasma PAI-1 may be an important risk factor for atherosclerotic vascular disease and are associated with premature myocardial infarction. The role of the endogenous plasminogen activation system in limiting thrombus formation following atherosclerotic plaque disruption is unknown. This study found that genetic deficiency for PAI-1, the primary physiologic regulator of tissue-type plasminogen activator (tPA), prolonged the time to occlusive thrombosis following photochemical injury to carotid atherosclerotic plaque in apolipoprotein E-deficient (apoE−/−) mice. However, anatomic analysis revealed a striking difference in the extent of atherosclerosis at the carotid artery bifurcation between apoE−/− mice and mice doubly deficient for apoE and PAI-1 (PAI-1−/−/apoE−/−). Consistent with a previous report, PAI-1+/+/apoE−/−and PAI-1−/−/apoE−/− mice developed similar atherosclerosis in the aortic arch. The marked protection from atherosclerosis progression at the carotid bifurcation conferred by PAI-1 deficiency suggests a critical role for PAI-1 in the pathogenesis of atherosclerosis at sites of turbulent flow, potentially through the inhibition of fibrin clearance. Consistent with this hypothesis, intense fibrinogen/fibrin staining was observed in atherosclerotic lesions at the carotid bifurcation compared to the aortic arch. These observations identify significant differences in the pathogenesis of atherosclerosis at varying sites in the vascular tree and suggest a previously unappreciated role for the plasminogen activation system in atherosclerosis progression at sites of turbulent flow.
Introduction
Human atherosclerosis is a complex, multifactorial disease involving repetitive vascular injury, lipid accumulation, platelet and fibrin deposition, and cellular migration and proliferation.1 Complications of atherosclerosis are the leading cause of death in industrialized societies. Disruption of atherosclerotic plaques leading to occlusive thrombosis is the immediate cause of most acute coronary syndromes.1Subocclusive thrombosis may also occur, contributing to plaque growth, as evidenced by the presence of extensive fibrin deposition in most complex atherosclerotic lesions.2,3 Plasma fibrinogen levels in humans have been shown to be an independent risk factor for myocardial infarction.4 Elevated fibrinogen may also affect the process of atherogenesis by leading to enhanced thrombosis and fibrin deposition within developing atherosclerotic lesions.2,3 Intravascular clearance of fibrin is predominantly mediated by plasmin, which is formed from plasminogen by the action of the plasminogen activators (PAs), tissue-type plasminogen activator (tPA), and urokinase-type plasminogen activator (uPA). Plasminogen activator inhibitor-1 (PAI-1) is the primary inhibitor of the PAs,5 and elevated levels of PAI-1 have been identified as a risk factor for myocardial infarction in humans.6
With continued advances in transgenic technology, the mouse has become a uniquely powerful model in which to study the complex genetic factors contributing to atherosclerosis progression. Targeted deletion of genes involved in lipoprotein metabolism have produced mice that develop atherosclerosis similar to that observed in humans.7-9Deficiency of apolipoprotein E (apoE) leads to an especially severe form of atherosclerosis that is accelerated with high-fat chow.7 Within the vascular tree of these mice, bifurcation sites are predisposed to the development of atherosclerosis10 similar to the pattern observed in humans.11
Transgenic mouse studies examining the role of fibrinogen and fibrin clearance in atherosclerosis have yielded conflicting results. Although mice doubly deficient for apoE and plasminogen develop accelerated atherosclerosis,12 deficiency of fibrinogen appears to have no significant effect on atherosclerotic lesion growth in the aortic arch of apoE−/− mice.13 While examining the effect of PAI-1 on the susceptibility of carotid plaques in apoE−/− mice to form occlusive thrombosis, we observed marked protection from carotid atherosclerosis in apoE−/−mice deficient in PAI-1. These observations identify significant differences in the pathogenesis of atherosclerosis at varying sites in the vascular tree and suggest a previously unappreciated role for the plasminogen activation system in atherosclerosis progression at sites of turbulent flow.
Materials and methods
Mice
The PAI-1–deficient mice (a gift of P. Carmeliet and D. Collen) were generated by homologous recombination as previously described.14 ApoE−/− mice8 were purchased from the Jackson Laboratory (Bar Harbor, ME). PAI-1−/−mice were back-crossed to C57BL/6J (for 5 generations) and apoE−/− mice were back-crossed to C57BL/6J mice (for 6 generations) before cross-breeding. Double heterozygous (PAI-1+/−/apoE+/−) mice were intercrossed to produce the double PAI-1−/−/apoE−/− mice, which were identified by polymerase chain reaction analysis of tail DNA specimens. Primer sequences for identification of PAI-1 and apoE wild-type and null alleles were as previously described.15 Mice were maintained on standard chow or Teklad Western diet (TD 88137). Because of unexpected differences in the extent of carotid atherosclerosis (see “Results”) between PAI-1−/−/apoE−/− and PAI-1+/+/apoE−/− mice on a normal diet that contradicted our previous studies, an additional group of mice that had been maintained on high-fat (Western) chow were also analyzed. This group of mice on the Western chow (gift of W. Fay) had also received a ferric chloride-induced injury to one carotid artery as part of a previous study. These mice were of the same C57BL/6J genetic background as the older mice. Only the aortic arch and uninjured carotid artery from these animals were analyzed. All animal care and experimental procedures complied with the “Principles of Laboratory and Animal Care” established by the National Society for Medical Research and were approved by the University of Michigan Committee on Use and Care of Animals.
Carotid arterial thrombosis protocol
Thirty-week-old male apoE−/− mice along with 30-week-old combined PAI-1−/−/apoE−/− mice were subjected to photochemical injury of the right carotid artery as previously described.16 Flow in the vessel was monitored for 150 minutes or until cessation of flow occurred, at which time the experiment was terminated.
Analysis of atherosclerotic lesions
At 18, 30, or 52 weeks of age, mice were perfusion fixed with zinc formalin under intraperitoneal pentobarbital anesthesia (100 mg/kg). The common carotid artery including the bifurcation of the internal and external carotid was dissected and embedded in paraffin. Serial sections at 50-μm intervals were inspected moving from the common carotid artery into the bifurcation. At the onset of the bifurcation, 10 sections were stained with hematoxylin and eosin and subjected to quantitative morphometric analysis as previously described.15 For quantitation of surface area occupied by atherosclerosis, the aorta and its major branches were stained with oil red O as previously described7 and then subjected to quantitative morphometry. The operator was aware of the mouse genotype during quantitation. Staining for fibrinogen/fibrin was performed with a polyclonal goat antimouse antibody15 as previously described.
Statistical analysis
The statistical significance of differences of time to occlusion and intimal lesion area between the various groups was determined using the Student 2-tailed t test. A P value of less than .05 was considered significant.
Results
Effect of PAI-1 deficiency on development of occlusive thrombosis following plaque injury
We recently demonstrated that the level of PAI-1 expression significantly modifies the rate of thrombus formation in the mouse carotid artery following photochemical injury.17 We have also shown that this type of injury can be performed to atherosclerotic lesions and that the time to occlusion is decreased in diseased compared to nondiseased arteries,16 suggesting that murine plaque is highly thrombogenic, similar to the human lesion.18 To examine the contribution of PAI-1 in this model for the acute thrombosis associated with plaque disruption, 30-week-old PAI-1−/−/apoE−/− mice were subjected to photochemical injury at the site of an atherosclerotic lesion just proximal to the carotid bifurcation. The mean time to occlusion in these mice (n = 6) was 65 ± 7 minutes, which is significantly prolonged compared to our previously reported occlusion time of 44 ± 5 minutes in 30-week-old PAI-1+/+/apoE−/− mice (n = 9)16(P < .03).
Effect of PAI-1 deficiency on development of atherosclerosis at the carotid bifurcation and aortic arch
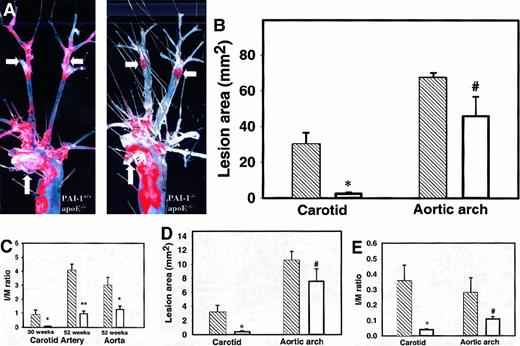
To examine potential anatomic differences in the atherosclerotic lesions at the carotid bifurcation, 52-week-old PAI-1−/−/apoE−/− or PAI-1+/+/apoE−/− male mice, maintained on a normal chow diet, were killed and the aorta with its major branches was dissected free of connective tissue and stained for lipid with Oil Red O.7 Although the extent of atherosclerosis involving the proximal aortic arch appeared similar between PAI-1−/−/apoE−/−and PAI-1+/+/apoE−/− mice, a marked difference in carotid disease was observed (Figure 1A). Total lesion surface area in the aortic arch and the carotid arteries as determined by quantitative morphometry is shown in Figure 1B. Consistent with our previous report,15 the mean lesion area in the aortic arch was not significantly different, although there was a trend toward less disease in the PAI-1−/−/apoE−/− group. However, marked differences in lesion surface area were observed in the carotid distribution. Quantitative analysis of intimal lesion area on histologic cross sections (Figure 1C) confirmed the dramatic protection against atherosclerosis at the carotid bifurcation afforded by PAI-1 deficiency. At 30 weeks, significant intimal thickening is evident in the carotid artery bifurcations of PAI-1+/+/apoE−/− mice maintained on normal chow, whereas little or no disease is observed in PAI-1−/−/apoE−/− mice at the same time point (Figure 1C). A more modest decrease in lesion area at the aortic arch was also observed in PAI-1−/−/apoE−/−mice compared to apoE−/− controls, achieving statistical significance at the 52-week time point.
Analysis of atherosclerosis.
(A) Oil red O staining of atherosclerotic lesions in aortic arch and carotid arteries of 52-week-old mice on normal chow. Atherosclerosis of the distal common carotid and carotid bifurcation is markedly diminished in the PAI-1−/−/apoE−/− mice compared to PAI-1+/+/apoE−/− mice, whereas atherosclerosis involving the aortic arch appears similar. Arrows show aortic arch (vertical) and carotid bifurcation (horizontal). (B) Quantitation of aortic and carotid arterial atherosclerosis in 52-week-old PAI-1−/−/apoE−/− mice (n = 8) (■) and PAI-1+/+/apoE−/− mice (n = 6) (▧) on normal chow. *P < .0003, #P > .1. (C) Cross-sectional area of atherosclerotic lesions in 30-week-old PAI-1−/−/apoE−/− (n = 10) and PAI-1+/+/apoE−/− mice (n = 7) maintained on normal chow and in 52-week-old PAI-1−/−/apoE−/− (n = 9) and PAI-1+/+/apoE−/− mice (n = 6) maintained on normal chow. Intima-to-media (I/M) ratio in PAI-1−/−/apoE−/− mice (■) and PAI-1+/+/apoE−/− mice (▧). *P < .01, **P < 2 × 10−8. (D) Quantification of lesion area in 18-week-old PAI-1−/−/apoE−/− mice (n = 5) (■) and PAI-1+/+/apoE−/− mice (n = 5) (▧) on high-fat chow. *P < .05, #P > .2. (E) I/M ratios in PAI-1−/−/apoE−/− mice (n = 5) (■) and PAI-1+/+/apoE−/− mice (n = 5) (▧) on high-fat chow. *P < .05, #P > .1.
Analysis of atherosclerosis.
(A) Oil red O staining of atherosclerotic lesions in aortic arch and carotid arteries of 52-week-old mice on normal chow. Atherosclerosis of the distal common carotid and carotid bifurcation is markedly diminished in the PAI-1−/−/apoE−/− mice compared to PAI-1+/+/apoE−/− mice, whereas atherosclerosis involving the aortic arch appears similar. Arrows show aortic arch (vertical) and carotid bifurcation (horizontal). (B) Quantitation of aortic and carotid arterial atherosclerosis in 52-week-old PAI-1−/−/apoE−/− mice (n = 8) (■) and PAI-1+/+/apoE−/− mice (n = 6) (▧) on normal chow. *P < .0003, #P > .1. (C) Cross-sectional area of atherosclerotic lesions in 30-week-old PAI-1−/−/apoE−/− (n = 10) and PAI-1+/+/apoE−/− mice (n = 7) maintained on normal chow and in 52-week-old PAI-1−/−/apoE−/− (n = 9) and PAI-1+/+/apoE−/− mice (n = 6) maintained on normal chow. Intima-to-media (I/M) ratio in PAI-1−/−/apoE−/− mice (■) and PAI-1+/+/apoE−/− mice (▧). *P < .01, **P < 2 × 10−8. (D) Quantification of lesion area in 18-week-old PAI-1−/−/apoE−/− mice (n = 5) (■) and PAI-1+/+/apoE−/− mice (n = 5) (▧) on high-fat chow. *P < .05, #P > .2. (E) I/M ratios in PAI-1−/−/apoE−/− mice (n = 5) (■) and PAI-1+/+/apoE−/− mice (n = 5) (▧) on high-fat chow. *P < .05, #P > .1.
The latter data contrast with our previous report15 in which absence or overexpression of PAI-1 produced no significant difference in aortic arch lesion thickness, when examined in both the apoE−/− and LDLR−/− murine atherosclerosis models. The latter studies were performed on a high-fat diet, which is known to accelerate the growth of atherosclerotic lesions and may have overwhelmed the effect of varying PAI-1 expression. To address the potential contribution of diet, we analyzed an additional group of 18-week-old mice (5 mice in each group) fed high-fat chow for 8 weeks (Figure 1D). Similar to the mice on regular chow, PAI-1 deficiency resulted in a marked decrease in lesion surface area at the distal common carotid artery compared to apoE−/− controls (3.2 ± 1.0 versus 0.42 ± 0.2,P < .03) However, consistent with our previous report, no significant differences were observed in the ascending aorta, although there was a trend toward less lesion area in PAI-1−/−/apoE−/− mice (10.5 ± 1.3 versus 7.5 ± 1.8, P = .2). A similar pattern was observed for the intimal lesion thickness (Figure 1E).
Fibrin/fibrinogen immunohistochemistry
No significant differences in lesion composition or morphology were evident among the genetically distinct experimental groups on routine histologic analysis. However, immunohistochemical staining for fibrin/fibrinogen revealed evidence of fibrin/fibrinogen deposition at all sites of lesion formation, considerably more extensive at the distal common carotid artery of PAI-1+/+/apoE−/− mice compared to the ascending aorta (Figure 2). PAI-1−/−/apoE−/− mice would be expected to exhibit less fibrin deposition in plaques compared to PAI-1+/+/apoE−/− mice; however, no major differences were noted between the 2 groups although direct comparisons are difficult due to differences in lesion size.
Fibrin/fibrinogen staining of atherosclerotic lesions in 52-week-old apoE−/− mice.
Left panel shows ascending aorta; right panel shows distal common carotid artery. Fibrin/fibrinogen, identified by red staining, is more extensive at the carotid bifurcation than the aortic arch. M indicates media; IEL, internal elastic lamina; P, plaque.
Fibrin/fibrinogen staining of atherosclerotic lesions in 52-week-old apoE−/− mice.
Left panel shows ascending aorta; right panel shows distal common carotid artery. Fibrin/fibrinogen, identified by red staining, is more extensive at the carotid bifurcation than the aortic arch. M indicates media; IEL, internal elastic lamina; P, plaque.
Discussion
Plasminogen activator inhibitor-1 is expressed at sites of vascular disease and has been proposed to play an important role in the pathogenesis of human atherosclerosis.19,20 Because PAI-1 is the primary regulator of fibrinolytic activity in vivo,21 deficiency of PAI-1 might be expected to enhance fibrinolysis and attenuate the growth of atherosclerotic plaques. However, PAI-1 also appears to regulate cellular migration via fibrin-independent mechanisms,22,23 with PAI-1 blocking cellular accumulation at sites of extrinsic vascular injury in several rodent models.24,25 Human clinical studies of the association between plasma PAI-1 level and atherosclerotic vascular disease have yielded conflicting results.6,26 A common polymorphism in the PAI-1 promoter resulting in alteredPAI-1 gene expression in vitro has been associated with increased cardiovascular risk in some studies though not confirmed in others.27-30
Our data suggest that the relative contribution of fibrin deposition and PA activity to the pathogenesis of atherosclerosis may vary considerably at different sites within the vasculature. Taken together with our previous results,15 these findings also suggest that the marked hypercholesterolemia and accelerated atherosclerosis associated with high-fat feeding in apoE−/− mice may obscure subtle contributions from nonlipid factors that are likely to be relevant in human disease, including alterations in hemostatic balance. The lesions that develop in the carotid arteries of PAI-1+/+/apoE−/− mice involve the distal common carotid artery and typically extend across the bifurcation into the internal and external carotids (Figure 1A). Bifurcation sites in humans are similarly predisposed to the development of atherosclerosis11 and unique hemodynamic forces may affect development of lesions at these sites.
Shear stress has been shown to increase the expression of tPA, which may affect fibrin clearance.31,32 Other factors contributing to fibrin turnover have been shown to correlate with the development of atherosclerosis at the carotid bifurcation in humans.33 These observations suggest that delayed fibrin clearance may provide an expanded matrix for accelerated plaque growth. Consistent with this hypothesis, accelerated atherosclerosis has also been reported in apoE−/− mice deficient in plasminogen.12 In contrast, fibrinogen deficiency fails to protect apoE−/− mice from atherosclerosis progression.13 However, the latter results were based on analysis restricted to the proximal aorta, and significant differences at the more distal sites studied in the current report cannot be excluded. The confounding effect of strain modifier genes in these and other previous reports34 could also obscure significant differences in plaque burden. We studied mice on the C57BL/6J background because this strain has become the standard inbred strain for atherosclerosis studies. It is possible that the effect of PAI-1 observed in these studies could vary on different genetic backgrounds. Dansky and coworkers34 have demonstrated marked differences in atherosclerotic burden in apoE−/− mice on the FVB/NJ background compared to the C57BL/6J background. Although the aorta has been the standard site for quantitative analysis of atherosclerosis in genetically modified mice,35 our results suggest that atherosclerosis progression at other sites in the vascular tree may be more relevant to the complex mechanisms underlying the pathogenesis of human coronary atherosclerosis.
Taking all of these results together, we propose that chronic low-grade fibrin deposition following repetitive vascular injury facilitates the growth of atherosclerotic plaques, particularly at sites of altered hemodynamics and turbulent flow. In addition to its direct role as a regulator of acute intravascular thrombosis,17 PAI-1 may also contribute to chronic plaque growth and thrombogenicity through its effect on fibrinolytic balance. These results suggest that pharmacologic inhibition of PAI-1 may be a useful therapeutic strategy, for preventing acute coronary thrombosis in susceptible vessels as well as delaying development of the primary atherosclerotic lesion.
Acknowledgments
We thank William Fay for providing 18-week-old mice on Western chow and Angela Yang for assistance with fibrin staining.
Supported by National Institutes of Health grants HL-035989 and HL-036195 (D.E.) and HL-57345 (D.G.). D.G. is a Howard Hughes Medical Institute investigator.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Daniel T. Eitzman, University of Michigan Medical Center, MSRB III Room 7301, 1150 Medical Center Drive, Ann Arbor, MI 48109-0644; e-mail: deitzman@umich.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal