Abstract
Laminins are a family of disulfide-linked heterotrimeric proteins consisting of 3 different subunits termed α, β, and γ chains. Combinations of 11 characterized laminin subunits (α1-α5, β1-β3, and γ1-γ3) generate at least 12 laminin isoforms, which can serve different functions. Although expression of laminin in the hematopoietic microenvironment has been known for many years, the nature of the laminin isoforms present in the human bone marrow is poorly characterized. The present study attempts to clarify this issue. Reverse transcriptase–polymerase chain reaction analysis of human bone marrow stromal cells suggested the expression of many laminin isoforms in the marrow. Northern blot and immunoblot analysis, however, showed that laminin-8/9 and laminin-10/11 are the most abundant laminin isoforms synthesized by human bone marrow stromal cells. Other isoforms, if present, certainly play a minor role in the hematopoietic microenvironment. Functionally, laminin-10/11 preparations showed strong adhesive interactions with human CD34+ cell lines. Antibodies against the β1 integrin subunit inhibited these interactions. Other laminin isoforms, especially laminin-1 and laminin-2/4, showed only weak or no adhesive interactions with the hematopoietic cell lines tested, explaining former negative results. In addition to its adhesion-mediating properties, laminin-10/11 preparations also showed a mitogenic activity for human hematopoietic progenitor cells. Taken together, these data suggest that laminin in the bone marrow plays a hitherto unexpected important function in the development of hematopoietic progenitor cells.
Introduction
The hematopoietic microenvironment of the bone marrow plays a fundamental role in regulating proliferation, differentiation, and migration of the developing blood cells.1-3 An essential part of the bone marrow microenvironment is its complex extracellular matrix (ECM), which is synthesized by nonhematopoietic stromal cells.4,5 ECM molecules including glycoproteins, proteoglycans, and the collagen family have been shown to be directly involved in controlling cell adhesion and proliferation of maturing hematopoietic cells as well as presentation of cytokines to hematopoietic progenitor cells.6-10
The laminins represent a large gene family of ECM molecules found predominantly in basement membranes of epithelial cells but also in interstitial tissues and embryonic mesenchyme.11,12 The heterotrimeric laminin molecules consist of 3 different subunit chains, which are termed α, β, and γ chains according to the current nomenclature.13 Twelve different laminin isoforms (LN-1 to LN-12) are proposed to be formed from 5 individual α chains, 3 β chains, and 3 γ chains.14,15 The most prominent and best-studied isoform is LN-1, consisting of α1, β1, and γ1 chains, and can easily be isolated from a murine tumor. However, analysis of the expression pattern of LN-1 revealed that it is restricted to a subset of basement membranes, whereas other laminin isoforms show a much broader tissue distribution.16-21Because it is now known that different laminin isoforms can serve distinct functions, it becomes evident that many functional studies performed with purified LN-1 have to be reinterpreted with respect to its specialized tissue distribution. Because of possible functional differences, it is important to characterize the chain compositions of the laminins expressed in the different tissues.
Laminins are expressed in the bone marrow microenvironment.22 Early studies did not distinguish between laminin isoforms because the used polyclonal antisera against LN-1 identified both the β1 and the γ1 laminin chains, which are present in 11 of the 12 proposed laminin isoforms. A recent study by Ekblom and coworkers defined the laminin isoforms found in mouse and rat bone marrow to be LN-2 (containing α2, β1, and γ1 chains), LN-8 (α4, β1, γ1), and LN-10 (α5, β1, γ1), but not LN-1.23 In human bone marrow, a different laminin isoform expression pattern was suggested by the detection of the laminin β2 chain, which was not detected in rat bone marrow.24Because these investigators failed to take the expression pattern of other identified human laminin chains into account, the chain composition of the laminins in the human bone marrow microenvironment remained unresolved.
A dominant feature of all laminin molecules is that they can act as strong adhesive substrates for many cell types. However, several studies indicate that hematopoietic cell types do not seem to adhere to LN-1.25-27 Another laminin isoform, LN-10/11, isolated from human placenta, on the contrary, is strongly adhesive for mouse and human hematopoietic cells.23,28 Interactions with laminins can be mediated by different cellular receptors, including members of the integrin family and dystroglycan.29 The identified interactions of human hematopoietic cells with LN-10/11 seemed to be mediated by β1 integrins, although the exact nature of the involved receptors has not been determined.28
In the present study we have analyzed the expression pattern of 11 different laminin chains in human bone marrow. This was performed in vitro using long-term bone marrow cultures (LTMCs) and established stromal cell lines from the marrow microenvironment but also in cryostat sections of native bone marrow tissue. Furthermore, different laminin preparations representing different laminin isoforms were used to study their functional interactions with human hematopoietic progenitor cells. Because cell adhesion can be directly linked to induction of cell proliferation, both mitogenic and adhesive properties of the different laminin isoforms for human hematopoietic cells have been tested.
Material and methods
Cell cultures
The human bone marrow stromal cell lines L87/4, L88/5, and HS-5 30 31 were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS). The hybridoma cell lines C4 and D18, purchased from the Developmental Studies Hybridoma Bank (Iowa City, IA), were cultured under the same conditions as the stromal cells. For the collection of conditioned media, the hybridoma cells were transferred into serum-free RPMI 1640 cell culture medium supplemented with 2% Ultroser (Gibco BRL, Eggenstein, Germany).
The human hematopoietic cell lines KG1a, U937, KU.812, and K562 were used in previous studies.6,8 32 Two B-cell lines, BOB and COX, were obtained after Epstein-Barr virus infection of peripheral B lymphocytes following standard procedures (C.A.M., unpublished data, 1982). The plasmocytoma cell lines, U266 and NCI-H929, and the pre-B-leukemic cell line, BV173, were from the German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany. The interleukin-3–dependent CD34+ TF-1 cell line, kindly provided by Dr Kitamura (University of Tokyo), was cultured with 2% conditioned medium from the carcinoma cell line, 5637, as an exogeneous source of interleukin-3.
Long-term bone marrow cultures
Bone marrow aspirates were obtained from healthy donors after informed consent according to the guidelines of the local ethic committee. Bone marrow mononuclear cells were isolated from aspirates by density gradient centrifugation on a Percoll cushion (1.077 g/mL). LTMCs were established in RPMI 1640 culture medium containing 12.5% FCS, 12.5% horse serum, 10−6 mol/L hydrocortisone, and antibiotics (Dexter conditions).
Isolation of CD34+ cells by MiniMACS
CD34+ progenitor cells were purified from bone marrow mononuclear cells using the MiniMACS cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Briefly, 5 × 107 mononuclear cells were resuspended in phosphate-buffered saline (PBS) containing human immunoglobulin and monoclonal hapten-conjugated anti-CD34 antibody. Labeled cells were washed, centrifuged, and resuspended in a buffer containing super-paramagnetic MACS Microbeads conjugated to an antihapten antibody. Magnetic separation was performed by applying labeled cells on positive selection columns. Columns were washed repeatedly, and CD34+ cells were eluted by removing the column from the magnetic field. The purity of the isolated CD34+ cells was determined by fluorescence-activated cell sorter analysis.
ECM components and antibodies
Different laminin preparations were used in the cell binding and proliferation studies. Murine laminin isolated from the Engelbreth-Holm-Swarm tumor, which consists mainly of the LN-1 isoform, was obtained from Biozol (Eching, Germany). Human merosin, purified from human placenta and consisting of LN-2/4,33was purchased from Life Technologies (Eggenstein, Germany). Another laminin preparation isolated from human placenta by mild pepsin digestion followed by affinity chromatography on monoclonal antibody (mAb) 4C7-coupled Sepharose was also obtained from Life Technologies. The composition of this human placental laminin preparation has been determined previously as LN-10/11 by Ferletta and Ekblom.34
Rabbit antiserum against LN-1 was that used in previous studies.17,35 The antiserum against human LN-10/11 was commercially available from Life Technologies. The rat mAb 4H8-2 was used to detect laminin α2 chain.36 Generation of an antiserum against the murine laminin α4 chain, which cross-reacts with the human homolog, has been described recently.37 A rabbit antiserum raised against the peptide sequence KPPVKRPELT corresponding to the human laminin α4 chain38 was used for Western blotting. The mouse mAb 4C7 to human laminin α5 chain was obtained from Roche (Mannheim, Germany). Mouse ascites against the human laminin β1 chain was from Life Technologies. Mouse mAbs to laminin β2 (clone C4) and laminin γ1 (clone D18) were isolated from conditioned hybridoma medium. The human endothelial-specific mAb Rb10 was a kind gift from Dr Rupert Hallmann (University of Erlangen-Nuremberg, Germany). The mAb to smooth muscle–specific α actin (α-SM actin, clone 1A4) was from Sigma (Deisenhofen, Germany). Function-blocking antibodies against various human integrin subunits were purchased from Coulter (β1 integrin, clone 4B4), Oncogene (α2 integrin, clone P1E6), Immunotech (α4 integrin, clone HP2/1; β3 integrin, clone SZ21), or Pharmingen (α3 integrin, clone P1B5; α5 integrin, clone IIA1; α6 integrin, clone GoH3). The synthetic hexapeptide, GRGDSP, containing the RGD adhesion sequence, and the control peptide, GRGESP, were from Life Technologies.
Purification of bone marrow laminin isoforms
Conditioned media of LTMCs were incubated overnight with DEAE Sephacel anion exchanger (Amersham-Pharmacia Biotech, Freiburg, Germany). After washing the DEAE Sephacel, bound proteins were eluted using 10 mmol/L Tris, pH 8.5, containing 1 mol/L NaCl. Contaminating fibronectin was removed by incubation with gelatin Sepharose 4B, and laminin isoforms were then purified by affinity chromatography on NHS-activated Sepharose to which the isolated laminin β2 and γ1 chain antibodies, C4 and D18, had been coupled. Bound laminin isoforms were eluted using a buffer containing 40 mmol/L sodium phosphate, pH 11.75, and 150 mmol/L NaCl. After immediate neutralization, the laminin-containing solution was concentrated by ultrafiltration. The protein content of the concentrated laminin preparation was determined by Micro BCA protein assay (Pierce, Rockford, IL).
Reverse transcriptase and polymerase chain reaction
Total RNA was isolated from the different cell types by acid guanidinium isothiocyanate phenol chloroform extraction.39Possible DNA contaminations of the RNA preparations were eliminated by deoxyribonuclease treatment. Expression of messenger RNA (mRNA) for human laminin α1-α5, β1-β3, and γ1-γ3 chains were analyzed using reverse transcriptase-polymerase chain reaction (RT-PCR) methodology. Based on published sequences for the human laminin chains (EMBL accession numbers for α1, α2, α3A, α3B, α4, α5: X58531, Z26653, X85107, L34156, S78569,Z95636; for β1-β3: M61916, S77512, L25541; and for γ1-γ3:J03202, Z15008, AF041835), specific primer pairs were designed with the aid of the HUSAR/GCG program from EMBL (Heidelberg, Germany). Reverse transcription was performed with Moloney murine leukemia virus RT (Gibco) with 1.0 μg oligo(dT)12-18. PCR conditions with 4 units of Taq polymerase (Perkin-Elmer, Weiterstadt, Germany) and 0.3 μmol/L of each primer included denaturation at 94°C for 40 seconds, annealing at 60°C for 1 minute, and polymerization at 72°C for 1 minute. A total of 35 cycles were run. Control experiments were performed using total RNA preparations without reverse transcription. The PCR products of the expected sizes were analyzed by gel electrophoresis in 2.0% agarose gels, purified using the PCR purification kit (Qiagen, Hilden, Germany), and sequenced with the ABI Prism DyeDeoxy terminator kit (Perkin-Elmer).
Northern blotting
Total RNA from bone marrow stromal cells were electrophoresed on agarose gels containing 2% formaldehyde. After transfer to Hybond N+ membranes (Amersham-Pharmacia), the RNAs were cross-linked to the membranes by UV irradiation. The membranes were prehybridized at 42°C in DIG Easy Hyb solution buffer according to the manufacturer's suggestions (Roche). Hybridization was performed overnight in the same solution with the following complementary DNA (cDNA) probes: a 2.5-kb EcoRI fragment of cDNA clone A1 corresponding to human laminin α1 chain,40 a 3.2-kbEcoRI fragment of clone M10-22 corresponding to human laminin α2 chain,41 a 3.6-kb EcoRI fragment of clone HL-40 corresponding to human laminin β1 chain,42 and a 1.4-kb human GAPDH cDNA probe as control. Complementary DNA probes for the human laminin α2, α3, α4, α5, β2, γ1, γ2, and γ3 chains were generated by subcloning the PCR amplification products using the Topo cloning kit (Invitrogen, NV Leek, the Netherlands). Isolated inserts were labeled with digoxigenin (DIG) using the DIG DNA labeling and detection kit (Roche). After hybridization the membranes were washed, blocked with 2% Boehringer blocking reagent, and developed with the chemiluminescence detection system using alkaline phosphatase–conjugated anti-DIG antibodies and CSPD as substrate for the phosphatase (Roche).
Immunohistochemistry
Native bone marrow samples taken from the sternum of hematologically healthy donors during thoracic surgery were frozen in Tissue-Tek embedding medium (Sakura, Zouterwoude, the Netherlands). Sections measuring 5 μm, prepared on a cryostat, as well as the long-term bone marrow stromal cells grown on glass coverslips, were fixed in methanol for 5 minutes at −20°C. After washing, the fixed cells or tissue sections were incubated with the primary antibodies for 1 hour. Bound antibodies were detected after washing by incubation with Cy3-conjugated or fluorescein isothiocynate–conjugated secondary antibodies (Dianova, Hamburg, Germany). Control stainings were performed by omitting the primary antibodies.
Immunoblotting
Conditioned media and cell extracts of human bone marrow stromal cells were analyzed by immunoblotting for laminin isoform chain composition. Media and cell extracts were boiled for 5 minutes with loading buffer containing dithiothreitol, separated on 5%-to-15% sodium dodecyl sulfate–polyacrylamide gradient gels, and transferred to nitrocellulose. After blocking, the filters were incubated with laminin chain–specific antibodies. Bound antibodies were detected either with alkaline phosphatase–conjugated antibodies (Dako Diagnostika, Hamburg, Germany) followed by colorimetric reaction with the Fast BCIP/NBT system (Sigma), or with horseradish peroxidase–conjugated antibodies (Dako) and the enhanced chemiluminescence reagent (Amersham-Pharmacia).
Cell proliferation assays
To analyze mitogenic activities of different laminin preparations, a nonradioactive cell proliferation assay (Roche) based on formazan formation was used. Using 96-well flat-bottom microtiter plates (Costar, Wiesbaden, Germany), 5 × 103 freshly isolated CD34+ cells per well or 5 × 104bone marrow mononuclear cells per well were cultured in a final volume of 100 μL per well RPMI 1640 culture medium supplemented with 10% FCS containing various amounts of different laminin preparations. After 6 days of incubation, the cell proliferation reagent WST-1 (Roche) was added to each well. After 1, 2, or 4 hours of incubation, the absorbance of the samples was measured against a background control at 440 nm. The optical density determined in each well was directly correlated with the number of viable cells in the assay. All experiments were carried out in triplicate.
Cell adhesion and inhibition assays
The assay was carried out as described previously.8Briefly, serial dilutions of the different laminin preparations were immobilized onto plastic by air drying. Nonspecific cell binding to plastic was blocked by subsequent incubation of the culture dishes with 1% human serum albumin in PBS. Cells in serum-free medium were permitted to adhere to the immobilized laminin preparations for 1 hour at 37°C. Nonadherent cells were removed by gently rinsing the dishes with PBS. Specific cell attachment was evaluated under an Axiovert microscope (Zeiss, Oberkochen, Germany).
To inhibit cell adhesion to immobilized laminin preparations, cells under study were preincubated with different anti-integrin antibodies (diluted 1:50) for 30 minutes under constant rotation. On the other hand, the immobilized laminin preparations were preincubated for 30 minutes with polyclonal antilaminin antisera. After these preincubation periods, the adhesion assays were performed in the presence of the respective antibodies as described above.
Results
Detection of individual laminin chains in human bone marrow stroma
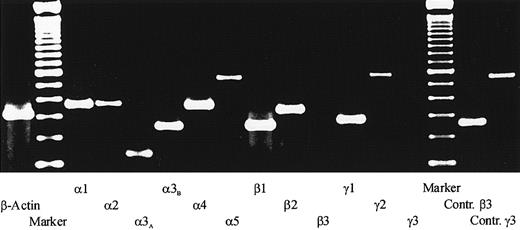
To determine which laminin chains are expressed by the heterogeneous bone marrow stromal cell population, RT-PCR analysis of the 5 human laminin α chains, the 3 β chains, and the 3 γ chains were performed. Using RNA from primary LTMCs, specific amplification products were obtained for all analyzed laminin chains except the β3 chain and the γ3 chain (Figure 1). Similar results were obtained with RNA preparations from the established human bone marrow stromal cell lines L87/4 and HS-5 (data not shown). The identities of the amplified products were confirmed by DNA sequencing. These results suggested that all laminin isoforms, with the exception of LN-5 and LN-12, which contain the β3 or the γ3 chains, respectively, could be present in the human bone marrow.
RT-PCR analysis of laminin chain expression.
Total RNA isolated from the adherent stromal layers of LTMCs was reverse transcribed, amplified with specific primer pairs for the human laminin α, β, and γ chains, and separated on a 2.0% agarose gel. The following specific amplification products were detected: a 357–base pair (bp) signal for the α1 chain, 368 bp for the α2 chain, 172 bp for the α3A chain, 253 bp for the α3B chain, 358 bp for the α4 chain, and 562 bp for the α5 chain; a 253-bp signal for the β1 chain and 337 bp for the β2 chain; a 283-bp signal for the γ1 chain and a 579-bp signal for the γ2 chain. No signals could be detected for the β3 chain and the γ3 chain, but positive controls with RNA from endothelial cells showed that the primer pairs for the β3 and the γ3 chain could amplify a 261-bp and a 556-bp signal, respectively. In addition, two 100-bp ladders (marker) and a β-actin control amplification are shown.
RT-PCR analysis of laminin chain expression.
Total RNA isolated from the adherent stromal layers of LTMCs was reverse transcribed, amplified with specific primer pairs for the human laminin α, β, and γ chains, and separated on a 2.0% agarose gel. The following specific amplification products were detected: a 357–base pair (bp) signal for the α1 chain, 368 bp for the α2 chain, 172 bp for the α3A chain, 253 bp for the α3B chain, 358 bp for the α4 chain, and 562 bp for the α5 chain; a 253-bp signal for the β1 chain and 337 bp for the β2 chain; a 283-bp signal for the γ1 chain and a 579-bp signal for the γ2 chain. No signals could be detected for the β3 chain and the γ3 chain, but positive controls with RNA from endothelial cells showed that the primer pairs for the β3 and the γ3 chain could amplify a 261-bp and a 556-bp signal, respectively. In addition, two 100-bp ladders (marker) and a β-actin control amplification are shown.
Northern blot analysis, however, revealed a more restricted expression pattern. Under stringent conditions, no hybridization signal for laminin α1, α2, or α3 chains could be detected in RNA preparations from the stromal cell lines L87/4, L88/5, and HS-5 or from stromal layers from LTMCs. Strong signals, however, could be detected for the laminin α4 chain in the stromal cell line HS-5 and in LTMC preparations (Figure 2). The stromal cell lines L87/4 and L88/5 did not show any signal for the laminin α4 chain, but strong signals for the laminin α5 and β1 chains were detected. Only faint bands for laminin α5 and β1 were observed for HS-5 and primary LTMCs. Prominent signals for the laminin β2 and γ1 chain could be observed in all analyzed stromal cells (Figure 2). These Northern blot data suggest the following: (1) Laminin isoforms containing the α4, α5, β1, β2, and γ1 chains are expressed by human bone marrow stromal cells. The isoforms LN-8/9 and LN-10/11 are therefore likely to be synthesized (Table1). (2) Because of the observed expression pattern, the stromal cell line HS-5 closely resembles a major cell type of the stromal cell population of LTMCs, whereas the stromal cell lines L87/4 and L88/5 may represent a stromal cell type that is found more scarcely in the marrow.
Northern blot analysis of laminin chain mRNA expression in human bone marrow stromal cells.
Twenty micrograms of total RNA isolated from the bone marrow–derived stromal cell lines L88/5 (a lanes), L87/4 (b lanes), and HS-5 (c lanes) as well as 10 μg of total RNA isolated from primary stromal cells of LTMCs (d lanes) were hybridized with DIG-labeled cDNA probes corresponding to the human laminin α2, α4, α5, β1, β2, and γ1 chains. The amount of RNA loaded per lane was determined by hybridization with a DIG-labeled cDNA probe for human GAPDH mRNA (←). The size of the detected laminin chain mRNAs was estimated using a DIG-labeled molecular weight marker whose largest fragment runs at 7 kb (⇐). Expression of laminin α4, α5, β1, β2, and γ1 chain mRNAs was found in the stromal cell line HS-5 (lane c) and the primary bone marrow stromal cells (lane d), whereas the stromal cell lines L88/5 and L87/4 express mainly laminin α5, β1, β2, and γ1 chain mRNAs. No expression of α2 chain mRNA was detected in any of the analyzed stromal cells.
Northern blot analysis of laminin chain mRNA expression in human bone marrow stromal cells.
Twenty micrograms of total RNA isolated from the bone marrow–derived stromal cell lines L88/5 (a lanes), L87/4 (b lanes), and HS-5 (c lanes) as well as 10 μg of total RNA isolated from primary stromal cells of LTMCs (d lanes) were hybridized with DIG-labeled cDNA probes corresponding to the human laminin α2, α4, α5, β1, β2, and γ1 chains. The amount of RNA loaded per lane was determined by hybridization with a DIG-labeled cDNA probe for human GAPDH mRNA (←). The size of the detected laminin chain mRNAs was estimated using a DIG-labeled molecular weight marker whose largest fragment runs at 7 kb (⇐). Expression of laminin α4, α5, β1, β2, and γ1 chain mRNAs was found in the stromal cell line HS-5 (lane c) and the primary bone marrow stromal cells (lane d), whereas the stromal cell lines L88/5 and L87/4 express mainly laminin α5, β1, β2, and γ1 chain mRNAs. No expression of α2 chain mRNA was detected in any of the analyzed stromal cells.
Composition of laminin isoforms and their presence in human bone marrow stromal cells
| Isoform . | Subunit composition . | Presence in stromal cells . |
|---|---|---|
| Laminin-1 | α1β1γ1 | − |
| Laminin-2 | α2β1γ1 | − |
| Laminin-3 | α1β2γ1 | − |
| Laminin-4 | α2β2γ1 | − |
| Laminin-5 | α3β3γ2 | − |
| Laminin-6 | α3β1γ1 | − |
| Laminin-7 | α3β2γ1 | − |
| Laminin-8 | α4β1γ1 | ++ |
| Laminin-9 | α4β2γ1 | ++ |
| Laminin-10 | α5β1γ1 | ++ |
| Laminin-11 | α5β2γ1 | ++ |
| Laminin-12 | α2β1γ3 | − |
| Isoform . | Subunit composition . | Presence in stromal cells . |
|---|---|---|
| Laminin-1 | α1β1γ1 | − |
| Laminin-2 | α2β1γ1 | − |
| Laminin-3 | α1β2γ1 | − |
| Laminin-4 | α2β2γ1 | − |
| Laminin-5 | α3β3γ2 | − |
| Laminin-6 | α3β1γ1 | − |
| Laminin-7 | α3β2γ1 | − |
| Laminin-8 | α4β1γ1 | ++ |
| Laminin-9 | α4β2γ1 | ++ |
| Laminin-10 | α5β1γ1 | ++ |
| Laminin-11 | α5β2γ1 | ++ |
| Laminin-12 | α2β1γ3 | − |
Immunofluorescence stainings of the adherent stromal layer of LTMCs with chain-specific antibodies against the laminin α2, α4, α5, and β2 chains showed strong signals for the α4, α5, and β2 chains, whereas laminin α2, as expected from the Northern blot data, was not expressed (Figure 3A-C). Even more prominent staining signals were obtained with an antiserum against LN-1, recognizing the α1, β1, and γ1 chains (Figure 3D), or with the mAbs against laminin β1 or γ1 chains (not shown). The staining signals were exclusively found in the cytoplasm of the stromal cells. No deposition of laminin isoforms in an extracellular meshwork was observed, indicating that the synthesized isoforms are secreted into the cell culture supernatants. Indeed, immunoblot analyses of conditioned media of LTMCs with the anti–LN-1 antiserum confirmed the secretion of laminin isoforms into the cell culture medium (data not shown).
Immunofluorescence stainings of LTMCs.
The adherent stromal layer of human LTMCs were stained with an antilaminin α4 chain antiserum (A); the antibody, 4C7, recognizing the α5 chain (B); the antibody, C4, against the β2 chain (C); or an antiserum against the LN-1 isoform, recognizing α1, β1, and γ1 chains (D). All 4 antibodies show strong staining signals. Note that the staining signals were restricted to the cytoplasmic part of the cells, and no extracellular deposition could be detected. (Original magnification × 270.)
Immunofluorescence stainings of LTMCs.
The adherent stromal layer of human LTMCs were stained with an antilaminin α4 chain antiserum (A); the antibody, 4C7, recognizing the α5 chain (B); the antibody, C4, against the β2 chain (C); or an antiserum against the LN-1 isoform, recognizing α1, β1, and γ1 chains (D). All 4 antibodies show strong staining signals. Note that the staining signals were restricted to the cytoplasmic part of the cells, and no extracellular deposition could be detected. (Original magnification × 270.)
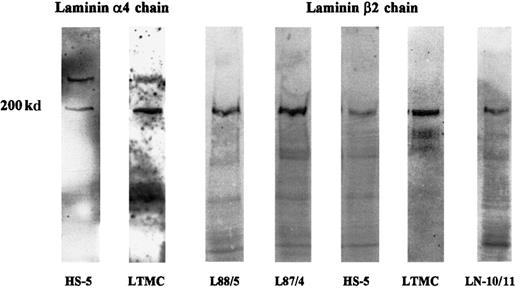
Expression of the β2 chain by human bone marrow stromal cells was verified by immunoblotting. In protein extracts of the stromal cell lines L87/4, L88/5, and HS-5 and of LTMCs, a strong band of 200 kd could be visualized (Figure 4). Using the antiserum against a short peptide sequence of the human laminin α4 chain, a specific band of 200 kd could be observed in extracts of LTMCs and the stromal cell line HS-5. In addition to the 200-kd band, a specific band of about 300 kd was observed in both extracts (Figure 4). As expected from the Northern blot data, no Western blot signal for the α4 chain could be found in extracts of the stromal cell lines L87/4 and L88/5 (data not shown).
Immunoblot analysis of laminin α4 and β2 chains in bone marrow stromal cells.
Protein extracts of the stromal cell lines, HS-5, L88/5, and L87/4 and of LTMCs were separated on 5%-to-15% gradient gels, transferred to nitrocellulose, and incubated with an antiserum against the synthetic peptide KPPVKRPELT corresponding to the human laminin α4 chain or with the mAb C4 against the laminin β2 chain. The antilaminin α4 chain antiserum detected 2 specific bands of 200 and 300 kd in HS-5 cells and in LTMCs but not in L87/4 and L88/5 cells (not shown). A 200-kd band corresponding to the β2 chain could be detected in all stromal cell lysates (L88/5, L87/4, HS-5, and LTMCs) and in an LN-10/11 preparation (LN-10/11), which was used as a positive control.
Immunoblot analysis of laminin α4 and β2 chains in bone marrow stromal cells.
Protein extracts of the stromal cell lines, HS-5, L88/5, and L87/4 and of LTMCs were separated on 5%-to-15% gradient gels, transferred to nitrocellulose, and incubated with an antiserum against the synthetic peptide KPPVKRPELT corresponding to the human laminin α4 chain or with the mAb C4 against the laminin β2 chain. The antilaminin α4 chain antiserum detected 2 specific bands of 200 and 300 kd in HS-5 cells and in LTMCs but not in L87/4 and L88/5 cells (not shown). A 200-kd band corresponding to the β2 chain could be detected in all stromal cell lysates (L88/5, L87/4, HS-5, and LTMCs) and in an LN-10/11 preparation (LN-10/11), which was used as a positive control.
Localization of laminin chains in human bone marrow
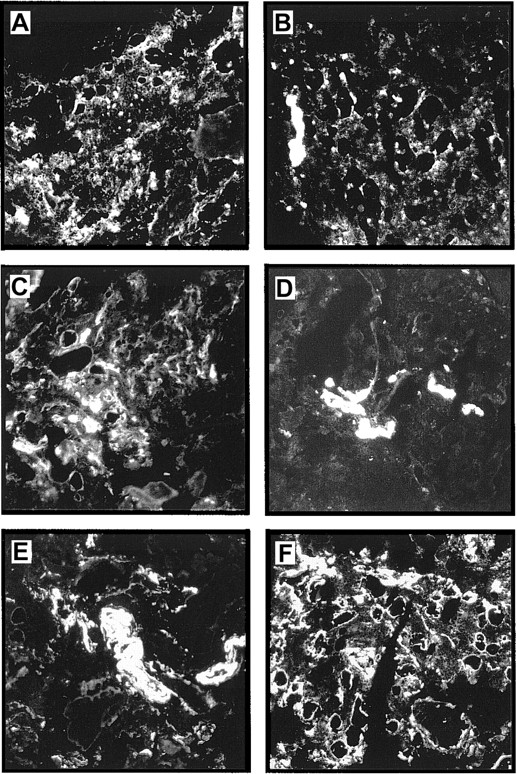
Immunostaining of human bone marrow cryostat sections with an antiserum against LN-1 showed widespread expression of laminins in the marrow. Strong staining signals could be observed in arteriolar walls, sinusoidal endothelial basement membranes, intersinusoidal interstitial connective tissue, and basement membranes of adipocytes (Figure5F). Similar staining patterns were observed with mAbs against the laminin β1 and γ1 chains, although at slightly different staining intensities (Figure 5C,E). A completely different expression pattern was observed for the laminin α5 and β2 chains. Expression of laminin β2 seemed to be restricted to the arteriolar walls, whereas laminin α5 could be found in arteriolar walls and sinusoidal cells (Figure 5B,D). The laminin α4 chain was detected mainly in intersinusoidal spaces (Figure 5A).
Expression of laminin chains in human bone marrow.
The micrographs show immunofluorescence stainings of bone marrow cryostat sections stained with the following antilaminin antibodies: (A) antiserum against the α4 chain; the staining is mainly found in intersinusoidal spaces; (B) mAb 4C7 against the α5 chain; expression is restricted to the endothelial cells; (C) mAb against the β1 chain; (D) mAb C4 against the β2 chain; the staining is restricted to the larger blood vessels; (E) mAb D18 against the γ1 chain; and (F) an antiserum against the LN-1 isoform recognizing α1, β1, and γ1 chains. Comparable staining patterns in intersinusoidal spaces and in basement membranes of adipocytes and endothelial cells are apparent in panels C, E, and F, although at different intensities. (Original magnification × 110.)
Expression of laminin chains in human bone marrow.
The micrographs show immunofluorescence stainings of bone marrow cryostat sections stained with the following antilaminin antibodies: (A) antiserum against the α4 chain; the staining is mainly found in intersinusoidal spaces; (B) mAb 4C7 against the α5 chain; expression is restricted to the endothelial cells; (C) mAb against the β1 chain; (D) mAb C4 against the β2 chain; the staining is restricted to the larger blood vessels; (E) mAb D18 against the γ1 chain; and (F) an antiserum against the LN-1 isoform recognizing α1, β1, and γ1 chains. Comparable staining patterns in intersinusoidal spaces and in basement membranes of adipocytes and endothelial cells are apparent in panels C, E, and F, although at different intensities. (Original magnification × 110.)
Double labeling of a bone marrow section with the antiserum against the laminin α4 chain and an endothelial-specific mAb showed that quite a few endothelial cells do not express the laminin α4 chain; however, some blood vessels were found to be laminin α4+ (Figure6A,B). A codistribution of the laminin α4 chain could also be observed with α-SM actin, indicating that intersinusoidal α-SM+ stromal cells or myoid cells lining sinuses also express laminin α4 (Figure 6C,D).
Localization of laminin α4 chain in human bone marrow.
Double labeling of bone marrow cryostat sections with antilaminin α4 antiserum (A,C) and the endothelial cell–specific antibody Rb10 (B) or the mAb against α-SM actin (D), respectively, revealed that the laminin α4 chain is mainly found outside the endothelial cells. The colocalization of laminin α4 chain and α-SM muscle actin indicates an expression of laminin α4 in intersinusoidal stromal cells and myoid cells lining sinuses. (Original magnification × 220.)
Localization of laminin α4 chain in human bone marrow.
Double labeling of bone marrow cryostat sections with antilaminin α4 antiserum (A,C) and the endothelial cell–specific antibody Rb10 (B) or the mAb against α-SM actin (D), respectively, revealed that the laminin α4 chain is mainly found outside the endothelial cells. The colocalization of laminin α4 chain and α-SM muscle actin indicates an expression of laminin α4 in intersinusoidal stromal cells and myoid cells lining sinuses. (Original magnification × 220.)
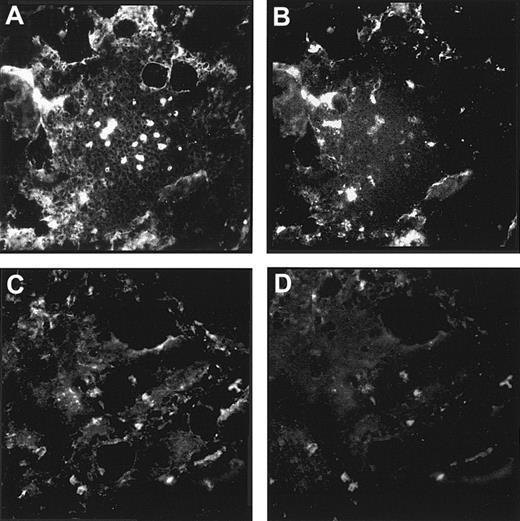
The codistribution of laminin α5 and laminin β2 in arteriolar walls was analyzed in consecutive bone marrow sections, because only 2 unlabeled murine mAbs against these 2 laminin chains were available. The observed staining pattern revealed a colocalization of laminin α5 and β2 chains in these larger blood vessels (Figure7A,B). In the arteriolar walls, the laminin α2 chain also could be detected. This was shown in a double staining with α-SM actin, which strongly labeled the arteriolar walls (Figure 7C,D). Outside the arteriolar smooth muscle cell layer, no laminin α2 expression could be observed in the marrow. Recently it has been suggested that human megakaryocytes may be a source of laminin expression within the human marrow.23 Antibodies against the integrin β3 chain can be used to localize megakaryocytes in the marrow because these antibodies strongly, although not exclusively, label these giant cell types. Double labeling of a bone marrow section with the LN-1 antiserum and a mAb against the β3 integrin chain revealed that no laminin isoforms containing β1 or γ1 chains are expressed by human megakaryocytes (Figure 7E,F). The expression pattern of laminin isoforms in the marrow is summarized in Table2.
Colocalization of laminin chains in arteriolar walls and in megakaryocytes.
(A,B) The 2 micrographs show 2 consecutive bone marrow cryostat sections stained with the antilaminin α5 chain antibody, 4C7 (A), and the antilaminin β2 chain antibody, C4 (B). A colocalization of these 2 laminin chains in arteriolar walls cells could be observed. (C,D) By double labeling of a bone marrow cryostat section, a coexpression of the laminin α2 chain (C) and of α-SM actin (D) could also be found in arteriolar walls of larger blood vessels within the marrow. Outside arteriolar walls no laminin α2 chain expression could be detected. (E,F) Double immunofluorescence staining of a bone marrow cryostat section with the polyclonal antiserum against the LN-1 isoform (E) and an anti–β3 integrin chain antibody (F) revealed that megakaryocytes (arrows in E and F) do not express laminin β1 or γ1 chains. (Original magnification × 220.)
Colocalization of laminin chains in arteriolar walls and in megakaryocytes.
(A,B) The 2 micrographs show 2 consecutive bone marrow cryostat sections stained with the antilaminin α5 chain antibody, 4C7 (A), and the antilaminin β2 chain antibody, C4 (B). A colocalization of these 2 laminin chains in arteriolar walls cells could be observed. (C,D) By double labeling of a bone marrow cryostat section, a coexpression of the laminin α2 chain (C) and of α-SM actin (D) could also be found in arteriolar walls of larger blood vessels within the marrow. Outside arteriolar walls no laminin α2 chain expression could be detected. (E,F) Double immunofluorescence staining of a bone marrow cryostat section with the polyclonal antiserum against the LN-1 isoform (E) and an anti–β3 integrin chain antibody (F) revealed that megakaryocytes (arrows in E and F) do not express laminin β1 or γ1 chains. (Original magnification × 220.)
Localization of laminin isoforms in human bone marrow
| Subunit . | Marrow cord . | Arteriolar walls . | Sinusoids/myoid cells . | Adipocytes . |
|---|---|---|---|---|
| α2 chain | − | + | − | − |
| α4 chain | + | − | + | + |
| α5 chain | − | + | + | − |
| β1 chain | + | + | + | + |
| β2 chain | − | + | − | − |
| γ1 chain | + | + | + | + |
| α-SM actin | − | + | + | − |
| Subunit . | Marrow cord . | Arteriolar walls . | Sinusoids/myoid cells . | Adipocytes . |
|---|---|---|---|---|
| α2 chain | − | + | − | − |
| α4 chain | + | − | + | + |
| α5 chain | − | + | + | − |
| β1 chain | + | + | + | + |
| β2 chain | − | + | − | − |
| γ1 chain | + | + | + | + |
| α-SM actin | − | + | + | − |
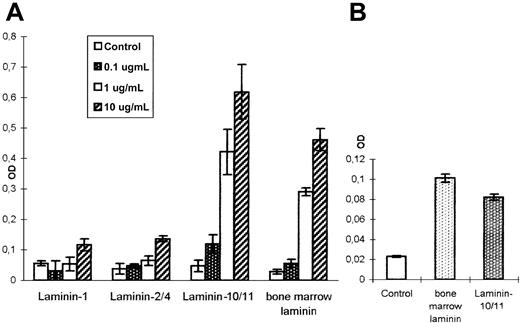
Mitogenic response of bone marrow cells to LN-10/11
To determine whether laminins have a proliferation-inducing influence on developing hematopoietic cells, 4 different laminin preparations were tested in a nonradioactive proliferation assay. LN-1 (α1β1γ1) and LN-2/4 (α2β1γ1/α2β2γ1) did not show any mitogenic effect on freshly isolated bone marrow mononuclear cells (Figure 8A). A laminin preparation isolated from LTMC supernatants by affinity chromatography as well as the LN-10/11 isoform, however, showed a strong mitogenic effect on bone marrow mononuclear cells that was dose-dependent. A more than 6-fold increase in cell proliferation could be obtained at a concentration of 1 μg/mL LN-10/11, which was further enhanced at higher concentrations (Figure 8A). Analyzing the kinetics of this proliferative response, a constant increase in cell numbers could be observed with increasing time in the presence of these 2 laminin preparations (data not shown). To determine the responsive cells in the heterogeneous bone marrow mononuclear cell preparation, CD34+ hematopoietic progenitor cells were isolated by MiniMACS technology. This highly enriched CD34+ cell population also responded to LN-10/11 and to the bone marrow laminin preparation with a proliferative burst (Figure 8B), indicating that the proliferative activity of the early hematopoietic progenitors can be influenced by interactions with laminin isoforms present within the human bone marrow.
Mitogenic activity of different laminin isoforms.
Freshly isolated human bone marrow mononuclear cells (A) and a CD34+ cell population highly enriched by MiniMACS separation (B) were incubated with different laminin isoforms and analyzed in a nonradioactive proliferation assay. No significant proliferation-inducing effect could be observed with LN-1 and LN-2/4 (A). However, LN-10/11 and a laminin preparation isolated from human LTMCs had a strong mitogenic effect on the heterogeneous mononuclear cell population (A) but also on the purified CD34+progenitor cells (B). The mitogenic effect of these 2 laminin preparations was dose-dependent. A more than 6-fold increase in mononuclear cell numbers could be detected at 1.0 μg/mL, which was further enhanced at 10 μg/mL (A).
Mitogenic activity of different laminin isoforms.
Freshly isolated human bone marrow mononuclear cells (A) and a CD34+ cell population highly enriched by MiniMACS separation (B) were incubated with different laminin isoforms and analyzed in a nonradioactive proliferation assay. No significant proliferation-inducing effect could be observed with LN-1 and LN-2/4 (A). However, LN-10/11 and a laminin preparation isolated from human LTMCs had a strong mitogenic effect on the heterogeneous mononuclear cell population (A) but also on the purified CD34+progenitor cells (B). The mitogenic effect of these 2 laminin preparations was dose-dependent. A more than 6-fold increase in mononuclear cell numbers could be detected at 1.0 μg/mL, which was further enhanced at 10 μg/mL (A).
Adhesive interactions of hematopoietic cells with LN-10/11 are mediated by β1 integrins
The ability of the laminin isoforms LN-1, LN-2/4, and LN-10/11 to support hematopoietic cell adhesion was tested in a conventional cell attachment assay using the CD34+ myeloid cell line KG1a (Figure 9) and the lymphoid cell line U266 (data not shown). Drops of different laminin concentrations were coated onto plastic dishes. After incubation for 1 hour, the unbound hematopoietic cells were carefully washed away. Even at very high coating concentrations (eg, up to 500 μg/mL), both cell lines did not adhere to LN-1. LN-2/4 was a weakly adhesive substrate for hematopoietic cells; only at high coating concentrations (100 μg/mL) was attachment of U266 and KG1a observed. In such cases, the round areas coated by LN-2/4 were evenly covered with attached cells. Outside the coated areas, only background binding could be detected (Figure 9). However, at lower coating concentrations (eg, 10 μg/mL) cell attachment of KG1a or U266 to LN-2/4 was not detectable. In contrast to LN-2/4, the LN-10/11 isoform is a strong adhesive component for hematopoietic cells. KG1a and U266 cells strongly attached to LN-10/11 coated at concentrations of 1.0 to 10 μg/mL (Figure 9). Only at 0.1 μg/mL was no cell attachment observed, indicating dose-dependent adhesion of the analyzed cell types to LN-10/11.
Cell attachment to different laminin preparations.
The CD34+ hematopoietic cell line KG1a was used to analyze adhesive interactions of the laminin isoforms LN-1, LN-2/4, and LN-10/11 in a conventional cell adhesion assay where the different laminin preparations were immobilized onto small areas of Petri dishes. LN-1 showed no cell adhesive capacity, even at a concentration of 100 μg/mL. A specific interaction of LN-2/4 with KG1a cells (represented by the white dots) was detected at 100 μg/mL, but at lower concentrations (eg, 10 μg/mL) no cell attachment to LN-2/4 was observed. LN-10/11, on the contrary, showed a strong adhesive activity at 10 μg/mL. Even at 1.0 μg/mL LN-10/11, cell attachment was only marginally diminished, and at 0.1 μg/mL no cell attachment could be observed, indicating concentration-dependent attachment. (Original magnification × 30.)
Cell attachment to different laminin preparations.
The CD34+ hematopoietic cell line KG1a was used to analyze adhesive interactions of the laminin isoforms LN-1, LN-2/4, and LN-10/11 in a conventional cell adhesion assay where the different laminin preparations were immobilized onto small areas of Petri dishes. LN-1 showed no cell adhesive capacity, even at a concentration of 100 μg/mL. A specific interaction of LN-2/4 with KG1a cells (represented by the white dots) was detected at 100 μg/mL, but at lower concentrations (eg, 10 μg/mL) no cell attachment to LN-2/4 was observed. LN-10/11, on the contrary, showed a strong adhesive activity at 10 μg/mL. Even at 1.0 μg/mL LN-10/11, cell attachment was only marginally diminished, and at 0.1 μg/mL no cell attachment could be observed, indicating concentration-dependent attachment. (Original magnification × 30.)
The specificity of binding to LN-10/11 was confirmed by inhibition with an antiserum against LN-10/11. Preincubation of the LN-10/11–coated plastic with the antiserum raised against LN-10/11 completely blocked adhesion of the hematopoietic cell types (Figure10). The adhesive interactions of U266 and KG1a cells with LN-10/11 were mediated by integrins of the β1 integrin family. Preincubation of the hematopoietic cells with the β1 integrin antibody, 4B4, completely inhibited adhesion to LN-10/11 (Figure 10). However, antibodies against the integrin α2-α6 chains did not inhibit cell adhesion to LN-10/11. The integrin-mediated interaction was not RGD-dependent, because addition of an RGD-containing hexapeptide did not interfere with adhesion (data not shown).
Integrin-mediated adhesion to LN-10/11.
Cell adhesion assays of the myeloblastic cell line, KG1a, to LN-10/11 coated at a concentration of 5 μg/mL. In the control experiment, without any inhibitory factor, strong adhesion of the KG1a cells to LN-10/11 could be observed. Addition of an antiserum against LN-10/11 (diluted 1:100) completely abolished cell adhesion (anti–LN-10/11). Preincubation of KG1a cells with the β1 integrin antibody, 4B4 (diluted 1:50), for half an hour also inhibited cell binding to LN-10/11 (anti–β1 integrin). (Original magnification × 37.)
Integrin-mediated adhesion to LN-10/11.
Cell adhesion assays of the myeloblastic cell line, KG1a, to LN-10/11 coated at a concentration of 5 μg/mL. In the control experiment, without any inhibitory factor, strong adhesion of the KG1a cells to LN-10/11 could be observed. Addition of an antiserum against LN-10/11 (diluted 1:100) completely abolished cell adhesion (anti–LN-10/11). Preincubation of KG1a cells with the β1 integrin antibody, 4B4 (diluted 1:50), for half an hour also inhibited cell binding to LN-10/11 (anti–β1 integrin). (Original magnification × 37.)
The adhesive capacity of the LN-10/11 isoform was then tested using a variety of myeloid and lymphoid cell lines. In addition to the CD34+ KG1a cell line, the myeloid cell lines TF-1, U937, and K562 also adhered to LN-10/11, whereas the megakaryoblastic cell line KU.812 did not. The Epstein-Barr virus–transformed B-lymphoid cell lines, BOB and COX, and the pre-B-leukemic cell line, BV173, did not adhere to LN-10/11, but both plasmocytoma cell lines (U266, NCI-H929) strongly adhered to LN-10/11 (Table3). This differential adhesion pattern of hematopoietic cell lines to LN-10/11 also demonstrated the specificity of the observed adhesive interactions.
Adhesion of hematopoietic cell lines to laminin-10/11
| Myeloid cell lines | |
| KG1a (myeloblastic, CD34+) | ++ |
| TF-1 (erythroid progenitor, CD34+) | + |
| U937 (promonocytic) | ++ |
| KU.812 (megakaryoblastic) | − |
| K562 (erythroleukemic) | ++ |
| Lymphoid cell lines | |
| BOB (B-lymphoid) | − |
| COX (B-lymphoid) | − |
| U266 (plasmocytoma) | ++ |
| NCI-H929 (plasmocytoma) | ++ |
| BV173 (pre-B-leukemic) | − |
| Myeloid cell lines | |
| KG1a (myeloblastic, CD34+) | ++ |
| TF-1 (erythroid progenitor, CD34+) | + |
| U937 (promonocytic) | ++ |
| KU.812 (megakaryoblastic) | − |
| K562 (erythroleukemic) | ++ |
| Lymphoid cell lines | |
| BOB (B-lymphoid) | − |
| COX (B-lymphoid) | − |
| U266 (plasmocytoma) | ++ |
| NCI-H929 (plasmocytoma) | ++ |
| BV173 (pre-B-leukemic) | − |
++ indicates strong binding (> 500 cells/mm2); +, intermediate binding (100-500 cells/mm2); −, no binding above background.
Discussion
The bone marrow provides a specified microenvironment for the development, growth, and differentiation of hematopoietic cells. Our present study provides new insights into the complex nature of laminin isoforms expressed in human bone marrow. We could demonstrate that human bone marrow stromal cells do not express laminin isoforms containing α1, α2, or α3 chains. Instead, synthesis of the laminin α4 and α5 chains together with the laminin β1, β2, and γ1 chains could be detected by Northern blotting and by immunofluorescence stainings using chain-specific antibodies, suggesting the secretion of LN-8/9 and LN-10/11 isoforms. The laminin chains synthesized in vitro by bone marrow stromal cells were also detected in native bone marrow cryostat sections, although at various locations. Finally, purified human laminin preparations containing LN-10/11 isoforms show strong adhesive and mitogenic activities toward developing hematopoietic cells, whereas other laminin isoforms, not present in the human bone marrow, show no or only weak functional interactions.
LTMCs are a widely used model to study myeloid development.1 In this in vitro system, proliferation and differentiation of the hematopoietic progenitor cells are strongly dependent on the formation of a heterogeneous adherent stromal cell layer. Analysis of the stromal cells of human LTMCs by RT-PCR revealed amplification products of all analyzed laminin chains, with the exception of the β3 and γ3 chains. Such a broad expression pattern was not expected and cannot be explained by the heterogeneity of the primary stromal cells in culture, because the clonal bone marrow stromal cell lines also showed similar results in RT-PCR analysis. A more likely explanation for this finding is the enormous sensitivity of RT-PCR, which can detect even trace amounts of mRNA.
The ability of the human stromal cells to synthesize all 5 laminin α chains, however, does not mean that all these chains are expressed at the same level. Northern blotting of RNA derived from primary stromal cells and 3 established human stromal cell lines showed that only laminin α4 and α5 chains are expressed at significant amounts, whereas the expression of the 3 other laminin α chains (α1, α2, and α3) falls below the detection limits of Northern blotting, indicating only minimal synthesis. These results are in partial agreement with data obtained from rodent bone marrow cultures.23 Whereas the laminin α4 and α5 chains can be detected in human and in murine bone marrow stromal cells, expression of the α1 and α2 chains was also detected in murine stromal cells in vitro. Another discrepancy concerns the laminin β2 chain, which was not expressed by rodent stromal cells but is strongly synthesized by human stromal cells in vitro.
The laminin isoform expression pattern in human bone marrow stromal cells as detected by Northern blotting was confirmed by immunofluorescence stainings and Western blotting using laminin chain–specific antibodies. Immunoblotting of stromal cell lysates with an antiserum against human laminin α4 showed 2 bands of 200 and 300 kd. Whereas a 200-kd band for laminin α4 has been reported in earlier studies,37 43 the existence of the 300-kd band has not been previously reported. Whether this band represents a splice variant or an unprocessed precursor form of the laminin α4 chain requires elucidation in further studies.
Remarkably, the laminin isoforms secreted by the stromal cells were not deposited in an extracellular meshwork, as is the case for many other ECM molecules, including fibronectin, tenascin, perlecan, or various collagen types.6,8,44,45 The reason for this is not clear, but it may have functional implications suggesting that laminin isoforms secreted by bone marrow stromal cells might be important for cell proliferation or cell migration of hematopoietic cells. Secretion into the cell culture supernatants rather than an extracellular deposition facilitates isolation of laminin isoforms. The human bone marrow stromal cell line HS-5 may be regarded as a suitable source for isolation of human LN-8/9 because it only synthesizes the α4, β1, β2, and γ1 chains. The stromal cell lines L87/4 and L88/5, which only synthesize α5, β1, β2, and γ1 chains can be used as a source for human LN-10/11, but other human cell lines, including adenocarcinomas and choriocarcinomas, have also been reported to produce LN-10/11.46 47 Measurements of the synthesized amounts of laminin isoforms (eg, by enzyme-linked immunosorbent assay) should indicate the appropriate cell lines for purification of different human laminin isoforms.
In native human bone marrow, laminin isoforms were found in the marrow cord, in arteriolar walls, and in basement membranes of endothelial sinusoidal cells and adipocytes. The laminin α4 chain was mainly detected in intersinusoidal spaces in the marrow cord and in some endothelial cells, in agreement with the findings of Gu and coworkers.23 Endothelial cells do not express α-SM actin, whereas myoid cells lining sinuses express α-SM actin.48 Our double-labeling experiments indicated that myoid cells express the laminin α4 chain. The laminin α5 chain has been reported to be the major laminin chain of mature blood vessels.49 In accordance with this, all arteriolar walls and endothelial sinusoidal cells in the human bone marrow were found to express laminin α5. Another laminin chain associated with vascular basement membranes is laminin β2.50 Immunoflourescence stainings of bone marrow sections indicated coexpression of laminin α5 and β2 in arteriolar walls. At other sites, no significant expression of laminin β2 could be detected in bone marrow cryostat sections. These findings are in contrast to a recent study of Vogel et al,24 who reported an expression of laminin β2 by megakaryocytes in isolated bone marrow mononuclear cell fractions. According to our present double immunofluoresence stainings of native bone marrow tissue, megakaryocytes also do not express laminin β1 or γ1 chains, making the expression of a laminin isoform by human megakaryocytes unlikely. Two human erythromegakaryocytic cell lines, however, are able to synthesize LN-8 in vitro.51 At present, we have no explanation for this discrepancy.
The finding that the laminin α1 chain is not expressed by human bone marrow stromal cells may explain why no significant interaction of several human hematopoietic cells or cell lines with the LN-1 isoform have been detected in the past.25-27 Functional analyses of LN-10/11 have only recently become possible since it has been shown that a widely used human laminin preparation purified by affinity chromatography from placenta with the laminin α5 specific antibody, 4C7, contains only LN-10/11 isoforms.34 The very recently reported production of recombinant LN-8 should make it possible to functionally analyze this laminin isoform in the near future.52
Using identical cell adhesion conditions for LN-1, LN-2/4, and LN-10/11, we demonstrated that LN-10/11 was a strong adhesive substrate for the 2 hematopoietic cell lines, KG1a and U266, which represent 2 distinct cell types present in bone marrow. LN-1, however, did not show any adhesive activity; LN-2/4 isoforms showed an intermediate activity. A similar finding has recently been reported for sickle red blood cells. Whereas normal red blood cells do not adhere to any laminin isoform, sickle red blood cells strongly adhered to LN-10/11 but not to human LN-2/4 or murine LN-1.53
The specificity of hematopoietic cell interactions with LN-10/11 was further shown by using lymphoid cell lines that represent different stages of B-lymphocyte development. BOB and COX cells representing mature B cells outside the bone marrow did not adhere to LN-10/11, whereas plasmocytoma cell lines, which represent plasma cells found in the marrow microenvironment, strongly adhered to LN-10/11.28
Inhibition assays revealed that the adhesive interactions of the human hematopoietic cells with LN-10/11 are mediated by integrins of the β1 family. The most prominent integrin laminin receptor, α6β1, however, is not responsible for binding of human hematopoietic cell lines because neither KG1a cells nor U266 cells express the α6 integrin chain. Human CD34+ hematopoietic progenitor cells also do not express significant amounts of α6 integrin.54 The present study and Kibler et al28 show that another laminin receptor, α3β1 integrin, is not responsible for binding of KG1a or U266 cells to LN-10/11. Therefore, the expression and involvement of other integrin alpha chains (eg, α7-α11), which have not been analyzed so far on human hematopoietic cells, have to be tested in the near future.
As shown recently for tenascin-C, ECM molecules can directly stimulate hematopoietic cell proliferation.10 Laminin isoforms can also induce a mitogenic response, as shown previously for nonhematopoietic cells with LN-1.55 The mitogenic effect observed in the present study for LN-10/11 on bone marrow mononuclear cells was also very striking. As expected from the expression pattern in the human bone marrow, LN-1 and LN-2/4 did not show any mitogenic effect on developing hematopoietic cells. Since bone marrow mononuclear cells represent a heterogeneous cell population, it is important to determine the individual cell types that can respond to the mitogenic stimuli. Whereas the early CD34+ hematopoietic progenitor cells were not able to give a mitogenic response to tenascin-C,10 these cells could respond to LN-10/11 with increased cell proliferation, indicating an important function for LN-10/11 in early hematopoietic progenitor cell development. A major challenge for the future will be to determine the receptors that transduce the mitogenic signals to the hematopoietic cells. Since the laminin preparations used have been purified from tissues or cell culture supernatants, it is possible that they are contaminated with growth factors that could be responsible for the observed mitogenic effects. Although we cannot exclude this possibility, the fact that the proliferation-inducing LN-10/11 isoform and the inactive LN-2/4 isoform were both isolated from human placenta makes this possibility unlikely. Furthermore, LN-1 was isolated from tumor tissue with high content of growth factors and did not show any mitogenic activity.
Taken together, the adhesive and mitogenic response of hematopoietic cells to LN-10/11 suggests important functions for this laminin isoform during hematopoiesis. Although there are species-dependent differences in laminin isoform expression in the bone marrow, the LN-10 isoform is expressed both in mouse and human. A careful examination of hematopoietic cell development in laminin α5 chain knockout mice that die late in embryogenesis56 might, therefore, reveal defects in the hematopoietic microenvironment.
Acknowledgments
The authors thank Ingrid Teufel and Jutta Hildenbrand for expert technical assistance. We are grateful to Drs Jeffrey Miner (Washington University, St Louis, MO), Rupert Hallmann (University of Erlangen), and Karl Tryggvason (Karolinska Institut, Stockholm, Sweden) for their kind donation of antibodies and cDNA probes. We also thank Dr Christoph Faul (University of Tübingen) for a constant supply of bone marrow samples.
Supported by grants from the Deutsche Forschungsgemeinschaft (Kl 709/2-3) and from the fortüne-program of the Medical Faculty of the University of Tübingen (grant 761-0-0).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Gerd Klein, University Medical Clinic, Dept II, Section for Transplantation Immunology and Immunohematology, ZMF (Center for Medical Research), Waldhörnlestrasse 22, 72072 Tübingen, Germany; email: gerd.klein@uni-tuebingen.de.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal