Abstract
The dynamics of cell cycle regulation were investigated during in vitro erythroid proliferation and differentiation of CD34+cord blood cells. An unusual cell cycle profile with a majority of cells in S phase (70.2%) and minority of cells in G1 phase (27.4%) was observed in burst-forming unit-erythrocytes (BFU-E)–derived erythroblasts from a 7-day culture of CD34+ cells stimulated with interleukin 3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), Steel factor, and Epo. Terminal erythroid differentiation was accompanied by a rapid increase of G0/G1 phase cells. Expression of cyclin E and cyclin-dependent kinase 2 (cdk2) correlated with the proportion of S phase cells. Cyclin D3 was moderately up-regulated during the proliferation phase, and both cyclin E and D3 were rapidly down-regulated during terminal differentiation. This suggests that the high proliferation potential of erythroblasts is associated with temporal up-regulation of cyclin E and cdk2.
Introduction
Cell proliferation, differentiation, and fate are controlled by the cell cycle, which depends on a highly ordered formation and activation of cyclin-cyclin–dependent kinase (cdk) complexes. Transition of cells from G1 to S phase depends on activation of kinase complexes containing D-type cyclins, on the catalytic subunit cdk4 or cdk6, and formation of a complex containing cyclin E and cdk2; S phase progress requires synthesis of cyclin A and activation of the cdk2/cyclin A complex; transition from G2 to M phase is initiated by the mitotic cdk complex composed of cyclin B and p34cdc2.1G1 cyclins, such as cyclin D and E, play crucial roles as rate-limiting activators in G1/S phase transition.2-6 Cyclin E binds to and activates cdk2 protein kinase, and in proliferating cells the assembly of catalytically active cyclin E-cdk2 complexes is directly related to the abundance of cyclin E protein.2
Hematopoiesis is a highly regulated process of cell proliferation and differentiation from a small number of quiescent stem cells, which give rise to nonproliferative and terminally differentiated mature lineage cells.7 Regulation of the cell cycle of stem/progenitor cells by cyclins, cdks, and cdk inhibitors may contribute to proliferation and differentiation of various lineage cells.8-12 Less information is known about cell cycle regulation in normal erythropoiesis. Cdc2, cdk2, cdk4, cyclin D1, and cyclin A are induced by multiple cytokines in human CD34+cells and chicken erythroid progenitors.13 14 Thus, we evaluated cell cycle regulatory proteins during erythroid proliferation and differentiation from normal CD34+ cord blood (CB) cells.
Study design
Recombinant human (rhu) Epo, Steel factor (SLF), and granulocyte colony-stimulating factor (G-CSF) were purchased from Amgen Corp and R & D Systems (Thousand Oaks, CA). Rhu granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin 3 (IL-3) were kind gifts from Immunex (Seattle, WA). Fluorescein isothiocyanate (FITC)-conjugated antihuman CD34 (Becton Dickinson Immunocytometry System, San Jose, CA), FITC-conjugated anti-CD71, antiglycophorin A (GPA), phycoerythrin (PE)-conjugated anti-CD34, and anti-CD117 (PharMingen, San Diego, CA) were used for flow cytometry analysis. For bivariate flow cytometry analysis, FITC-conjugated mouse or rabbit antibodies against cyclin A, B1, D2, D3 (Santa Cruz Biotechnology, Santa Cruz, CA), and FITC-conjugated mouse or rabbit anticyclin D1, E, cdk 2, and cdc2 (PharMingen) were used. For immunoblot analysis, monoclonal or polyclonal mouse or rabbit antibodies against cyclin D1, D2, D3, E, B1, A, cdk2, cdk4, and cdc2 were purchased from Santa Cruz Biotechnology.
Cells were obtained from CB scheduled for discard. Eighty percent to 95% pure CD34+ cells were isolated with MACS beads (Miltenyi Biotec, Auburn, CA).15CD34+ cells were cultured either in suspension or semisolid culture for differentiation. To prepare burst-forming unit-erythrocytes (BFU-E)–derived cells, 200 cells/mL were plated in semisolid media.15
Cells were stained with 50 μg/mL propidium iodide (PI, Sigma, St Louis, MO) and analyzed by FACscan flow cytometry (Becton Dickinson) for cell cycle status, stained with PE- or FITC-conjugated anti-CD34, anti-CD71, or anti-GPA antibodies and analyzed for cell surface antigens. Cells were fixed and permeabilized using Cytofix/Cytoperm reagent (PharMingen). Intracellular staining was performed with 10 μg/mL FITC-conjugated antibodies against cyclin D1, D2, D3, E, A, B1, cdk2, and cdc2, and cells were counterstained with PI for bivariate flow cytometry analysis.16
Equal amounts of protein in cell lysates were separated by 10% SDS-PAGE, and electroblotted onto Immobilon-P membranes (Millipore, Bedford, MA). Membranes were incubated with antibodies against cell cycle regulatory proteins, followed by incubation with horseradish peroxidase–conjugated goat antirabbit or goat antimouse antibodies, and proteins were detected by Enhanced Chemi-luminescence (ECL)–Western blotting system (Amersham Life Sciences).
Results and discussion
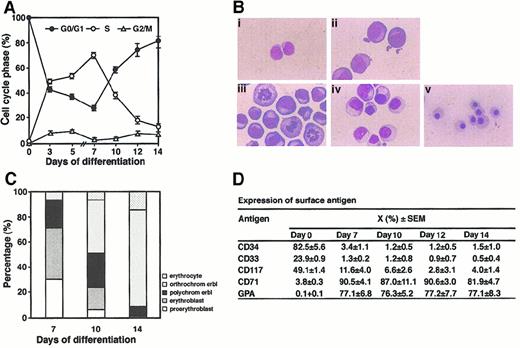
Freshly separated CD34+ cells were quiescent with 99.9% of cells in G1/G0 phase (Figure1). On stimulation with cytokines, CD34+ cells entered S phase. The proportion of S phase cells increased 50% to 55%, whereas G0/G1 phase cells decreased 48% and 36%, respectively, at day 3 and 5 of suspension cultures (Figure1A). An unusual cell cycle profile with a majority of cells in S phase (70.2%) and a minority of cells in G1 phase (27.4%) was observed in BFU-E–derived erythroblasts from day 7 cultures. After 7 days of culture, the proportion of S phase cells rapidly decreased and G1 phase cells increased. Only 15% of BFU-E–derived cells remained in S phase and 80.1% of cells were in G0/G1 phase on day 14, suggesting terminal differentiation of erythroid progenitor cells and final exit from the cell cycle. Fresh CD34+ cells are small and contain little cytoplasm (Figure 1B,C). After culture in the presence of cytokines, cell size increased. Ninety-eight percent of cells resembled proerythroblasts at day 4. Seventy-five percent of day 7 BFU-E–derived cells were proerythroblasts and erythroblasts, small numbers were orthochromatic erythroblasts, almost no mature red blood cells were detected, and cell size decreased after 7 days. In BFU-E–derived cells, the percentage of orthochromatic erythroblasts increased on day 10 to a maximal level on day 14, at which time some mature erythrocytes were also present and increased from that noted on day 10 (Figure 1C), indicating that these cells underwent terminal differentiation. Day 7 BFU-E–derived cells have a large proliferative capacity and can generate many CFU-E colonies (data not shown). Expression ofc-kit and CD34 antigen decreased with cell maturation, whereas differentiated cells expressed CD71 and GPA, indicating a more mature nature (Figure 1D).
Cell cycle status and morphology during erythroid differentiation of CB CD34+ cells.
(A) Freshly separated CD34+ cells (2 × 104/mL) (day 0), or cells from suspension culture of CD34+ cells in the presence of IL-3 (200 units), GM-CSF (200 units), SLF (50 ng) and Epo (1 unit) per milliliter for 3 and 5 days or from BFU-E–derived cells from semisolid cultures after 7, 10, 12, and 14 days of growth in the presence of IL-3, GM-CSF, SLF, Epo, vitamin B12 (10 μg, Sigma), folic acid (15 μg, Sigma), and insulin (10 μg, Sigma) per milliliter were used for cell cycle analysis. Results are the mean ± SEM from 5 experiments. (B) Wright-Giemsa stained day 0 CD34+ cells (i) and cells from suspension culture after 4 days (ii), or from BFU-E–derived colonies grown in the presence of cytokines for 7 (iii), 10 (iv), and 14 (v) days (original magnification × 1000). (C) Morphologic classification of BFU-E–derived cells. Cell types were determined by examining 500 cells per time point and are expressed as a percentage. (D) Expression of surface antigen in day 0 CD34+ cells and BFU-E–derived cells. Results are the mean percentage of positive cells ± SEM from 5 experiments.
Cell cycle status and morphology during erythroid differentiation of CB CD34+ cells.
(A) Freshly separated CD34+ cells (2 × 104/mL) (day 0), or cells from suspension culture of CD34+ cells in the presence of IL-3 (200 units), GM-CSF (200 units), SLF (50 ng) and Epo (1 unit) per milliliter for 3 and 5 days or from BFU-E–derived cells from semisolid cultures after 7, 10, 12, and 14 days of growth in the presence of IL-3, GM-CSF, SLF, Epo, vitamin B12 (10 μg, Sigma), folic acid (15 μg, Sigma), and insulin (10 μg, Sigma) per milliliter were used for cell cycle analysis. Results are the mean ± SEM from 5 experiments. (B) Wright-Giemsa stained day 0 CD34+ cells (i) and cells from suspension culture after 4 days (ii), or from BFU-E–derived colonies grown in the presence of cytokines for 7 (iii), 10 (iv), and 14 (v) days (original magnification × 1000). (C) Morphologic classification of BFU-E–derived cells. Cell types were determined by examining 500 cells per time point and are expressed as a percentage. (D) Expression of surface antigen in day 0 CD34+ cells and BFU-E–derived cells. Results are the mean percentage of positive cells ± SEM from 5 experiments.
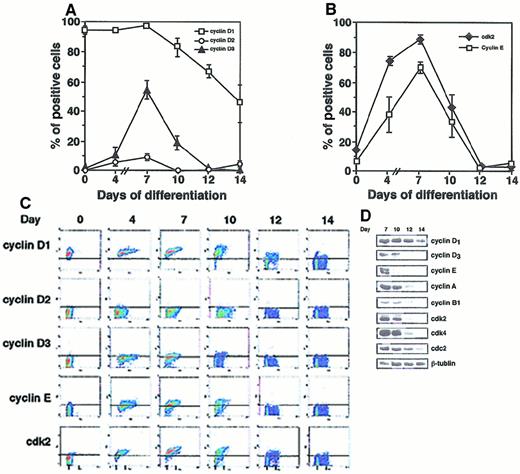
Proliferation of early erythroblasts is possibly due to a rapid transition of cells from the G1 to S phase. Therefore, we first observed G1 cyclins by flow cytometry (Figure2). Fresh CD34+ cells expressed high levels of cyclin D1, but not cyclins D2 or D3 (Figure2A). Cyclin D3, but not cyclin D2, was moderately up-regulated in day 4 BFU-E–derived cells and expressed at maximal levels in day 7 BFU-E–derived cells, after which cyclin D3 decreased. Expression of cyclin E correlated dramatically with S phase cells (Figure 2B,C). Expression of cyclins E and D3 rapidly decreased during terminal differentiation after 10 days, whereas cyclin D1 was moderately down-regulated. This was confirmed by immunoblot analysis (Figure 2D). Increases in cyclins D3 and E were associated with the G1/S phase transition and remained at high levels during the S and G2/M phases (Figure 2C). Increased expression of cyclins A and B1 was seen after cells entered the cell cycle with maximal expression during the S and G2/M phases. Expression of these cyclins decreased on terminal differentiation of erythroid progenitors (Figure 2D). Increases of cyclins D3 and E were associated with initiation of the G1/S phase transition, and they remained at high levels during the S and G2/M phases (Figure 2C). Cyclin binding and activation of cdk is essential for cell cycle regulation. Expression of cdk2 was remarkably similar to cyclin E (Figure 2B,C,D). Western blot analysis confirmed this similar pattern, and cdk2 and cdk4 both decreased with terminal differentiation (Figure 2D). In summary, our results suggest that an expansion phase preceding terminal erythroid differentiation is tightly associated with the expression of high levels of cyclin E and cdk2, and that down-regulation of cyclin E and cdk2 might be essential for initiation of terminal erythroid differentiation.
Expression of cyclin proteins during erythroid differentiation of CB CD34+ cells.
(A) and (B) Cells were analyzed by bivariate cell cycle analysis. Results are the mean ± SEM from 4 experiments. (C) Scatter density diagrams of bivariate flow cytometric analysis of cell cycle proteins. G0/G1 (2N) and G2/M (4N) phase populations are indicated. Above the solid horizontal lines are cutoffs for positive staining based on the isotype controls. One of 5 representative experiments is shown. (D) Expression of cyclin and cdk proteins determined by immunoblot analysis. Equal amounts of total cell protein were subjected to SDS/PAGE and immunoblotting, with tublin used as control. Results are 1 of 3 representative experiments.
Expression of cyclin proteins during erythroid differentiation of CB CD34+ cells.
(A) and (B) Cells were analyzed by bivariate cell cycle analysis. Results are the mean ± SEM from 4 experiments. (C) Scatter density diagrams of bivariate flow cytometric analysis of cell cycle proteins. G0/G1 (2N) and G2/M (4N) phase populations are indicated. Above the solid horizontal lines are cutoffs for positive staining based on the isotype controls. One of 5 representative experiments is shown. (D) Expression of cyclin and cdk proteins determined by immunoblot analysis. Equal amounts of total cell protein were subjected to SDS/PAGE and immunoblotting, with tublin used as control. Results are 1 of 3 representative experiments.
Acknowledgment
We thank Patricia Mantel for her help in preparing the manuscript.
Supported by Public Health Service Grants R01 HL 56416, and R01 DK 53674 from the National Institutes of Health to H.E.B., and by grants from the Phi Beta Psi Sorority to L.L.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Li Lu, The Walther Oncology Center, Indiana University School of Medicine, 1044 W Walnut St, R4, Rm 302, Indianapolis, IN, 46202-5121.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal