The recent Blood focus on hematology describing the role of bisphosphonates in the therapy of multiple myeloma, and the stimulation of γδ T cells by bisphosphonates and the induction of antiplasma cell activity in multiple myeloma,1,2 also cites several possible mechanisms for the beneficial effects observed in the treatment of human malignancies by bisphosphonates.3,4 These include the inhibition of cellular functions, induction of apoptosis, and change in the local concentrations and expressions of growth factors, cytokines, adhesion molecules, and so forth. But we propose that another mechanism may be involved, namely, the inhibition of matrix metalloproteinase (MMP) activity by bisphosphonates.5 There may also be other MMP-related mechanisms involved, such as TIMP- or substrate-related mechanisms, as shown by Farina et al6 and Makowski et al,7 respectively.
Recently, Diehl et al8 reported on significant decrease of breast cancer metastases both in bone and in soft tissues of patients receiving adjunctive clodronate therapy. Extensive literature supports a role for various MMPs in cancer growth and metastasis, especially collagenases-1 and -3 (MMP-1 and MMP-13),9 the gelatinases (MMP-2 and MMP-9), and the membrane-type MMPs (MT-MMPs); futhermore, several MMPs have been shown to be expressed by multiple myeloma. MMPs can collectively degrade the extracellular matrix and basement membrane, which act as barriers to tumor spread and growth. Therefore, identification of pharmacologically potential agents that might inhibit human cell–derived MMPs has long seemed a reasonable therapeutic goal for modulation and down-regulation of metastases formation and bone destruction in these pathological states.1,2 10
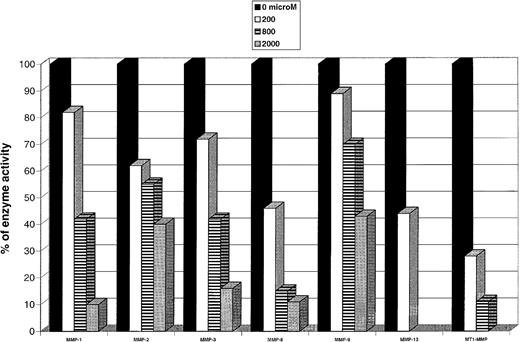
Our data show (Figure 1) that clodronate (and several other bisphosphonates; data not shown) can inhibit in vitro the activities of cancer-related enzymes MMP-2, MMP-9, MMP-13, and MT1-MMP (and several other MMPs).5,11,12 These drugs also inhibit reconstituted basement membrane-invasion of malignant cell lines (capable of expressing these MMPs) in a dose-dependent manner and at concentrations attainable in vivo.5 In our study with patients receiving clodronate therapy, a slight but significant decrease in the salivary collagenase levels was observed after 3 weeks, demonstrating a down-regulating effect of MMPs by clodronate in vivo.13 We propose that the beneficial effects of the bisphosphonates on the metastatic process may be related to inhibition and down-regulation of various genetically distinct MMPs that are crucial in the escape of malignant cells into and out of circulation, and destruction of local tissue at a metastatic site.
Representative data demonstrating dose-dependent inhibition of various MMP activities by clodronate.
The activity of MMPs were assayed by a urokinase-based activity assay.5
Representative data demonstrating dose-dependent inhibition of various MMP activities by clodronate.
The activity of MMPs were assayed by a urokinase-based activity assay.5

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal