We have read with great interest the recent article by Zain et al.1 We have made similar observations regarding the dual effect of thrombin in human tumor cells2 and would like to note the following.
The dual effects of thrombin (ie, inducing proliferation at concentrations less than 0.5 U/mL and apoptosis at concentrations of at least 0.5 U/mL) are effective on a wide variety of tumors. We have observed these effects on all the human tumor cell lines that we have examined (HeLa, U937, Jurkat, Daudi, CEM, Raji, HL60, Caco2, KSY-1, K562, MCF-7, MDA-231, 1301, CA46, BL41, etc). It should be noted that similar dual effects of thrombin have been observed on human neurons. But the concentration required to induce apoptosis in these cells is 80- to 200-fold higher than that required in the tumor cells.3,4 Zain et al1 did not mention these effects of thrombin on neurons.
The normal human peripheral blood mononuclear cells (PBMC) are relatively resistant to the proapoptotic effects of thrombin.
The thrombin-induced apoptosis in these tumor cells occurs without consistent changes in the expression of bcl-2, p53, or p21 proteins.
It is highly unlikely that all the above tumor cell lines that we tested and found susceptible to the thrombin-induced apoptosis express PAR-1. It may suggest the involvement of receptors/molecules other than PAR-1. Interestingly, we were also unable to observe apoptosis when the tumor cells were incubated with the PAR-1 activating hexapeptide (SFLLRN) at high concentrations, which could be due to the degradation by proteases as suggested by Zain et al.1
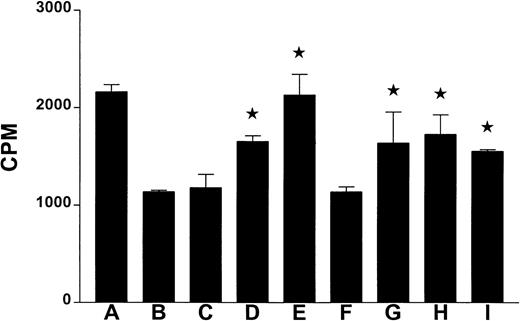
In an attempt to learn about the signaling pathways involved in the thrombin-induced apoptosis of the tumor cells, we incubated the U937 tumor cells with thrombin with or without some known inhibitors of cell signaling. As shown in Figure 1, genistein, H-7, wortmanin, cytochalasin D, and DEVD-CHO significantly prevented the growth inhibitory effects of thrombin. This suggests the involvement of caspases, protein kinase C, PI3-K, unspecified tyrosine kinases, and an intact cytoskeleton for the thrombin-induced apoptosis in the human tumor cells. Interestingly, similar observations were made regarding the signaling pathways involved in the protective and proapototic effects of thrombin on neurons.3 4
Effect of signal transduction inhibitors on the thrombin-induced apoptosis.
The human tumor cells (U937) were incubated in triplicate in the wells of a 96-well microculture plate (1 × 104 cells/well) with or without the presence of thrombin (0.5 U/mL), and/or the indicated inhibitors for 16 hours. The microcultures were pulsed with 0.037 MBq (1 μCi) of 3H-thymidine for 8 hours, and the3H-thymidine uptake was determined using liquid scintillation counting. Each bar represents average CPM ± SE from triplicate microcultures. The star on the bar indicates that the inhibitor was able to significantly (P ≤ .05; Studentt test) block the thrombin-induced inhibition of proliferation/apoptosis. The letters A-I on the x-axis indicate the inhibitors used in the microcultures: (A) no thrombin; (B) 0.5 U/mL thrombin; (C) 0.5 μmol/L herbimycin A (an inhibitor of phospholipase D, tyrosine kinases, and anti-CD3 antibody mediated apoptosis); (D) 100 μmol/L genistein (a broad spectrum inhibitor of protein tyrosine kinases); (E) 10 μmol/L H-7 (a potent inhibitor of PKC); (F) 100 μmol/L HA-1004 (an inhibitor of CaM kinase II, myosin light chain kinase, and PKA); (G) 1 μmol/L wortmanin (an inhibitor of PI3-kinase, and phospholipase D); (H) 0.5 μg/mL cytochalasin D (an inhibitor of function of the cytoskeletal protein actin); and (I) 0.3 μM DEVD-CHO (a cell permeable inhibitor of caspases 3, 6, 7, 8, and 10). Microcultures C-I were incubated in the presence of thrombin (0.5 U/mL). All these inhibitors were from Calbiochem (San Diego, CA).
Effect of signal transduction inhibitors on the thrombin-induced apoptosis.
The human tumor cells (U937) were incubated in triplicate in the wells of a 96-well microculture plate (1 × 104 cells/well) with or without the presence of thrombin (0.5 U/mL), and/or the indicated inhibitors for 16 hours. The microcultures were pulsed with 0.037 MBq (1 μCi) of 3H-thymidine for 8 hours, and the3H-thymidine uptake was determined using liquid scintillation counting. Each bar represents average CPM ± SE from triplicate microcultures. The star on the bar indicates that the inhibitor was able to significantly (P ≤ .05; Studentt test) block the thrombin-induced inhibition of proliferation/apoptosis. The letters A-I on the x-axis indicate the inhibitors used in the microcultures: (A) no thrombin; (B) 0.5 U/mL thrombin; (C) 0.5 μmol/L herbimycin A (an inhibitor of phospholipase D, tyrosine kinases, and anti-CD3 antibody mediated apoptosis); (D) 100 μmol/L genistein (a broad spectrum inhibitor of protein tyrosine kinases); (E) 10 μmol/L H-7 (a potent inhibitor of PKC); (F) 100 μmol/L HA-1004 (an inhibitor of CaM kinase II, myosin light chain kinase, and PKA); (G) 1 μmol/L wortmanin (an inhibitor of PI3-kinase, and phospholipase D); (H) 0.5 μg/mL cytochalasin D (an inhibitor of function of the cytoskeletal protein actin); and (I) 0.3 μM DEVD-CHO (a cell permeable inhibitor of caspases 3, 6, 7, 8, and 10). Microcultures C-I were incubated in the presence of thrombin (0.5 U/mL). All these inhibitors were from Calbiochem (San Diego, CA).
The relative resistance of normal human cells to the thrombin-induced apoptosis, as compared to the tumor cells, suggests that thrombin or its mimetic peptides may be potentially useful as anticancer agents and in the future may lead to novel thrombin-based anticancer therapies.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal